Vitamins in Gynecologic Malignancies
Abstract
1. Introduction
2. Methodology
3. Vitamin A
3.1. Mechanism of Action
3.2. Ovarian Cancer
3.3. Endometrial Cancer
3.4. Cervical Cancer
4. Vitamin D
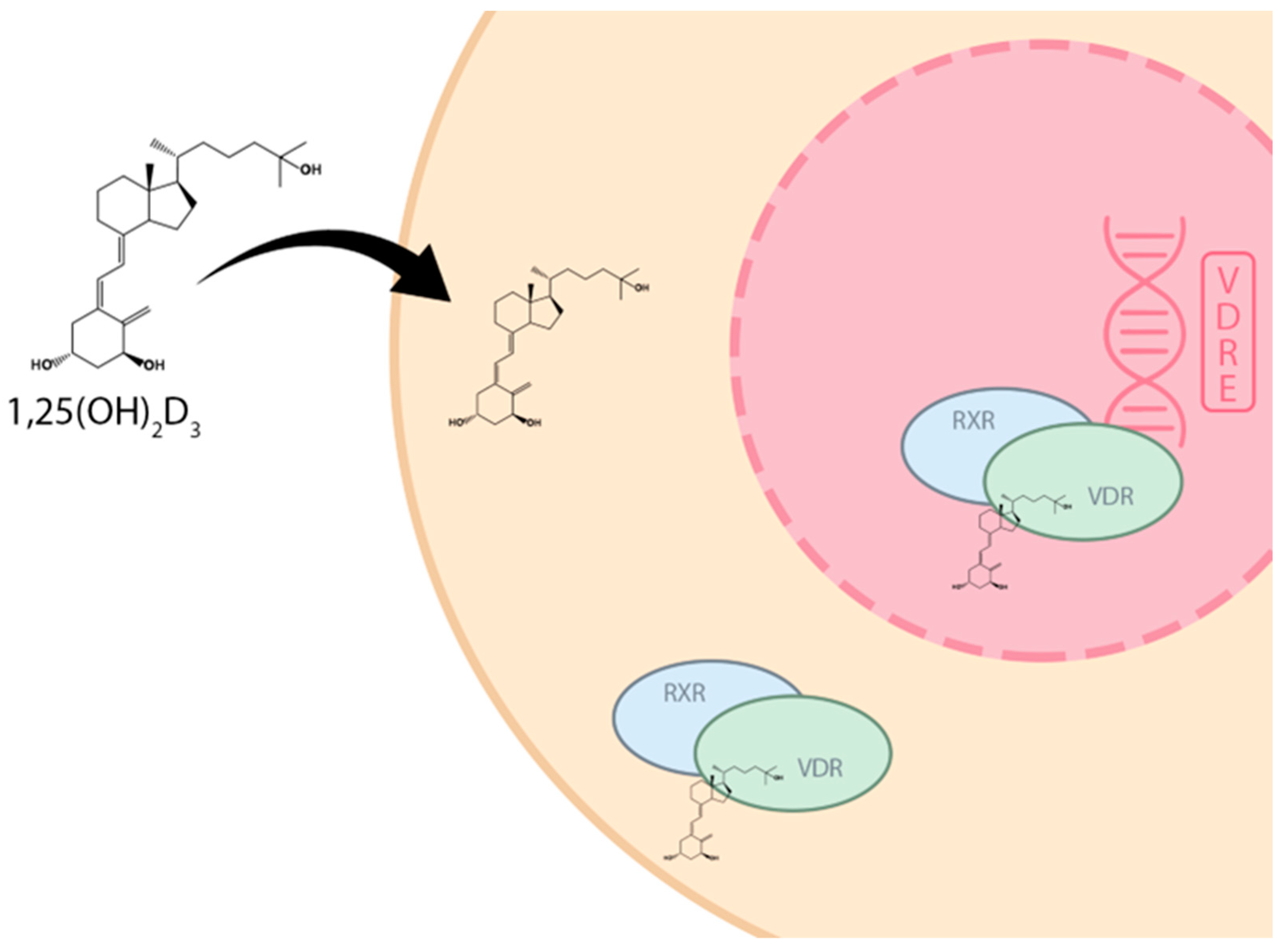
4.1. Mechanism of Action
4.2. Endometrial Cancer
4.3. Ovarian Cancer
4.4. Cervical Cancer
5. B Vitamins
6. Vitamin C, E and K
7. Potential Risks and Side Effects
8. Limitations
9. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- ECIS—European Cancer Information System. Available online: https://ecis.jrc.ec.europa.eu (accessed on 4 January 2024).
- Tergas, A.I.; Wright, J.D. Cancer Prevention Strategies for Women. Obstet. Gynecol. 2019, 134, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Staples, J.N.; Duska, L.R. Cancer Screening and Prevention Highlights in Gynecologic Cancer. Obstet. Gynecol. Clin. N. Am. 2019, 46, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, F.A.; Magni, F.; Bosco, M.; Biancotto, G.; Zorzato, P.C.; Laganà, A.S.; Chiantera, V.; Raffaelli, R.; Franchi, M.; Uccella, S.; et al. The Role of Micronutrients in Human Papillomavirus Infection, Cervical Dysplasia, and Neoplasm. Healthcare 2023, 11, 1652. [Google Scholar] [CrossRef] [PubMed]
- Koshiyama, M. The Effects of the Dietary and Nutrient Intake on Gynecologic Cancers. Healthcare 2019, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhao, R.; Wang, Y.; Ma, H.; Yu, M.; Chen, X.; Zhang, D.; Ma, S.; Liu, B.; Cai, H. Dietary Vitamin A Intake and Circulating Vitamin A Concentrations and the Risk of Three Common Cancers in Women: A Meta-Analysis. Oxid. Med. Cell. Longev. 2022, 2022, 7686405. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; He, C. Dietary vitamin A intake and the risk of ovarian cancer: A meta-analysis. Biosci. Rep. 2020, 40, BSR20193979. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.Q.; Gao, X.P.; Yu, X.X.; Zeng, Y.F.; Li, S.N.; Naicker, N.; Joseph, T.; Cao, W.T.; Liu, Y.H.; Zhu, S.; et al. Effects of dairy products, calcium and vitamin D on ovarian cancer risk: A meta-analysis of twenty-nine epidemiological studies. Br. J. Nutr. 2020, 124, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Kuznia, S.; Zhu, A.; Akutsu, T.; Buring, J.E.; Camargo CAJr Cook, N.R.; Chen, L.J.; Cheng, T.D.; Hantunen, S.; Lee, I.M.; Manson, J.E. Efficacy of vitamin D3 supplementation on cancer mortality: Systematic review and individual patient data meta-analysis of randomised controlled trials. Ageing Res. Rev. 2023, 87, 101923. [Google Scholar] [CrossRef]
- FDA Approves Firstline Arsenic Trioxide for Acute Promyelocytic Leukemia. Available online: https://ashpublications.org/ashclinicalnews/news/3653/FDA-Approves-Firstline-Arsenic-Trioxide-for-Acute (accessed on 4 January 2024).
- Young, M.J.; Wu, Y.H.; Chiu, W.T.; Weng, T.Y.; Huang, Y.F.; Chou, C.Y. All-trans retinoic acid downregulates ALDH1-mediated stemness and inhibits tumour formation in ovarian cancer cells. Carcinogenesis 2015, 36, 498–507. [Google Scholar] [CrossRef]
- Whitworth, J.M.; Londoño-Joshi, A.I.; Sellers, J.C.; Oliver, P.J.; Muccio, D.D.; Atigadda, V.R.; Straughn, J.M., Jr.; Buchsbaum, D.J. The impact of novel retinoids in combination with platinum chemotherapy on ovarian cancer stem cells. Gynecol. Oncol. 2012, 125, 226–230. [Google Scholar] [CrossRef]
- Ezawa, S.; Suzuki, N.; Ohie, S.; Higashiguchi, A.; Hosoi, F.; Kitazato, K.; Susumu, N.; Aoki, D. A synthetic retinoid, TAC-101 (4-[3,5-bis (trimethylsilyl) benzamido] benzoic acid), plus cisplatin: Potential new therapy for ovarian clear cell adenocarcinoma. Gynecol. Oncol. 2008, 108, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Hunsu, V.O.; Facey, C.O.B.; Fields, J.Z.; Boman, B.M. Retinoids as Chemo-Preventive and Molecular-Targeted Anti-Cancer Therapies. Int. J. Mol. Sci. 2021, 22, 7731. [Google Scholar] [CrossRef] [PubMed]
- Doldo, E.; Costanza, G.; Ferlosio, A.; Passeri, D.; Bernardini, S.; Scioli, M.G.; Mazzaglia, D.; Agostinelli, S.; Del Bufalo, D.; Czernobilsky, B.; et al. CRBP-1 expression in ovarian cancer: A potential therapeutic target. Anticancer Res. 2014, 34, 3303–3312. [Google Scholar] [PubMed]
- Connolly, R.M.; Nguyen, N.K.; Sukumar, S. Molecular pathways: Current role and future directions of the retinoic acid pathway in cancer prevention and treatment. Clin. Cancer Res. 2013, 19, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Alvarez Secord, A.; Sayer, R.; Snyder, S.A.; Broadwater, G.; Rodriguez, G.C.; Berchuck, A.; Blackwell, K. The relationship between serum vascular endothelial growth factor, persistent disease, and survival at second-look laparotomy in ovarian cancer. Gynecol. Oncol. 2004, 94, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Kalitin, N.N.; Karamysheva, A.F. RARα mediates all-trans-retinoic acid-induced VEGF-C, VEGF-D, and VEGFR3 expression in lung cancer cells. Cell Biol. Int. 2016, 40, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Prabhala, R.H.; Garewal, H.S.; Hicks, M.J.; Sampliner, R.E.; Watson, R.R. The effects of 13-cis-retinoic acid and beta-carotene on cellular immunity in humans. Cancer 1991, 67, 1556–1560. [Google Scholar] [CrossRef] [PubMed]
- Bono, M.R.; Tejon, G.; Flores-Santibañez, F.; Fernandez, D.; Rosemblatt, M.; Sauma, D. Retinoic Acid as a Modulator of T Cell Immunity. Nutrients 2016, 8, 349. [Google Scholar] [CrossRef]
- Recchia, F.; Saggio, G.; Cesta, A.; Candeloro, G.; Nuzzo, A.; Lombardo, M.; Carta, G.; Rea, S. Interleukin-2 and 13-cis retinoic acid as maintenance therapy in advanced ovarian cancer. Int. J. Oncol. 2005, 27, 1039–1046. [Google Scholar] [CrossRef]
- Brewer, M.; Kirkpatrick, N.D.; Wharton, J.T.; Wang, J.; Hatch, K.; Auersperg, N.; Utzinger, U.; Gershenson, D.; Bast, R.; Zou, C. 4-HPR modulates gene expression in ovarian cells. Int. J. Cancer 2006, 119, 1005–1013. [Google Scholar] [CrossRef]
- Holmes, W.F.; Soprano, D.R.; Soprano, K.J. Comparison of the mechanism of induction of apoptosis in ovarian carcinoma cells by the conformationally restricted synthetic retinoids CD437 and 4-HPR. J. Cell. Biochem. 2003, 89, 262–278. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Formelli, F.; Cantù, M.G.; Parma, G.; Gasco, M.; Argusti, A.; Santinelli, A.; Montironi, R.; Cavadini, E.; Baglietto, L.; et al. A phase I-II preoperative biomarker trial of fenretinide in ascitic ovarian cancer. Cancer Epidemiol. Biomarkers Prev. 2006, 15, 1914–1919. [Google Scholar] [CrossRef]
- Suzuki, N.; Aoki, D.; Oie, S.; Horiuchi, M.; Hasegawa, Y.; Ezawa, S.; Suzuki, A.; Susumu, N.; Hosoi, F.; Kitazato, K.; et al. A novel retinoid, 4-[3,5-bis (trimethylsilyl) benzamido] benzoic acid (TAC-101), induces apoptosis of human ovarian carcinoma cells and shows potential as a new antitumor agent for clear cell adenocarcinoma. Gynecol. Oncol. 2004, 94, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, K.; Utsunomiya, H.; Miki, Y.; Hanihara, M.; Fue, M.; Takagi, K.; Nishimoto, M.; Suzuki, F.; Yaegashi, N.; Suzuki, T.; et al. Retinoic Acid Receptor β: A Potential Therapeutic Target in Retinoic Acid Treatment of Endometrial Cancer. Int. J. Gynecol. Cancer 2017, 27, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Suzuki, T.; Moriya, T.; Utsunomiya, H.; Sugawara, A.; Konno, R.; Sato, S.; Sasano, H. Retinoid receptors in the human endometrium and its disorders: A possible modulator of 17 beta-hydroxysteroid dehydrogenase. J. Clin. Endocrinol. Metab. 2001, 86, 2721–2727. [Google Scholar] [CrossRef][Green Version]
- Mittal, N.; Malpani, S.; Dyson, M.; Ono, M.; Coon, J.S.; Kim, J.J.; Schink, J.C.; Bulun, S.E.; Pavone, M.E. Fenretinide: A novel treatment for endometrial cancer. PLoS ONE 2014, 9, e110410. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.H.; Yin, P.; Xue, Q.; Yilmaz, B.; Dawson, M.I.; Bulun, S.E. Retinoic acid (RA) regulates 17beta-hydroxysteroid dehydrogenase type 2 expression in endometrium: Interaction of RA receptors with specificity protein (SP) 1/SP3 for estradiol metabolism. J. Clin. Endocrinol. Metab. 2008, 93, 1915–1923. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, B.; Zhang, B.; Wang, Z. Vitamin A and risk of cervical cancer: A meta-analysis. Gynecol. Oncol. 2012, 124, 366–373. [Google Scholar] [CrossRef]
- Helm, C.W.; Lorenz, D.J.; Meyer, N.J.; Rising, W.W.; Wulff, J.L. Retinoids for preventing the progression of cervical intra-epithelial neoplasia. Cochrane Database Syst. Rev. 2013, 2013, CD003296. [Google Scholar] [CrossRef]
- Sanusi, R.S. Outcome of Combined Neoadjuvant Chemotherapy and Vitamin A in Advanced Cervical Carcinoma: A Randomized Double-Blind Clinical Trial. Asian Pac. J. Cancer Prev. 2019, 20, 2213–2218. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.M.; Shin, E.A. Exploring vitamin D metabolism and function in cancer. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, A.; Grant, W.B. Vitamin D and Cancer: An Historical Overview of the Epidemiology and Mechanisms. Nutrients 2022, 14, 1448. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawy, A.; Malki, A. Vitamin D Signaling in Inflammation and Cancer: Molecular Mechanisms and Therapeutic Implications. Molecules. 2020, 25, 3219. [Google Scholar] [CrossRef] [PubMed]
- Ao, T.; Kikuta, J.; Ishii, M. The Effects of Vitamin D on Immune System and Inflammatory Diseases. Biomolecules 2021, 11, 1624. [Google Scholar] [CrossRef] [PubMed]
- Cermisoni, G.C.; Alteri, A.; Corti, L.; Rabellotti, E.; Papaleo, E.; Viganò, P.; Sanchez, A.M. Vitamin D and Endometrium: A Systematic Review of a Neglected Area of Research. Int. J. Mol. Sci. 2018, 19, 2320. [Google Scholar] [CrossRef] [PubMed]
- Bergadà, L.; Pallares, J.; Maria Vittoria, A.; Cardus, A.; Santacana, M.; Valls, J.; Cao, G.; Fernàndez, E.; Dolcet, X.; Dusso, A.S.; et al. Role of local bioactivation of vitamin D by CYP27A1 and CYP2R1 in the control of cell growth in normal endometrium and endometrial carcinoma. Lab. Investig. 2014, 94, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Zelenko, Z.; Aghajanova, L.; Irwin, J.C.; Giudice, L.C. Nuclear receptor, coregulator signaling, and chromatin remodeling pathways suggest involvement of the epigenome in the steroid hormone response of endometrium and abnormalities in endometriosis. Reprod. Sci. 2012, 19, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Bokhari, A.A.; Lee, L.R.; Raboteau, D.; Turbov, J.; Rodriguez, I.V.; Pike, J.W.; Hamilton, C.A.; Maxwell, G.L.; Rodriguez, G.C.; Syed, V. Progesterone potentiates the growth inhibitory effects of calcitriol in endometrial cancer via suppression of CYP24A1. Oncotarget 2016, 7, 77576–77590. [Google Scholar] [CrossRef]
- Anderson, M.G.; Nakane, M.; Ruan, X.; Kroeger, P.E.; Wu-Wong, J.R. Expression of VDR and CYP24A1 mRNA in human tumors. Cancer Chemother. Pharmacol. 2006, 57, 234–240. [Google Scholar] [CrossRef]
- Yan, L.; Gu, Y.; Luan, T.; Miao, M.; Jiang, L.; Liu, Y.; Li, P.; Zeng, X. Associations between serum vitamin D and the risk of female reproductive tumors: A meta-analysis with trial sequential analysis. Medicine 2018, 97, e0360. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.N.; Lennox, G. Highlighting obesity as a risk factor for endometrial cancer. CMAJ 2021, 193, E58. [Google Scholar] [CrossRef] [PubMed]
- Vanlint, S. Vitamin D and obesity. Nutrients 2013, 5, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.S.; Bowles, S.; Evans, A.L. Vitamin D in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, G.C.; Turbov, J.; Rosales, R.; Yoo, J.; Hunn, J.; Zappia, K.J.; Lund, K.; Barry, C.P.; Rodriguez, I.V.; Pike, J.W.; et al. Progestins inhibit calcitriol-induced CYP24A1 and synergistically inhibit ovarian cancer cell viability: An opportunity for chemoprevention. Gynecol. Oncol. 2016, 143, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Song, Z.; Guo, Y.; Luo, X.; Li, X.; Wu, X.; Gong, Y. 1α,25-Dihydroxyvitamin D3 Improves Follicular Development and Steroid Hormone Biosynthesis by Regulating Vitamin D Receptor in the Layers Model. Curr. Issues Mol. Biol. 2023, 45, 256. [Google Scholar] [CrossRef] [PubMed]
- Grzeczka, A.; Graczyk, S.; Skowronska, A.; Skowronski, M.T.; Kordowitzki, P. Relevance of Vitamin D and Its Deficiency for the Ovarian Follicle and the Oocyte: An Update. Nutrients 2022, 14, 3712. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Zhang, X.; Tang, J.; Kasiappan, R.; Jinwal, U.; Li, P.; Hann, S.; Nicosia, S.V.; Wu, J.; Zhang, X.; et al. The coupling of epidermal growth factor receptor down regulation by 1alpha,25-dihydroxyvitamin D3 to the hormone-induced cell cycle arrest at the G1-S checkpoint in ovarian cancer cells. Mol. Cell. Endocrinol. 2011, 338, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Li, P.; Fornace, A.J., Jr.; Nicosia, S.V.; Bai, W. G2/M arrest by 1,25-dihydroxyvitamin D3 in ovarian cancer cells mediated through the induction of GADD45 via an exonic enhancer. J. Biol. Chem. 2003, 278, 48030–48040. [Google Scholar] [CrossRef]
- Hou, Y.F.; Gao, S.H.; Wang, P.; Zhang, H.M.; Liu, L.Z.; Ye, M.X.; Zhou, G.M.; Zhang, Z.L.; Li, B.Y. 1α,25(OH)₂D₃ Suppresses the Migration of Ovarian Cancer SKOV-3 Cells through the Inhibition of Epithelial-Mesenchymal Transition. Int. J. Mol. Sci. 2016, 17, 1285. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Chen, P.; Li, X.; Li, M.; Guo, H.; Li, J.; Chu, R.; Wang, H. Polymorphisms in the vitamin D Receptor (VDR) and the risk of ovarian cancer: A meta-analysis. PLoS ONE 2013, 8, e66716. [Google Scholar] [CrossRef] [PubMed]
- van Etten, E.; Verlinden, L.; Giulietti, A.; Ramos-Lopez, E.; Branisteanu, D.D.; Ferreira, G.B.; Overbergh, L.; Verstuyf, A.; Bouillon, R.; Roep, B.O.; et al. The vitamin D receptor gene FokI polymorphism: Functional impact on the immune system. Eur. J. Immunol. 2007, 37, 395–405. [Google Scholar] [CrossRef]
- Paucarmayta, A.; Taitz, H.; McGlorthan, L.; Casablanca, Y.; Maxwell, G.L.; Darcy, K.M.; Syed, V. Progesterone-Calcitriol Combination Enhanced Cytotoxicity of Cisplatin in Ovarian and Endometrial Cancer Cells In Vitro. Biomedicines 2020, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Czogalla, B.; Deuster, E.; Liao, Y.; Mayr, D.; Schmoeckel, E.; Sattler, C.; Kolben, T.; Hester, A.; Fürst, S.; Burges, A.; et al. Cytoplasmic VDR expression as an independent risk factor for ovarian cancer. Histochem. Cell Biol. 2020, 154, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Meyberg, R.; Axt-Fliedner, R.; Villena-Heinsen, C.; Tilgen, W.; Schmidt, W.; Reichrath, J. Vitamin D receptor (VDR) expression is not a prognostic factor in cervical cancer. Anticancer Res. 2002, 22, 299–304. [Google Scholar] [PubMed]
- Cázares-Ordoñez, V.; González-Duarte, R.J.; Díaz, L.; Ishizawa, M.; Uno, S.; Ortíz, V.; Ordoñez-Sánchez, M.L.; Makishima, M.; Larrea, F.; Avila, E. A cis-acting element in the promoter of human ether à go-go 1 potassium channel gene mediates repression by calcitriol in human cervical cancer cells. Biochem. Cell Biol. 2015, 93, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, Y.; Kong, D.; Papukashvili, D.; Rcheulishvili, N.; Zhao, H.; Li, Y.; Hou, C.; Ma, J.; Lu, X.; et al. Vitamin D Receptor Gene Polymorphisms and the Risk of CIN2+ in Shanxi Population. Biomed. Res. Int. 2022, 2022, 6875996. [Google Scholar] [CrossRef] [PubMed]
- Gnagnarella, P.; Pasquali, E.; Serrano, D.; Raimondi, S.; Disalvatore, D.; Gandini, S. Vitamin D receptor polymorphism FokI and cancer risk: A comprehensive meta-analysis. Carcinogenesis 2014, 35, 1913–1919. [Google Scholar] [CrossRef]
- Vahedpoor, Z.; Jamilian, M.; Bahmani, F.; Aghadavod, E.; Karamali, M.; Kashanian, M.; Asemi, Z. Effects of Long-Term Vitamin D Supplementation on Regression and Metabolic Status of Cervical Intraepithelial Neoplasia: A Randomized, Double-Blind, Placebo-Controlled Trial. Horm. Cancer 2017, 8, 58–67. [Google Scholar] [CrossRef]
- Punchoo, R.; Dreyer, G.; Pillay, T.S. 25-Hydroxycholecalciferol Inhibits Cell Growth and Induces Apoptosis in SiHa Cervical Cells via Autocrine Vitamin D Metabolism. Biomedicines 2023, 11, 871. [Google Scholar] [CrossRef]
- Lyon, P.; Strippoli, V.; Fang, B.; Cimmino, L. B Vitamins and One-Carbon Metabolism: Implications in Human Health and Disease. Nutrients 2020, 12, 2867. [Google Scholar] [CrossRef]
- Koushik, A.; Wang, M.; Anderson, K.E.; van den Brandt, P.; Clendenen, T.V.; Eliassen, A.H.; Freudenheim, J.L.; Genkinger, J.M.; Håkansson, N.; Marshall, J.R.; et al. Intake of vitamins A, C, and E and folate and the risk of ovarian cancer in a pooled analysis of 10 cohort studies. Cancer Causes Control. 2015, 26, 1315–1327. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, Q.; Yang, J. The effect of folate intake on ovarian cancer risk: A meta-analysis of observational studies. Medicine 2021, 100, e22605. [Google Scholar] [CrossRef]
- Lu, J.; Trabert, B.; Liao, L.M.; Pfeiffer, R.M.; Michels, K.A. Dietary intake of nutrients involved in folate-mediated one-carbon metabolism and risk for endometrial cancer. Int. J. Epidemiol. 2019, 48, 474–488. [Google Scholar] [CrossRef]
- Uccella, S.; Mariani, A.; Wang, A.H.; Vierkant, R.A.; Robien, K.; Anderson, K.E.; Cerhan, J.R. Dietary and supplemental intake of one-carbon nutrients and the risk of type I and type II endometrial cancer: A prospective cohort study. Ann. Oncol. 2011, 22, 2129–2136. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.C.; Maddocks, O.D.K. One-carbon metabolism in cancer. Br. J. Cancer. 2017, 116, 1499–1504. [Google Scholar] [CrossRef]
- Pieroth, R.; Paver, S.; Day, S.; Lammersfeld, C. Folate and Its Impact on Cancer Risk. Curr. Nutr. Rep. 2018, 7, 70–84. [Google Scholar] [CrossRef]
- Ferraro, S.; Biganzoli, G.; Calcaterra, V.; Zuccotti, G.; Biganzoli, E.M.; Plebani, M. The relevance of establishing method-dependent decision thresholds of serum folate in pregnancy and lactation: When the laboratory stewardship meets the health-care needs. Clin. Chem. Lab. Med. 2022, 60, 1493–1495. [Google Scholar] [CrossRef] [PubMed]
- Wien, T.N.; Pike, E.; Wisløff, T.; Staff, A.; Smeland, S.; Klemp, M. Cancer risk with folic acid supplements: A systematic review and meta-analysis. BMJ Open 2012, 2, e000653. [Google Scholar] [CrossRef]
- Gersekowski, K.; Ibiebele, T.I.; Australian Ovarian Cancer Study Group; Doherty, J.A.; Harris, H.R.; Goodman, M.T.; Terry, K.L.; Wu, A.H.; Bandera, E.V.; Qin, B.; et al. Folate Intake and Ovarian Cancer Risk among Women with Endometriosis: A Case-Control Study from the Ovarian Cancer Association Consortium. Cancer Epidemiol. Biomarkers Prev. 2023, 32, 1087–1096. [Google Scholar] [CrossRef]
- Yazaki, S.; Kojima, Y.; Yoshida, H.; Takamizawa, S.; Kitadai, R.; Nishikawa, T.; Shimoi, T.; Sudo, K.; Saito, A.; Okuma, H.S.; et al. High expression of folate receptor alpha is associated with poor prognosis in patients with cervical cancer. J. Gynecol. Oncol. 2022, 33, e82. [Google Scholar] [CrossRef]
- van Dam, G.M.; Themelis, G.; Crane, L.M.; Harlaar, N.J.; Pleijhuis, R.G.; Kelder, W.; Sarantopoulos, A.; de Jong, J.S.; Arts, H.J.; van der Zee, A.G.; et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: First in-human results. Nat. Med. 2011, 17, 1315–1319. [Google Scholar] [CrossRef]
- Arthur, R.S.; Kirsh, V.A.; Rohan, T.E. Dietary B-Vitamin Intake and Risk of Breast, Endometrial, Ovarian and Colorectal Cancer among Canadians. Nutr. Cancer. 2019, 71, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.X.; Zhu, F.F.; Chen, C.; Zhang, Y.X.; Lv, X.L.; Li, J.W.; Luo, S.P.; Gao, J. Association of Thiamine Intake with Human Papillomavirus (HPV) Infection in American Women: A Secondary Data Analysis Based on the National Health and Nutrition Examination Survey from 2003 to 2016. Med. Sci. Monit. 2020, 26, e924932. [Google Scholar] [CrossRef]
- Piyathilake, C.J.; Macaluso, M.; Chambers, M.M.; Badiga, S.; Siddiqui, N.R.; Bell, W.C.; Edberg, J.C.; Partridge, E.E.; Alvarez, R.D.; Johanning, G.L. Folate and vitamin B12 may play a critical role in lowering the HPV 16 methylation-associated risk of developing higher grades of CIN. Cancer Prev. Res. 2014, 7, 1128–1137. [Google Scholar] [CrossRef]
- Agudelo, M.C.; Agudelo, S.; Lorincz, A.; Ramírez, A.T.; Castañeda, K.M.; Garcés-Palacio, I.; Zea, A.H.; Piyathilake, C.; Sanchez, G.I. Folate deficiency modifies the risk of CIN3+ associated with DNA methylation levels: A nested case-control study from the ASCUS-COL trial. Eur. J. Nutr. 2024, 63, 563–572. [Google Scholar] [CrossRef]
- Peitz, J.G.; Adebamowo, C.A.; Adebamowo, S.N. Association between Serum Folate and Vaginal High-Risk Human Papillomavirus Infections in United States Women. J. Nutr. 2024, 154, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Fu, Q.; Kao, Y.H.; Tseng, T.S.; Reiss, K.; Cameron, J.E.; Ronis, M.J.; Su, J.; Nair, N.; Chang, H.M.; et al. Antioxidants Associated With Oncogenic Human Papillomavirus Infection in Women. J. Infect. Dis. 2021, 224, 1520–1528. [Google Scholar] [CrossRef]
- Flatley, J.E.; McNeir, K.; Balasubramani, L.; Tidy, J.; Stuart, E.L.; Young, T.A.; Powers, H.J. Folate status and aberrant DNA methylation are associated with HPV infection and cervical pathogenesis. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 2782–2789. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sedjo, R.L.; Fowler, B.M.; Schneider, A.; Henning, S.M.; Hatch, K.; Giuliano, A.R. Folate, vitamin B12, and homocysteine status. findings of no relation between human papillomavirus persistence and cervical dysplasia. Nutrition 2003, 19, 497–502. [Google Scholar] [CrossRef]
- Sedjo, R.L.; Inserra, P.; Abrahamsen, M.; Harris, R.B.; Roe, D.J.; Baldwin, S.; Giuliano, A.R. Human papillomavirus persistence and nutrients involved in the methylation pathway among a cohort of young women. Cancer Epidemiol. Biomarkers Prev. 2002, 11, 353–359. [Google Scholar] [PubMed]
- Majumder, D.; Nath, P.; Debnath, R.; Maiti, D. Understanding the complicated relationship between antioxidants and carcinogenesis. J. Biochem. Mol. Toxicol. 2021, 35, e22643. [Google Scholar] [CrossRef] [PubMed]
- Bandera, E.V.; Gifkins, D.M.; Moore, D.F.; McCullough, M.L.; Kushi, L.H. Antioxidant vitamins and the risk of endometrial cancer: A dose-response meta-analysis. Cancer Causes Control. 2009, 20, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Rosner, B.; Willett, W.C.; Hankinson, S.E. Antioxidant intake and risk of endometrial cancer: Results from the Nurses’ Health Study. Int. J. Cancer 2011, 128, 1169–1178. [Google Scholar] [CrossRef]
- Gu, J.H.; Gong, T.T.; Wu, Q.J.; Liu, F.H.; Wen, Z.Y.; Gao, C.; Wei, Y.F.; Yang, Z. Association Between Pre-diagnostic Dietary Supplements Intake and Ovarian Cancer Survival: Findings From a Prospective Cohort Study in Chinese Women. Front. Nutr. 2021, 8, 758178. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Fei, H.; Xu, S.; Wen, J.; Ye, L.; Su, Z. Association about dietary vitamin C intake on the risk of ovarian cancer: A meta-analysis. Biosci. Rep. 2020, 40, BSR20192385. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Zhou, H.; Meng, F.; Tian, T.; Xu, J.; Yan, F. Association of vitamin E on the risk of ovarian cancer: A meta-analysis. Biosci. Rep. 2019, 39, BSR20193311. [Google Scholar] [CrossRef] [PubMed]
- Barchitta, M.; Maugeri, A.; La Mastra, C.; Rosa, M.C.; Favara, G.; Lio, R.M.S.; Agodi, A. Dietary Antioxidant Intake and Human Papillomavirus Infection: Evidence from a Cross-Sectional Study in Italy. Nutrients 2020, 12, 1384. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Zheng, Z.; Chen, W. Association between serum vitamin C and HPV infection in American women: A cross-sectional study. BMC Womens Health 2022, 22, 404. [Google Scholar] [CrossRef]
- Cao, D.; Shen, K.; Li, Z.; Xu, Y.; Wu, D. Association between vitamin C Intake and the risk of cervical neoplasia: A meta-analysis. Nutr. Cancer 2016, 68, 48–57. [Google Scholar] [CrossRef]
- Kuiper, C.; Molenaar, I.G.; Dachs, G.U.; Currie, M.J.; Sykes, P.H.; Vissers, M.C. Low ascorbate levels are associated with increased hypoxia-inducible factor-1 activity and an aggressive tumor phenotype in endometrial cancer. Cancer Res. 2010, 70, 5749–5758. [Google Scholar] [CrossRef]
- Ma, Y.; Chapman, J.; Levine, M.; Polireddy, K.; Drisko, J.; Chen, Q. High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci. Transl. Med. 2014, 6, 222ra18. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, S.; Zhou, L.; Zhao, M.; Zhu, X. Effect of vitamin E supplementation on uterine cervical neoplasm: A meta-analysis of case-control studies. PLoS ONE 2017, 12, e0183395. [Google Scholar] [CrossRef]
- Myung, S.K.; Ju, W.; Kim, S.C.; Kim, H.; Korean Meta-analysis (KORMA) Study Group. Vitamin or antioxidant intake (or serum level) and risk of cervical neoplasm: A meta-analysis. BJOG 2011, 118, 1285–1291. [Google Scholar] [CrossRef]
- Abraham, A.; Kattoor, A.J.; Saldeen, T.; Mehta, J.L. Vitamin E and its anticancer effects. Crit. Rev. Food Sci. Nutr. 2019, 59, 2831–2838. [Google Scholar] [CrossRef]
- Donnelly, J.; Appathurai, A.; Yeoh, H.L.; Driscoll, K.; Faisal, W. Vitamin E in Cancer Treatment: A Review of Clinical Applications in Randomized Control Trials. Nutrients 2022, 14, 4329. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, E.A.; Evans, C.V.; Ivlev, I.; Rushkin, M.C.; Thomas, R.G.; Martin, A.; Lin, J.S. Vitamin and Mineral Supplements for the Primary Prevention of Cardiovascular Disease and Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2022, 327, 2334–2347. [Google Scholar] [CrossRef]
- Didier, A.J.; Stiene, J.; Fang, L.; Watkins, D.; Dworkin, L.D.; Creeden, J.F. Antioxidant and Anti-Tumor Effects of Dietary Vitamins A, C, and E. Antioxidants 2023, 12, 632. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, A.; Yang, J.; Zhao, W.; Wang, Z.; Wang, W.; Wang, J.; Song, J.; Li, L.; Lv, W.; et al. Dietary nutrient intake related to higher grade cervical intraepithelial neoplasia risk: A Chinese population-based study. Nutr. Metab. 2020, 17, 100. [Google Scholar] [CrossRef]
- Markowska, A.; Antoszczak, M.; Markowska, J.; Huczyński, A. Role of Vitamin K in Selected Malignant Neoplasms in Women. Nutrients 2022, 14, 3401. [Google Scholar] [CrossRef]
- Zhu, G.; Li, Z.; Tang, L.; Shen, M.; Zhou, Z.; Wei, Y.; Zhao, Y.; Bai, S.; Song, L. Associations of Dietary Intakes with Gynecological Cancers: Findings from a Cross-Sectional Study. Nutrients 2022, 14, 5026. [Google Scholar] [CrossRef] [PubMed]
- Gummin, D.D.; Mowry, J.B.; Beuhler, M.C.; Spyker, D.A.; Rivers, L.J.; Feldman, R.; Brown, K.; Pham, N.P.T.; Bronstein, A.C.; DesLauriers, C. 2022 Annual Report of the National Poison Data System® (NPDS) from America’s Poison Centers®: 40th Annual Report. Clin. Toxicol. 2023, 61, 717–939. [Google Scholar] [CrossRef] [PubMed]
- Carazo, A.; Macáková, K.; Matoušová, K.; Krčmová, L.K.; Protti, M.; Mladěnka, P. Vitamin A Update: Forms, Sources, Kinetics, Detection, Function, Deficiency, Therapeutic Use and Toxicity. Nutrients 2021, 13, 1703. [Google Scholar] [CrossRef]
- Janoušek, J.; Pilařová, V.; Macáková, K.; Nomura, A.; Veiga-Matos, J.; Silva, D.D.D.; Remião, F.; Saso, L.; Malá-Ládová, K.; Malý, J.; et al. Vitamin D: Sources, physiological role, biokinetics, deficiency, therapeutic use, toxicity, and overview of analytical methods for detection of vitamin D and its metabolites. Crit. Rev. Clin. Lab. Sci. 2022, 59, 517–554. [Google Scholar] [CrossRef] [PubMed]
- Elango, G.; Venkataraman, D.D.; Venkata Rao, S.; Ravi Kiran, V.S. Hypervitaminosis. Int. J. Biomed. Res. 2015, 6, 151–154. [Google Scholar] [CrossRef]
- Ferraro, S.; Biganzoli, G. The relevance of maternal folate levels during pregnancy. Clin. Nutr. 2022, 41, 1146–1147. [Google Scholar] [CrossRef]

| Cancer | Estimates of Cancer Incidence in 2022 in Europe | Estimates of Cancer Mortality in 2022 in Europe | Estimated Relative Change of Incidence from 2022 to 2040 | Estimated Relative Change of Mortality from 2022 to 2040 |
|---|---|---|---|---|
| Corpus uteri | 5.40% | 3.00% | 13.00% | 24.80% |
| Ovary | 3.20% | 4.80% | 12.90% | 19.20% |
| Cervix uteri | - | - | 2.60% | 12.00% |
| Vitamin | Potential Risks and Side Effects |
|---|---|
| Vitamin A | An acute form: headache, visual disturbances, vomiting, short-term loss of consciousness, dizziness, irritability, gastrointestinal disturbance, fever, skin rashes Chronic forms: hepatotoxicity and teratogenicity, kidney failure, arrhythmia, arthralgia, deterioration of vision, lipid disorders, dermatological disorders (including carotenodermia) |
| Vitamin D | greater risk of pancreatic cancer, cardiovascular events, tremors, muscle pain, subcutaneous hemorrhages, dehydration, dental enamel hypoplasia |
| Vitamin E | bleeding, hemorrhages, aggravation of angina, hypertension, atherosclerosis, gastrointestinal disturbance, fatigue, weakness, headache, dysregulation of immune system, delayed wound healing |
| Vitamin K | reduction in blood sugar levels, changes in blood clotting times, hemolytic anemia, jaundice, liver damage |
| Vitamin C | kidney disease, stomach ulcer, disturbance of the pancreas, gallstones, kidney stones, gastrointestinal disturbances (diarrhea, nausea, vomiting, heartburn, stomachache), increased blood pressure, hormonal disorders, leukopenia, insomnia, headache, deterioration of vision, feelings of weakness, dizziness, allergic reactions to skin |
| Vitamin B1 (thiamine) | interference in the thyroid function, heart failure, paralysis, restlessness, convulsions |
| Vitamin B2 (riboflavin) | hepatotoxicity, cardiomyopathies, vomiting, hypotension, fatigue, photophobia, paresthesia, itching, cracks and ulcers in the corners of the mouth |
| Vitamin B3 (niacin) | hepatotoxicity, aggravation of bronchial asthma, hypotension, dizziness, gout, fasting hyperglycemia, gastrointestinal disturbances, dermatological disorders, insomnia |
| Vitamin B6 (pyridoxine) | neurological symptoms, gastrointestinal disturbances, photosensitivity, skin rashes |
| Vitamin B9 (folic acid) | increased risk of diabetes (in interaction with lowest levels of vitamin B12), gastrointestinal disturbances, sleep disorders, dermatological disorders |
| Vitamin B12 (cobalamin) | cardiovascular disorders: congestive heart failure, pulmonary edema, palpitations, allergic reactions, paresthesia |
| Reference | Vitamin/Vitamin Metabolite | Role |
|---|---|---|
| OVARIAN CANCER | ||
| Young et al. [11] | ATRA | ATRA decreases signaling in the ALDH1/FoxM1/Notch1 pathway. ATRA reduces the proportion of ALDH1-positive cells, unlike paclitaxel, which targets the ALDH1-negative cell population. |
| Whitworth et al. [12] | 9cUAB130 | Combined treatment with 9cUAB130 and carboplatin achieves greater cytotoxicity against A2780 cells, as well as a decrease in the expression of CSCs markers. |
| Ezawa et al. [13], Suzuki et al. [26] | TAC-101 | The combination of cisplatin and TAC-101 allows a significant reduction in the volume of clear cell ovarian cancer, both in cisplatin-sensitive tumors and in RMG-I and RMG-II tumors. |
| Prabhala et al. [20], Bono et al. [21] | RA | Retinoids, together with IL-2, increase the synthesis of the anti-tumor IFN-γ. |
| Recchia et al. [22] | RA | The combination of IL-2 and 13-cis-retinoic acid has shown efficacy as maintenance immunotherapy in patients who have achieved clinical benefit after treatment with either liposomal doxorubicin or oxaliplatin chemotherapy. |
| Brewer et al. [23], Holmes et al. [24] | 4-HPR, CD437 | 4-HPR and CD437 promote apoptosis of ovarian cancer cells by increasing the activity of caspase-3 and caspase-9 enzymes in both ATRA-sensitive (CAOV-3) and resistant (SKOV-3) cells, as well as increasing the expression of proapoptotic genes and mitochondria uncoupling protein in OVCA433 cells. |
| Colombo et al. [25] | 4-HPR | The use of 4-HPR in the preoperative period does not provide significant clinical benefit. |
| Paucarmayta et al. [55] | 1α,25(OH)₂D₃ | The association of progesterone and calcitriol with cisplatin increases the efficacy of anticancer therapy. |
| Chen et al. [50], Jiang et al. [51] | 1α,25(OH)₂D₃ | 1α,25(OH)₂D₃ has the ability to stop uncontrolled growth of ovarian cancer cells at the G1/S and G2/M checkpoint of the cell cycle. |
| Hou et al. [52] | 1α,25(OH)₂D₃ | When SKOV-3 cells are stimulated with TGF-β1, 1α,25(OH)₂D₃ effectively suppresses their migration and invasion, while also promoting the adoption of an epithelial phenotype. This is achieved by 1α,25(OH)₂D₃ through its inhibition of cell migration, which it accomplishes by reducing the expression of EMT factors. |
| Kuznia et al. [9] | 1α,25(OH)₂D₃ | Vitamin D administered daily reduced cancer mortality by 12 %. |
| ENDOMETRIAL CANCER | ||
| Tsuji et al. [27] | ATRA | ATRA inhibits proliferation and induces apoptosis of RL95-2 cells while affecting either RARα or RARβ, with the effect mainly on RARβ expression. |
| Ito et al. [28] | RA | RA allows increased expression of RARβ relative to RARα in endometrioid adenocarcinoma cells. |
| Mittal et al. [29] | 4-HPR | 4-HPR by increasing STRA6 gene expression allows RA to increase uptake, which induces apoptosis of endometrial cancer cells. |
| Cheng et al. [30] | RA | The expression of HSD17B2, an enzyme that plays a crucial role in the metabolic conversion of hormones critical for the transition of the endometrium from the progesterone-dependent secretory phase to the estrogen-dependent proliferative phase, is induced by retinoic acid. |
| CERVICAL CANCER | ||
| Sanusi et al. [33] | Vitamin A | Adding vitamin A to NAC achieves greater reduction in cervical cancer volume. |
| Vahedpoor et al. [61] | Vitamin D | Administering one dose of 50,000 IU of vitamin D every two weeks for a period of six months was found to have a supportive effect on the regression of dysplastic lesions in individuals diagnosed with CIN1. |
| Punchoo et al. [62] | 25(OH)D₃ | Already physiological doses of 25-hydroxyvitamin D are sufficient to inhibit proliferation and to stimulate apoptosis in cells of the SiHa lineage. |
| Reference | Article Type | Vitamin/Vitamin Metabolite and Daily Dose (If Reported) | Cancer Risk | Measure of Association |
|---|---|---|---|---|
| OVARIAN CANCER | ||||
| Wang et al. [7] | Meta-analysis | Vitamin A | Intake of vitamin A was inversely associated with risk of ovarian cancer, especially among North Americans. | RR = 0.816 (95% CI 0.723–0.920) |
| Liao et al. [8] | Meta-analysis | Vitamin D | Intake of vitamin D was inversely associated with risk of ovarian cancer. | RR = 0.80 (95% CI 0.67–0.95) |
| Gersekowski et al. [72] | Case-control study | Folate: 400+ μg | Higher dietary folate intake was associated with an increased risk of ovarian cancer for women with endometriosis. No association for women without endometriosis. | OR = 1.37 (95% CI 1.01–1.86) |
| Arthur et al. [75] | Case-control study | Folate: >560.7 μg Vitamin B6: >2.9 mg | Higher dietary folate intake was inversely associated with risk of ovarian cancer. Higher dietary intake of vitamin B6 was inversely associated with ovarian cancer risk. | Folate: HRq4 vs. q1 = 0.39 (95% CI: 0.19–0.80) Vitamin B6: HRq4 vs. q1 = 0.49 (95% CI: 0.24–0.98 |
| ENDOMETRIAL CANCER | ||||
| Arthur et al. [75] | Case-control study | Folate: >614.9 μg | Higher dietary folate intake was inversely associated with risk of endometrial cancer. | HRq4 vs. q1 = 0.52 (95% CI 0.29–0.93) |
| Bandera et al. [85] | Meta-analysis | Beta-carotene, Vitamin C Vitamin E | Beta-carotene is associated with a 12% risk reduction in endometrial cancer, vitamin C is associated with a 15% risk reduction, and vitamin E is associated with a 9% risk reduction. | Beta-carotene: OR = 0.88 (95% CI: 0.79–0.98), Vitamin C: OR = 0.85 (95% CI: 0.73–0.98), Vitmain E: OR = 0.91 (95% CI: 0.84–0.99) |
| Zhu et al. [103] | Vitamin B12: 3.17 (1.78–5.14) Data were presented as median with range. | Intake of vitamin B12 was inversely associated with risk of endometrial cancer. | OR = 0.812 (95% CI: 0.714, 0.925) | |
| CERVICAL CANCER | ||||
| Zhou et al. [76] | Secondary data analysis | Thiamine: 2 mg | An increase of every 1-unit rise in thiamine intake is associated with a 18% decrease in HPV infection. | β = 0.82 (95% CI: 0.78–0.86) |
| Barchitta et al. [90] | Cross-Sectional Study | Vitamin A: 1097.59 IU (538.14), Vitamin C: 116.71 mg (107.55), Vitamin E: 37.97 mg (23.44) Data were presented as median with interquartile range. | Higher dietary intake of vitamin A, C and E intake was inversely associated with risk of hrHPV infection. | Composite Dietary Antioxidant Index (CDAI): OR = 0.39 (95% CI: 0.18–0.85) |
| Zheng et al. [91] | Cross-Sectional Study | Vitamin C | Negative association between vitamin C intake and HPV infection in women 25 years of age and older. | OR = 0.7 (95% CI: 0.52–0.94) |
| Cao et al. [92] | Meta-analysis | Vitamin C: 50 mg | Increased vitamin C intake by 50 mg/day was related to the reduced risk of cervical neoplasia. | OR = 0.92 (95% CI: 0.89–0.94) |
| Hu et al. [95] | Meta-analysis | Vitamin E | Intake of vitamin E was inversely associated with risk of cervical neoplasia. | OR = 0.58 (95% CI: 0.47–0.72 |
| Myung et al. [96] | Meta-analysis | Vitamin B12, Vitamin C, Vitamin E, Beta-carotene | Intake of vitamin E was inversely associated with risk of cervical neoplasia. | Vitamin B12: OR = 0.35 (95% CI: 0.19–0.63), Vitamin C: OR = 0.67 (95% CI: 0.55–0.82), Vitamin E: OR = 0.56 (95% CI: 0.35–0.88), Beta-karoten: OR = 0.68 (95% CI: 0.55–0.84) |
| Wang et al. [101] | Cohort study | Folate: 358.9 μg (283.8–836.5), Vitamin B6: 1.9 mg (1.6–4.2), Vitamin C: 59.4 mg (43.2–148.2), Niacin: 187.2 mg (127.7–560.6), Vitamin K: 187.2 μg (127.7–560.6) Data were presented as median with range. | The risk of CIN2+ was associated with low dietary intake of folate, vitamins B6, C, niacin, and vitamin K. | Folate: OR = 1.55 (95% CI: 1.03–2.33); Vitamin B6: OR = 1.63 (95% CI: 1.08–2.46), Vitamin C: OR = 1.59 (95% CI: 1.05–2.42), Vitamin B3: OR = 1.65 (95% CI: 1.08–2.51), Vitamin K: OR = 1.60 (95% CI: 1.05–2.44) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wierzbowska, N.; Olszowski, T.; Chlubek, D.; Kozłowski, M.; Cymbaluk-Płoska, A. Vitamins in Gynecologic Malignancies. Nutrients 2024, 16, 1392. https://doi.org/10.3390/nu16091392
Wierzbowska N, Olszowski T, Chlubek D, Kozłowski M, Cymbaluk-Płoska A. Vitamins in Gynecologic Malignancies. Nutrients. 2024; 16(9):1392. https://doi.org/10.3390/nu16091392
Chicago/Turabian StyleWierzbowska, Natalia, Tomasz Olszowski, Dariusz Chlubek, Mateusz Kozłowski, and Aneta Cymbaluk-Płoska. 2024. "Vitamins in Gynecologic Malignancies" Nutrients 16, no. 9: 1392. https://doi.org/10.3390/nu16091392
APA StyleWierzbowska, N., Olszowski, T., Chlubek, D., Kozłowski, M., & Cymbaluk-Płoska, A. (2024). Vitamins in Gynecologic Malignancies. Nutrients, 16(9), 1392. https://doi.org/10.3390/nu16091392





