Tremella fuciformis Berk Alleviated Atherosclerosis Symptoms via Nuclear Factor-Kappa B-Mediated Inflammatory Response in ApoE−/− Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
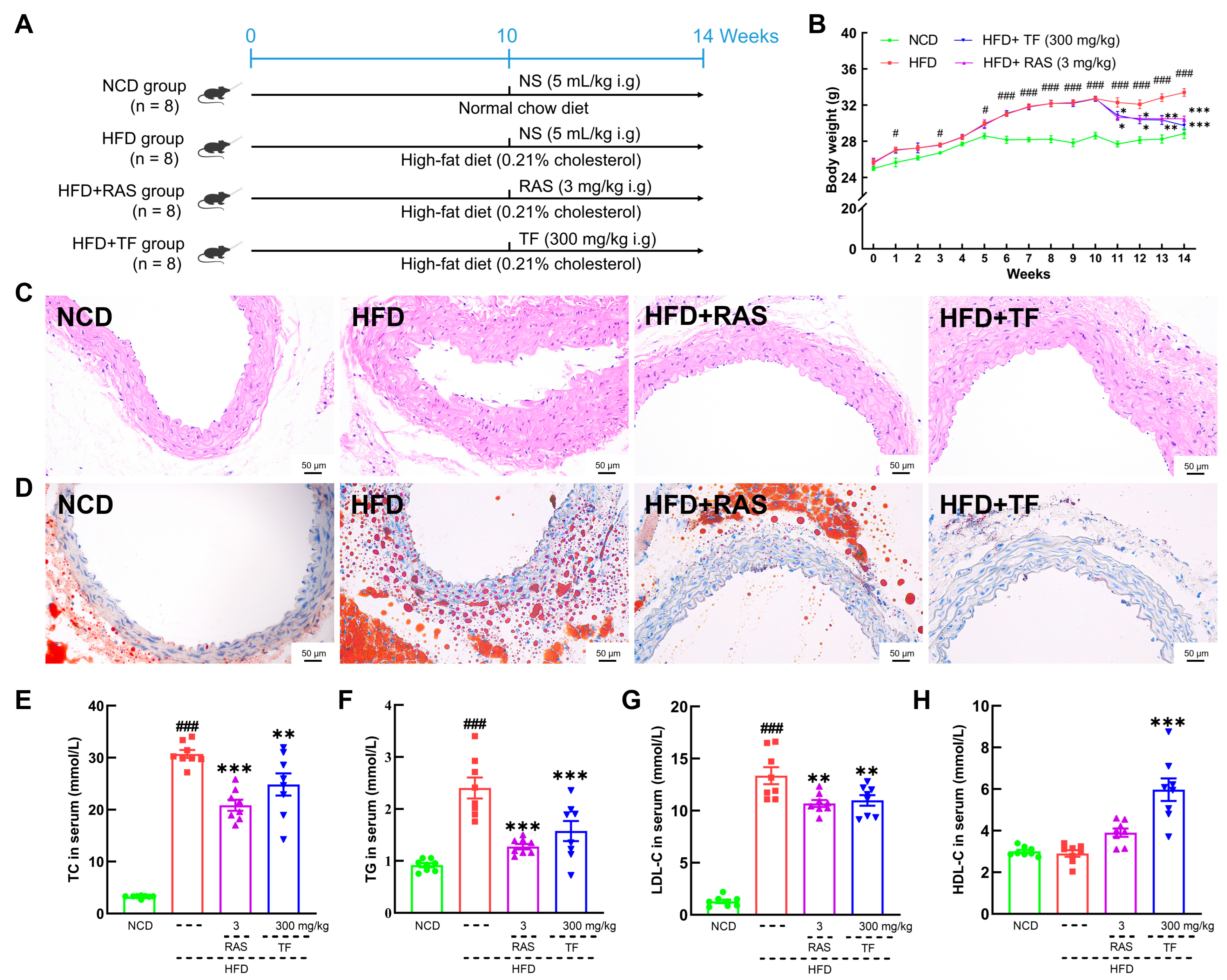
2.2. Animals and Experiment Design
2.3. Organ and Adipose Tissue Indices Analysis
2.4. Histological Analysis
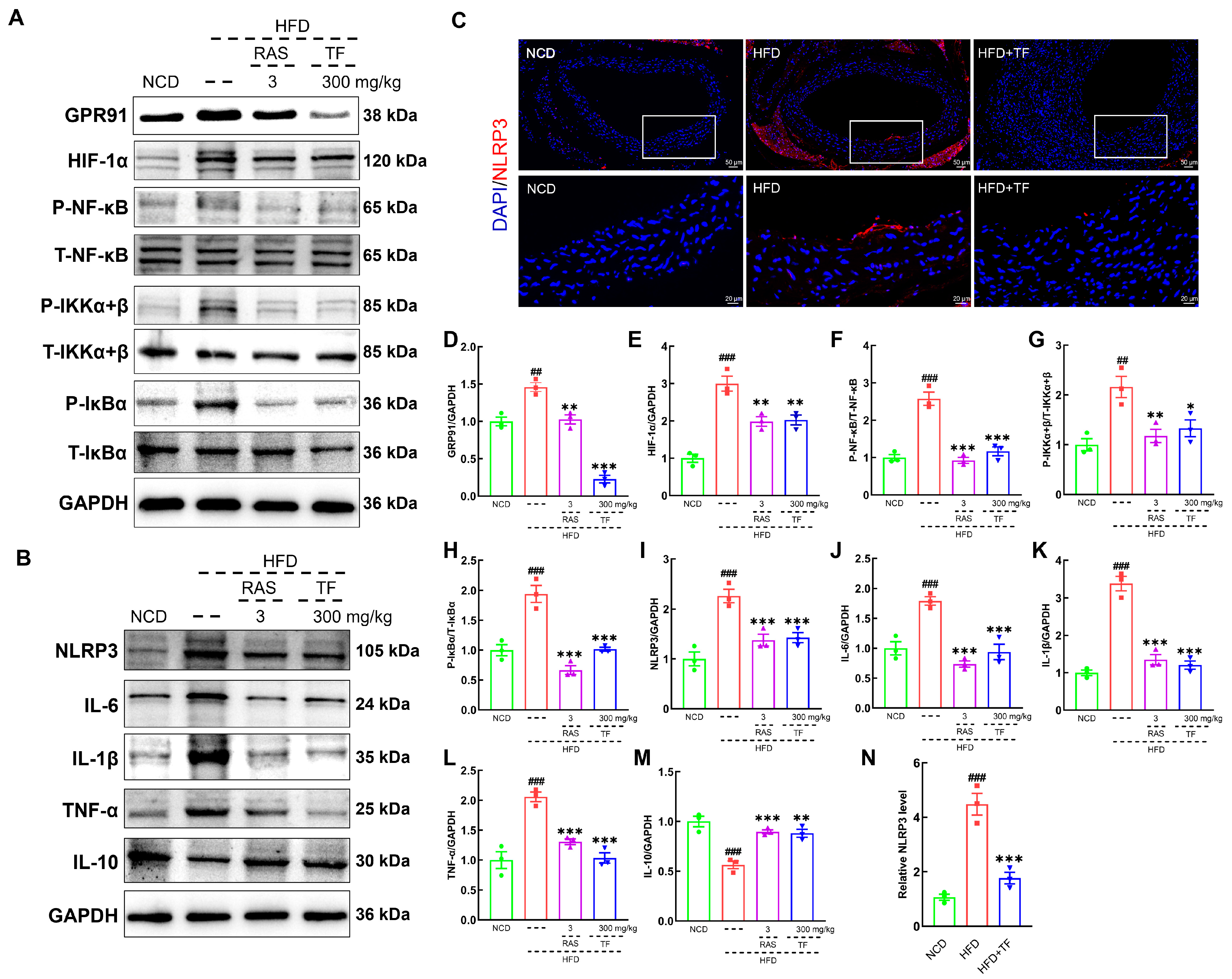
2.5. Immunofluorescence Staining
2.6. Serum Lipid Detection
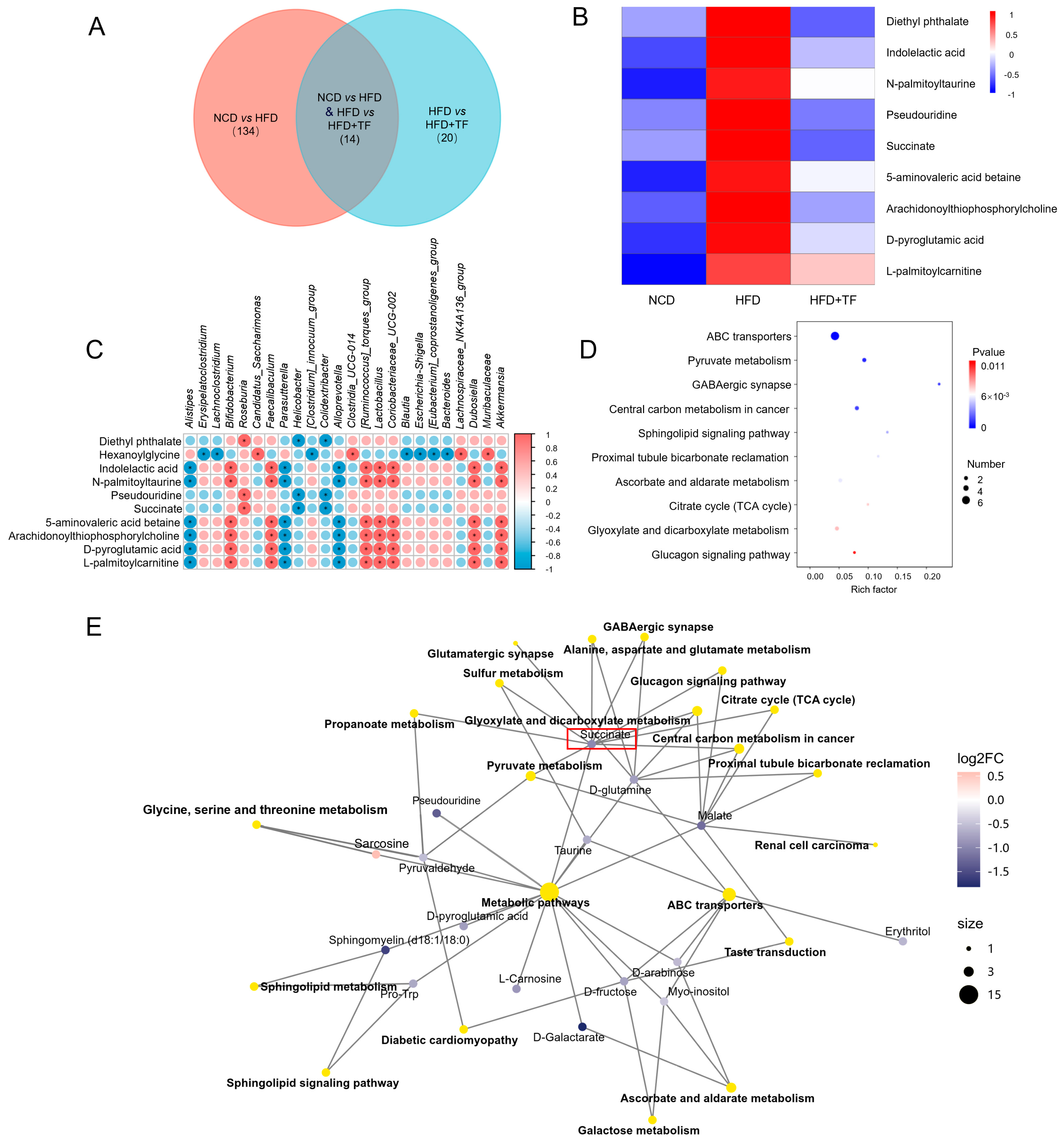
2.7. Gut Microbiota Analysis
2.8. Non-Targeted Metabolomics Analysis
2.9. Western Blot
2.10. Statistical Analysis
3. Results
3.1. T. fuciformis Alleviated Atherosclerosis Progression in HFD-Induced ApoE−/− Mice
3.2. T. fuciformis Regulated Gut Microbes in HFD-Induced ApoE−/− Mice
3.3. T. fuciformis Altered Serum Metabolite Levels in HFD-Induced ApoE−/− Mice
3.4. T. fuciformis Alleviated Aortic Inflammation via NF-κB Signaling Pathway in HFD-Induced ApoE−/− Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saigusa, R.; Winkels, H.; Ley, K. T cell subsets and functions in atherosclerosis. Nat. Rev. Cardiol. 2020, 17, 387–401. [Google Scholar] [CrossRef]
- Falk, E. Pathogenesis of Atherosclerosis. J. Am. Coll. Cardiol. 2006, 47, C7–C12. [Google Scholar] [CrossRef]
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Barone Gibbs, B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 2024, 149, e347–e913. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef]
- Björkegren, J.L.M.; Lusis, A.J. Atherosclerosis: Recent developments. Cell 2022, 185, 1630–1645. [Google Scholar] [CrossRef]
- Malekmohammad, K.; Bezsonov, E.E.; Rafieian-Kopaei, M. Role of Lipid Accumulation and Inflammation in Atherosclerosis: Focus on Molecular and Cellular Mechanisms. Front. Cardiovasc. Med. 2021, 8, 707529. [Google Scholar] [CrossRef]
- Ranjit, N.; Diez-Roux, A.V.; Shea, S.; Cushman, M.; Seeman, T.; Jackson, S.A.; Ni, H. Psychosocial factors and inflammation in the multi-ethnic study of atherosclerosis. Arch. Intern. Med. 2007, 167, 174–181. [Google Scholar] [CrossRef]
- Attiq, A.; Afzal, S.; Ahmad, W.; Kandeel, M. Hegemony of inflammation in atherosclerosis and coronary artery disease. Eur. J. Pharmacol. 2024, 966, 176338. [Google Scholar] [CrossRef]
- Yu, X.H.; Zheng, X.L.; Tang, C.K. Nuclear Factor-κB Activation as a Pathological Mechanism of Lipid Metabolism and Atherosclerosis. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 70, pp. 1–30. [Google Scholar]
- Chen, T.L.; Zhang, X.D.; Zhu, G.L.; Liu, H.F.; Chen, J.R.; Wang, Y.; He, X.L. Quercetin inhibits TNF-α induced HUVECs apoptosis and inflammation via downregulating NF-kB and AP-1 signaling pathway in vitro. Medicine 2020, 99, e22241. [Google Scholar] [CrossRef]
- Yuan, L.; Li, Y.; Chen, M.T.; Xue, L.; Wang, J.; Ding, Y.; Gu, Q.H.; Zhang, J.M.; Zhao, H.; Xie, X.Q.; et al. Therapeutic applications of gut microbes in cardiometabolic diseases: Current state and perspectives. Appl. Microbiol. Biotechnol. 2024, 108, 156. [Google Scholar] [CrossRef]
- Liang, Y.; Fu, J.; Shi, Y.; Jiang, X.; Lu, F.; Liu, S. Integration of 16S rRNA sequencing and metabolomics to investigate the modulatory effect of ginsenoside Rb1 on atherosclerosis. Heliyon 2024, 10, e27597. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Jonsson, A.L.; Bäckhed, F. Role of gut microbiota in atherosclerosis. Nat. Rev. Cardiol. 2017, 14, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, C.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoglu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Montastruc, J.L. Rhabdomyolysis and statins: A pharmacovigilance comparative study between statins. Br. J. Clin. Pharmacol. 2023, 89, 2636–2638. [Google Scholar] [CrossRef] [PubMed]
- Bellosta, S.; Corsini, A. Statin drug interactions and related adverse reactions. Expert Opin. Drug Saf. 2012, 11, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Pop, G.; Farcas, A.; Butuca, A.; Morgovan, C.; Arseniu, A.M.; Pumnea, M.; Teodoru, M.; Gligor, F.G. Post-Marketing Surveillance of Statins-A Descriptive Analysis of Psychiatric Adverse Reactions in EudraVigilance. Pharmaceuticals 2022, 15, 1536. [Google Scholar] [CrossRef] [PubMed]
- Hamza, A.; Mylarapu, A.; Krishna, K.V.; Kumar, D.S. An insight into the nutritional and medicinal value of edible mushrooms: A natural treasury for human health. J. Biotechnol. 2024, 381, 86–99. [Google Scholar] [CrossRef]
- González-Ibáñez, L.; Meneses, M.E.; Sánchez-Tapia, M.; Pérez-Luna, D.; Torres, N.; Torre-Villalvazo, I.; Bonilla, M.; Petlacalco, B.; Castillo, I.; López-Barradas, A.; et al. Edible and medicinal mushrooms (Pleurotus ostreatus, Ustilago maydis, Ganoderma lucidum) reduce endoplasmic reticulum stress and inflammation in adipose tissue of obese Wistar rats fed with a high fat plus saccharose diet. Food Funct. 2023, 14, 5048–5061. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Tang, J.; Gao, H.L.; Xu, Y.F.; Han, Y.L.; Shang, H.Q.; Lu, Y.Z.; Qin, C.A. Ganoderma lucidum triterpenoids and polysaccharides attenuate atherosclerotic plaque in high-fat diet rabbits. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1929–1938. [Google Scholar] [CrossRef]
- Deng, Y.; Guo, L.; Lin, L.; Li, Y.; Zhang, J.; Zhang, Y.; Yuan, B.; Ke, L.; Xie, B.; Ming, R. Meiosis in an asymmetric dikaryotic genome of Tremella fuciformis Tr01 facilitates new chromosome formation. Genome Biol. 2023, 24, 280. [Google Scholar] [CrossRef]
- He, G.; Chen, T.C.; Huang, L.F.; Zhang, Y.Y.; Feng, Y.J.; Qu, S.K.; Yin, X.J.; Liang, L.; Yan, J.; Liu, W. Tremella fuciformis polysaccharide reduces obesity in high-fat diet-fed mice by modulation of gut microbiota. Front. Microbiol. 2022, 13, 1073350. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Hu, Y.; Wang, D.; Guo, L.; Yang, S.; Fan, Y.; Zhao, B.; Wang, Y.; Abula, S. Optimization of sulfated modification conditions of tremella polysaccharide and effects of modifiers on cellular infectivity of NDV. Int. J. Biol. Macromol. 2011, 49, 44–49. [Google Scholar] [CrossRef]
- Wu, Y.J.; Wei, Z.X.; Zhang, F.M.; Linhardt, R.J.; Sun, P.L.; Zhang, A.Q. Structure, bioactivities and applications of the polysaccharides from Tremella fuciformis mushroom: A review. Int. J. Biol. Macromol. 2019, 121, 1005–1010. [Google Scholar] [CrossRef]
- Khan, T.J.; Xu, X.F.; Xie, X.L.; Dai, X.M.; Sun, P.N.; Xie, Q.D.; Zhou, X.L. Tremella fuciformis Crude Polysaccharides Attenuates Steatosis and Suppresses Inflammation in Diet-Induced NAFLD Mice. Curr. Issues Mol. Biol. 2022, 44, 1224–1234. [Google Scholar] [CrossRef]
- Li, X.G.; Zhang, F.Y.; Jiang, C.X.; Jiang, J.; Hou, Y.H.; Zhang, J.W. Structural analysis, in vitro antioxidant and lipid-lowering activities of purified Tremella fuciformis polysaccharide fractions. Process Biochem. 2023, 133, 99–108. [Google Scholar] [CrossRef]
- Curtiss, L.K.; Boisvert, W.A. Apolipoprotein E and atherosclerosis. Curr. Opin. Lipidol. 2000, 11, 243–251. [Google Scholar] [CrossRef]
- Imaizumi, K. Diet and Atherosclerosis in Apolipoprotein E-Deficient Mice. Biosci. Biotechnol. Biochem. 2011, 75, 1023–1035. [Google Scholar] [CrossRef]
- Pendse, A.A.; Arbones-Mainar, J.M.; Johnson, L.A.; Altenburg, M.K.; Maeda, N. Apolipoprotein E knock-out and knock-in mice: Atherosclerosis, metabolic syndrome, and beyond. J. Lipid Res. 2009, 50, S178–S182. [Google Scholar] [CrossRef]
- Huang, Z.Q.; Shen, S.R.; Han, X.; Li, W.X.; Luo, W.; Lin, L.M.; Xu, M.J.; Wang, Y.; Huang, W.J.; Wu, G.J.; et al. Macrophage DCLK1 promotes atherosclerosis via binding to IKKβ and inducing inflammatory responses. EMBO Mol. Med. 2023, 15, e17198. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, W.; Lei, L.; Feng, T.; Hu, Y.; Liu, P.; Li, Y.; Sheng, R.; Zhang, Y.; Li, S.; et al. Antirheumatic drug leflunomide attenuates atherosclerosis by regulating lipid metabolism and endothelial dysfunction via DHODH/AMPK signaling pathway. Int. J. Biol. Sci. 2024, 20, 3725–3741. [Google Scholar] [CrossRef]
- Tu, T.C.; Liu, H.; Liu, Z.H.; Liang, Y.Y.; Tan, C.J.; Feng, D.; Zou, J. Amelioration of Atherosclerosis by lycopene is linked to the modulation of gut microbiota dysbiosis and related gut-heart axis activation in high-fat diet-fed ApoE−/− mice. Nutr. Metab. 2023, 20, 53. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Kong, F.E.; Wang, C.X.; Li, L.Z.; Peng, S.C.; Wang, D.; Li, C.T. The amelioration of a purified Pleurotus abieticola polysaccharide on atherosclerosis in ApoE−/− mice. Food Funct. 2024, 15, 79–95. [Google Scholar] [CrossRef]
- Benton, H.P.; Ivanisevic, J.; Mahieu, N.G.; Kurczy, M.E.; Johnson, C.H.; Franco, L.; Rinehart, D.; Valentine, E.; Gowda, H.; Ubhi, B.K.; et al. Autonomous Metabolomics for Rapid Metabolite Identification in Global Profiling. Anal. Chem. 2015, 87, 884–891. [Google Scholar] [CrossRef]
- Martinet, W.; Coornaert, I.; Puylaert, P.; De Meyer, G.R.Y. Macrophage Death as a Pharmacological Target in Atherosclerosis. Front. Pharmacol. 2019, 10, 306. [Google Scholar] [CrossRef]
- Yang, M.; Liu, X.; Jiang, M.; Hu, J.; Xiao, Z. TAX1BP1/A20 inhibited TLR2-NF-κB activation to induce tolerant expression of IL-6 in endothelial cells. Int. Immunopharmacol. 2024, 139, 112789. [Google Scholar] [CrossRef]
- Al-Hawary, S.I.S.; Jasim, S.A.; Romero-Parra, R.M.; Bustani, G.S.; Hjazi, A.; Alghamdi, M.I.; Kareem, A.K.; Alwaily, E.R.; Zabibah, R.S.; Gupta, J.; et al. NLRP3 inflammasome pathway in atherosclerosis: Focusing on the therapeutic potential of non-coding RNAs. Pathol.-Res. Pract. 2023, 246, 154490. [Google Scholar] [CrossRef]
- Chu, T.; Wang, Y.; Wang, S.; Li, J.; Li, Z.; Wei, Z.; Li, J.; Bian, Y. Kaempferol regulating macrophage foaming and atherosclerosis through piezo1-mediated MAPK/NF-κB and Nrf2/HO-1 signaling pathway. J. Adv. Res. 2024. [Google Scholar] [CrossRef]
- Roth, C.L.; Molica, F.; Kwak, B.R. Browning of White Adipose Tissue as a Therapeutic Tool in the Fight against Atherosclerosis. Metabolites 2021, 11, 319. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, X.; Wang, W.; Lei, L.; Sheng, R.; Li, S.; Luo, J.; Liu, H.; Zhang, J.; Han, X.; et al. ASGR1 Deficiency Inhibits Atherosclerosis in Western Diet-Fed ApoE−/− Mice by Regulating Lipoprotein Metabolism and Promoting Cholesterol Efflux. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 2428–2449. [Google Scholar] [CrossRef]
- Xiao, Y.J.; Xu, Y.H.; Liu, X.D.; Cheng, S.J.; Wei, R.; Zhao, W.F.; Zhao, C.S. Simultaneous Rosiglitazone Release and Low-Density Lipoprotein Removal by Chondroitin Sodium Sulfate/Cyclodextrin/Poly(acrylic acid) Composite Adsorbents for Atherosclerosis Therapy. Biomacromolecules 2024, 25, 3141–3152. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M. CD36, a scavenger receptor implicated in atherosclerosis. Exp. Mol. Med. 2014, 46, e99. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dubland, J.A.; Allahverdian, S.; Asonye, E.; Sahin, B.; Jaw, J.E.; Sin, D.D.; Seidman, M.A.; Leeper, N.J.; Francis, G.A. Smooth Muscle Cells Contribute the Majority of Foam Cells in ApoE (Apolipoprotein E)-Deficient Mouse Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 876–887. [Google Scholar] [CrossRef]
- Akivis, Y.; Alkaissi, H.; McFarlane, S.I.; Bukharovich, I. The Role of Triglycerides in Atherosclerosis: Recent Pathophysiologic Insights and Therapeutic Implications. Curr. Cardiol. Rev. 2024, 20, 39–49. [Google Scholar] [CrossRef]
- Wang, X.; Cui, J.; Gu, Z.; Guo, L.; Liu, R.; Guo, Y.; Qin, N.; Yang, Y. Aged garlic oligosaccharides modulate host metabolism and gut microbiota to alleviate high-fat and high-cholesterol diet-induced atherosclerosis in ApoE−/− mice. Food Chem. 2025, 463, 141409. [Google Scholar] [CrossRef]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria with Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef]
- Vourakis, M.; Mayer, G.; Rousseau, G. The Role of Gut Microbiota on Cholesterol Metabolism in Atherosclerosis. Int. J. Mol. Sci. 2021, 22, 8074. [Google Scholar] [CrossRef]
- Wang, T.W.; Ye, Y.L.; Ji, J.; Zhang, S.; Yang, X.X.; Xu, J.Y.; Wang, J.S.; Chen, Z.Y.; Xia, B.G.; Shen, H.F.; et al. Astilbin from Smilax glabra Roxb. alleviates high-fat diet-induced metabolic dysfunction. Food Funct. 2022, 13, 5023–5036. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Liu, W.J.; Lv, Y.Q.; Liu, K.; Wang, Y.; Meng, S.L.; Kang, T.; Bao, Y.C.; Meng, H.C. Supplementation with Natto and Red Yeast Rice Alters Gene Expressions in Cholesterol Metabolism Pathways in ApoE−/− Mice with Concurrent Changes in Gut Microbiota. Nutrients 2023, 15, 973. [Google Scholar] [CrossRef]
- Yoshida, N.; Emoto, T.; Yamashita, T.; Watanabe, H.; Hayashi, T.; Tabata, T.; Hoshi, N.; Hatano, N.; Ozawa, G.; Sasaki, N.; et al. Bacteroides vulgatus and Bacteroides dorei Reduce Gut Microbial Lipopolysaccharide Production and Inhibit Atherosclerosis. Circulation 2018, 138, 2486–2498. [Google Scholar] [CrossRef] [PubMed]
- Bu, J.; Wang, Z.H. Cross-Talk between Gut Microbiota and Heart via the Routes of Metabolite and Immunity. Gastroenterol. Res. Pract. 2018, 2018, 6458094. [Google Scholar] [CrossRef]
- Song, L.N.; Ji, W.Y.; Cao, X. Integrated analysis of gut microbiome and its metabolites in ACE2-knockout and ACE2-overexpressed mice. Front. Cell. Infect. Microbiol. 2024, 14, 1404678. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Basu, S.; Ghosh, S.; Guria, S.; Mukherjee, S. Diethyl phthalate, a plasticizer, induces adipocyte inflammation and apoptosis in mice after long-term dietary administration. J. Biochem. Mol. Toxicol. 2024, 38, e23561. [Google Scholar] [CrossRef] [PubMed]
- van Dam, A.D.; Boon, M.R.; Berbée, J.F.P.; Rensen, P.C.N.; van Harmelen, V. Targeting white, brown and perivascular adipose tissue in atherosclerosis development. Eur. J. Pharmacol. 2017, 816, 82–92. [Google Scholar] [CrossRef]
- Mills, E.; O’Neill, L.A.J. Succinate: A metabolic signal inflammation. Trends Cell Biol. 2014, 24, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, G.J.; He, Y.N.; Chen, J.; Yan, J.Y.; Zhang, Q.; Li, L.Q.; Cai, X. Cellular succinate metabolism and signaling in inflammation: Implications for therapeutic intervention. Front. Immunol. 2024, 15, 1404441. [Google Scholar] [CrossRef]
- Zhen, Y.H.; Yang, J.Q.; Song, J.; Xing, Z.Y.; Zheng, J.H. Silencing ARL11 relieved atherosclerotic inflammation and lipid deposition via retraining JAK2/STAT1 pathway. Atherosclerosis 2024, 398, 118564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, X.; Bian, F.; Wu, P.; Xing, S.; Xu, G.; Li, W.; Chi, J.; Ouyang, C.; Zheng, T.; et al. TNF-α promotes early atherosclerosis by increasing transcytosis of LDL across endothelial cells: Crosstalk between NF-κB and PPAR-γ. J. Mol. Cell. Cardiol. 2014, 72, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Boisvert, W.A. Interleukin-10 protects against atherosclerosis by modulating multiple atherogenic macrophage function. Thromb. Haemost. 2015, 113, 505–512. [Google Scholar] [CrossRef]
- Takeda, N.; Manabe, I.; Shindo, T.; Iwata, H.; Iimuro, S.; Kagechika, H.; Shudo, K.; Nagai, R. Synthetic retinoid Am80 reduces scavenger receptor expression and atherosclerosis in mice by inhibiting IL-6. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Hu, Y.W.; Sha, Y.H.; Gao, J.J.; Ma, X.; Li, S.F.; Zhao, J.Y.; Qiu, Y.R.; Lu, J.B.; Huang, C.; et al. Ox-LDL Upregulates IL-6 Expression by Enhancing NF-κB in an IGF2-Dependent Manner in THP-1 Macrophages. Inflammation 2015, 38, 2116–2123. [Google Scholar] [CrossRef]
- Sun, X.H.; He, S.L.; Wara, A.K.M.; Icli, B.; Shvartz, E.; Tesmenitsky, Y.; Belkin, N.; Li, D.Z.; Blackwell, T.S.; Sukhova, G.K.; et al. Systemic Delivery of MicroRNA-181b Inhibits Nuclear Factor-κB Activation, Vascular Inflammation, and Atherosclerosis in Apolipoprotein E-Deficient Mice. Circ. Res. 2014, 114, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Ben-Neriah, Y. Phosphorylation meets ubiquitination: The control of NF- kappa B activity. Annu. Rev. Immunol. 2000, 18, 621–663. [Google Scholar] [CrossRef]
- Li, S.H.; Li, Q.J.; Zhou, Q.F.; Li, S.Q.; Wang, S.Q.; Yao, Q.; Ouyang, C.H.; Liu, C.; Li, M.C. Attenuating Atherosclerosis through Inhibition of the NF-κB/NLRP3/IL-1β Pathway-Mediated Pyroptosis in Vascular Smooth Muscle Cells (VSMCs). Cardiovasc. Ther. 2024, 2024, 1506083. [Google Scholar] [CrossRef]
- Lan, Q.; Chen, J.; Yang, Y. Chromofungin mitigates free fatty acids-induced endothelial inflammation via inhibition of NOD-like receptor thermal protein domain-associated protein 3 mediated by adenosine 5′-monophosphate-activated protein kinase. Biotechnol. Appl. Biochem. 2024, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shao, C.; Zhou, H.; Yu, L.; Bao, Y.; Mao, Q.; Yang, J.; Wan, H. Salvianolic acid B inhibits atherosclerosis and TNF-α-induced inflammation by regulating NF-κB/NLRP3 signaling pathway. Phytomedicine 2023, 119, 155002. [Google Scholar] [CrossRef] [PubMed]
- González, L.; Rivera, K.; Andia, M.E.; Rodriguez, G.M. The IL-1 Family and Its Role in Atherosclerosis. Int. J. Mol. Sci. 2023, 24, 17. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Zhang, Q.; Xie, R.; Zhao, J.; Han, Z.; Li, Y.; Yu, H.; Zhang, Y. Tremella fuciformis Berk Alleviated Atherosclerosis Symptoms via Nuclear Factor-Kappa B-Mediated Inflammatory Response in ApoE−/− Mice. Nutrients 2025, 17, 160. https://doi.org/10.3390/nu17010160
Dong Y, Zhang Q, Xie R, Zhao J, Han Z, Li Y, Yu H, Zhang Y. Tremella fuciformis Berk Alleviated Atherosclerosis Symptoms via Nuclear Factor-Kappa B-Mediated Inflammatory Response in ApoE−/− Mice. Nutrients. 2025; 17(1):160. https://doi.org/10.3390/nu17010160
Chicago/Turabian StyleDong, Yihao, Qinchun Zhang, Rui Xie, Jundi Zhao, Zhihua Han, Yu Li, Han Yu, and Yongfeng Zhang. 2025. "Tremella fuciformis Berk Alleviated Atherosclerosis Symptoms via Nuclear Factor-Kappa B-Mediated Inflammatory Response in ApoE−/− Mice" Nutrients 17, no. 1: 160. https://doi.org/10.3390/nu17010160
APA StyleDong, Y., Zhang, Q., Xie, R., Zhao, J., Han, Z., Li, Y., Yu, H., & Zhang, Y. (2025). Tremella fuciformis Berk Alleviated Atherosclerosis Symptoms via Nuclear Factor-Kappa B-Mediated Inflammatory Response in ApoE−/− Mice. Nutrients, 17(1), 160. https://doi.org/10.3390/nu17010160




