The Impact of a Nutritional Intervention on Glycemic Control and Cardiovascular Risk Markers in Type 2 Diabetes
Abstract
1. Introduction
1.1. Epidemiology
1.2. Nutritional Management
1.3. Personalization/Nutritional Precision
1.4. Dietary Patterns
1.5. Mediterranean Diet
1.6. DASH Diet (Dietary Approaches to Stop Hypertension)
1.7. Sustainable and Economically Viable Strategies
1.8. Literature Gap
2. Objectives
Specific Objectives
3. Materials and Methods
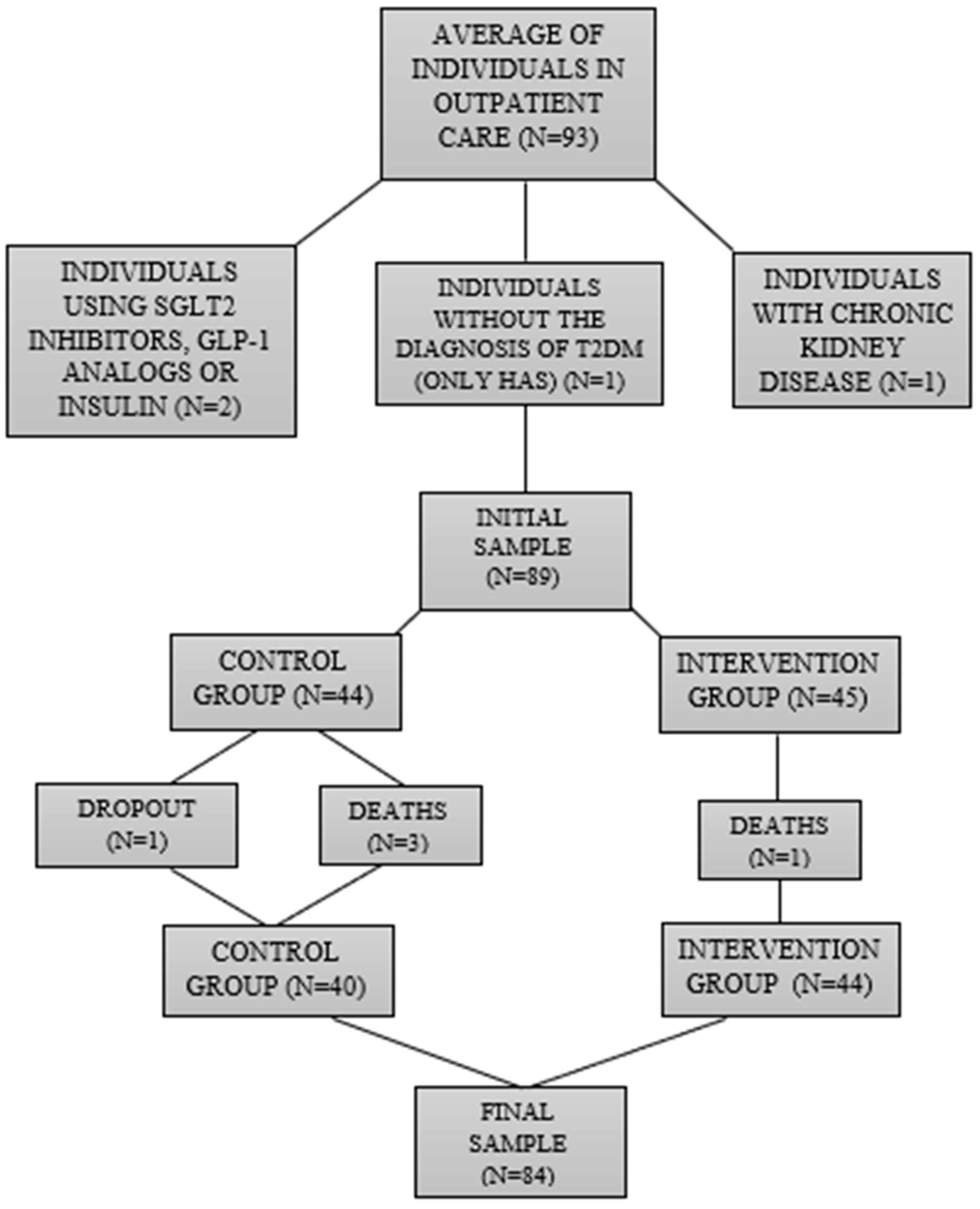
3.1. Case Study/Participants
3.1.1. The Inclusion Criteria for Participants Were as Follows
- -
- Age: Between 18 and 80 years old.
- -
- Diagnosis: Confirmed to have type 2 diabetes (T2D), indicated by fasting blood glucose levels ≥126 mg/dL and a glycated hemoglobin ≥ 6.5%.
- -
- Gender: Both male and female participants are included, aiming to expand the sample number and collect as many participants as possible.
- -
- Commitment: An availability to participate in quarterly meetings for a duration of 36 months.
- -
- Nutritional Status: A Body Mass Index (BMI) greater than 24.9 kg/m2.
- -
- Lifestyle: Sedentary.
3.1.2. The Exclusion Criteria for Participants Were as Follows
- -
- Individuals who had difficulties answering the requested instruments.
- -
- Those who demonstrated impediments to regular data collection.
- -
- Individuals without a confirmed diagnosis of type 2 diabetes (T2D).
- -
- Those who were using insulin therapy.
- -
- Individuals who were using Sodium-Glucose Transport Protein (SGLT-2) inhibitors and/or Glucagon-like peptide-1 (GLP-1) analogs.
- -
- Those diagnosed with Chronic Kidney Disease (CKD).
- -
- Individuals who were eutrophic or malnourished.
- -
- Those who practiced physical exercise for more than 150 min during the week.
3.2. Materials and Procedures
3.2.1. Study Design and General Information
3.2.2. Nutritional Interventions
3.2.3. Detailing of Protocols and Procedures
- (1)
- Nutritional Anamnesis Protocol: Analyzes the clinical and dietary parameters, which assist in the careful evaluation of the participant, and can even alert for some risk signs and diseases that are related to their diet [64].
- (2)
- Protocol for anthropometric, biochemical, and clinical evaluation: Analyzes the anthropometric, biochemical, clinical, and other general parameters’ information that assists in the complete evaluation of the participant [65].
- (3)
- Dietary evaluation protocol (habitual food recall): Analyzes the patient’s usual food intake, allowing for the careful evaluation of both quantitative and qualitative aspects and secondarily calculations, estimates, possible deficiencies, and excesses from the patient [64].
- (4)
- Sociodemographic research protocol: Provides general data, such as age, sex, socioeconomic level, race, profession, and other details. To designate social classes, the following classifications were considered: Class A (those who earn more than 20 minimum wages); Class B (from 10 to 20 minimum wages); Class C (from 4 to 10 minimum wages); Class D (from 2 to 4 minimum wages); and Class E (receives up to 2 minimum wages) [66]. In 2020 the value of a minimum wage was BRL 1039. Note: BRL 1039 are equivalent to approximately USD 207 or EUR 191, considering the values of currencies that were quoted at the end of 2020.
- (5)
- Nutritional guidelines for participants with T2D (APPENDIX 1 in Supplementary Materials): Offers guidelines, tips, and general clarifications for disease control directed at participants with T2D.
- (6)
- An individualized food plan was prepared and delivered at the time of consultation (APPENDIX 2 in Supplementary Materials).
Qualitative Aspects:
Energy Calculations and Estimate Formulas:
- (a)
- The basal metabolic rate (BMR) of patients was calculated using the Mifflin-St Jeor Formula [67] since the groups were largely composed of individuals with an obesity nutritional status: BMR (men) = (10 × weight in kg) + (6.25 × height in cm) − (5 × age in years) + 5; BMR (women) = (10 × weight in kg) + (6.25 × height in cm) − (5 × age in years) − 161.
- (b)
- The adjusted weight (AW) formula was also used for patients with obesity AW (kg) = [current weight (kg)-ideal weight (kg)] × 0.25 + ideal weight (kg) [68].
- (c)
- The total energy expenditure (TEE) was calculated using the formula TEE = BMR × PAL. The PAL (physical activity level) was considered to be 1.2, since all selected patients were sedentary [69].
- (d)
- The total energy value (TEV) of the baseline was calculated from the estimate of the total daily caloric value collected in the habitual food recall of the participants. For the calculation of the TEV of the new food plan, a deficit of 500 kcal was estimated in relation to the TEV baseline, remembering that all calculations were adjusted to the individuality and objectives of each patient. All values (BMR, TEE, and TEV) were expressed in calories (Kcal).
Dietary Prescription
- (a)
- (b)
- (c)
- (d)
- (e)
- (f)
- Supplementary documents: a food diary [70] and “10 Steps to Healthy Eating” [25]. A food diary is used by nutritionists to maximize the participant’s self-knowledge in relation to their eating habits, involving an awareness of the quality, quantity, and frequency of food intake. The participant was instructed to note all the times of their meals, as well as the types of food, their ingested quantities and/or the customized other items of their preference, thus working on the principle of food autonomy [70]. The “10 Steps to Healthy Eating” are key points summarized in the last chapter of the “Dietary Guidelines for the Brazilian Population”, consisting of practical, easy, and economically accessible strategies to be applied in the participants’ day-to-day life [25].
3.2.4. Follow-Up
3.2.5. Anthropometric Evaluation
3.2.6. Evaluation of Cardiovascular Parameters
3.2.7. Evaluation of Laboratory Parameters
3.2.8. Evaluation of Medications
3.2.9. Evaluation of Physical Exercises
3.2.10. COVID-19 Pandemic
3.2.11. Assessment of Eating Window and Sleep
3.2.12. Ethical Aspects
3.2.13. Construction, Research Management, and Databases—REDCAP FAMERP/FUNFARME
3.2.14. Statistical Analysis
4. Results
4.1. Quantitative Results
4.1.1. Sociodemographic Data and Another Baseline Information
4.1.2. General Lifestyle Data, Anthropometric, Biochemical, and Cardiovascular Parameters at Baseline
4.1.3. Comparison of Groups (Control and Intervention) with Their Own Metrics between Baseline and the 12th Month for Anthropometric, Biochemical, Cardiovascular Parameters, and General Lifestyle Data
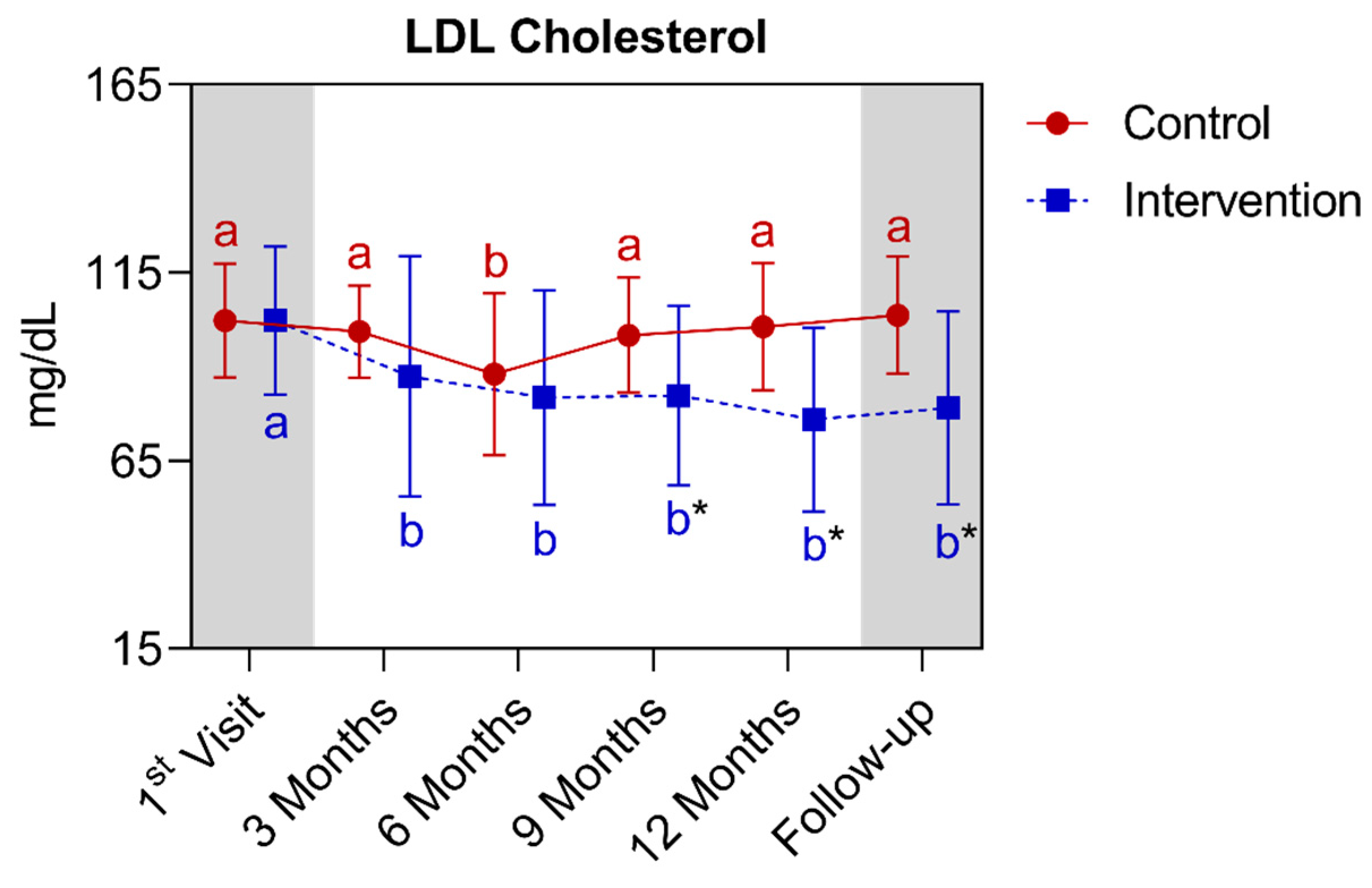
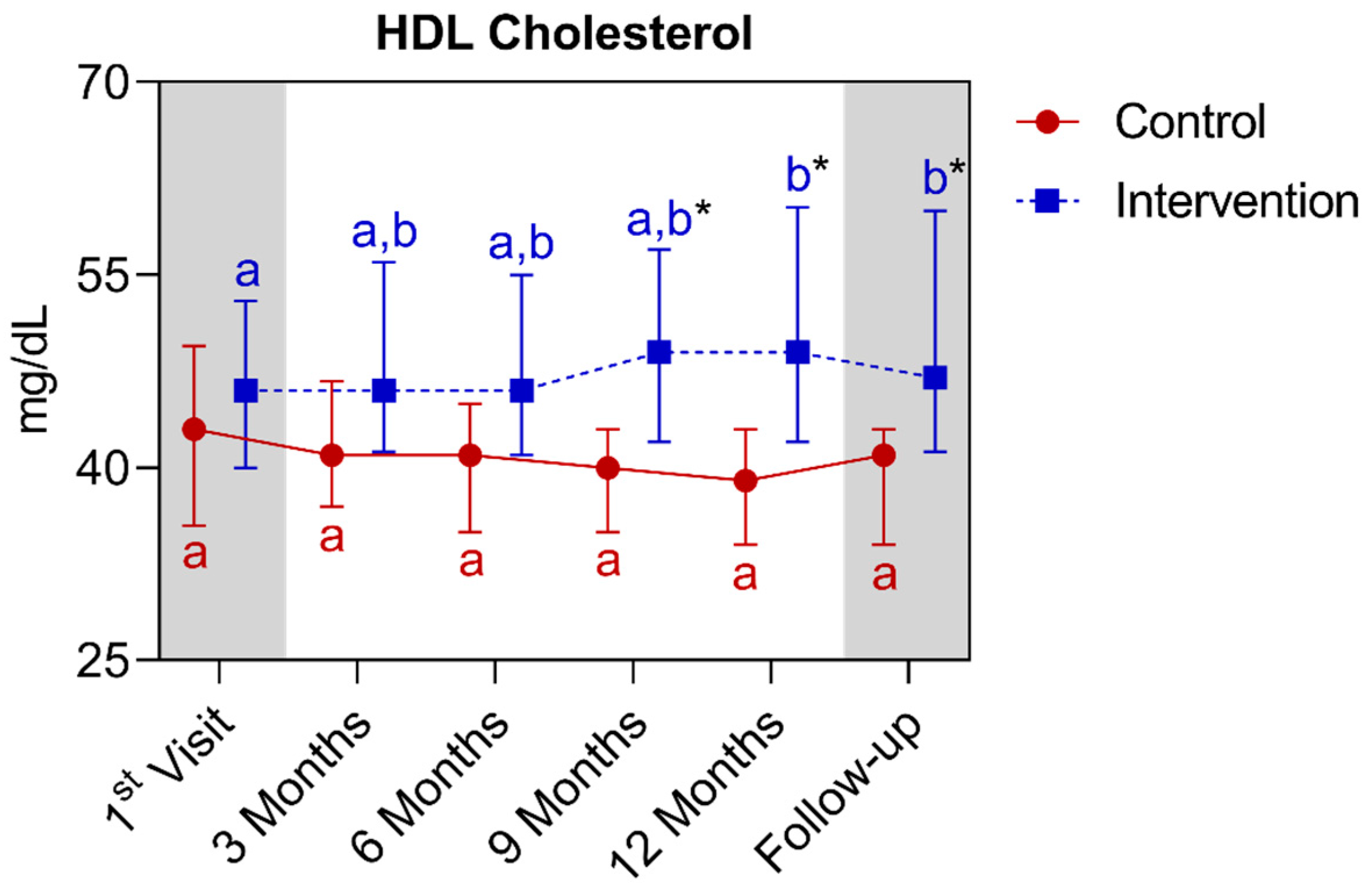
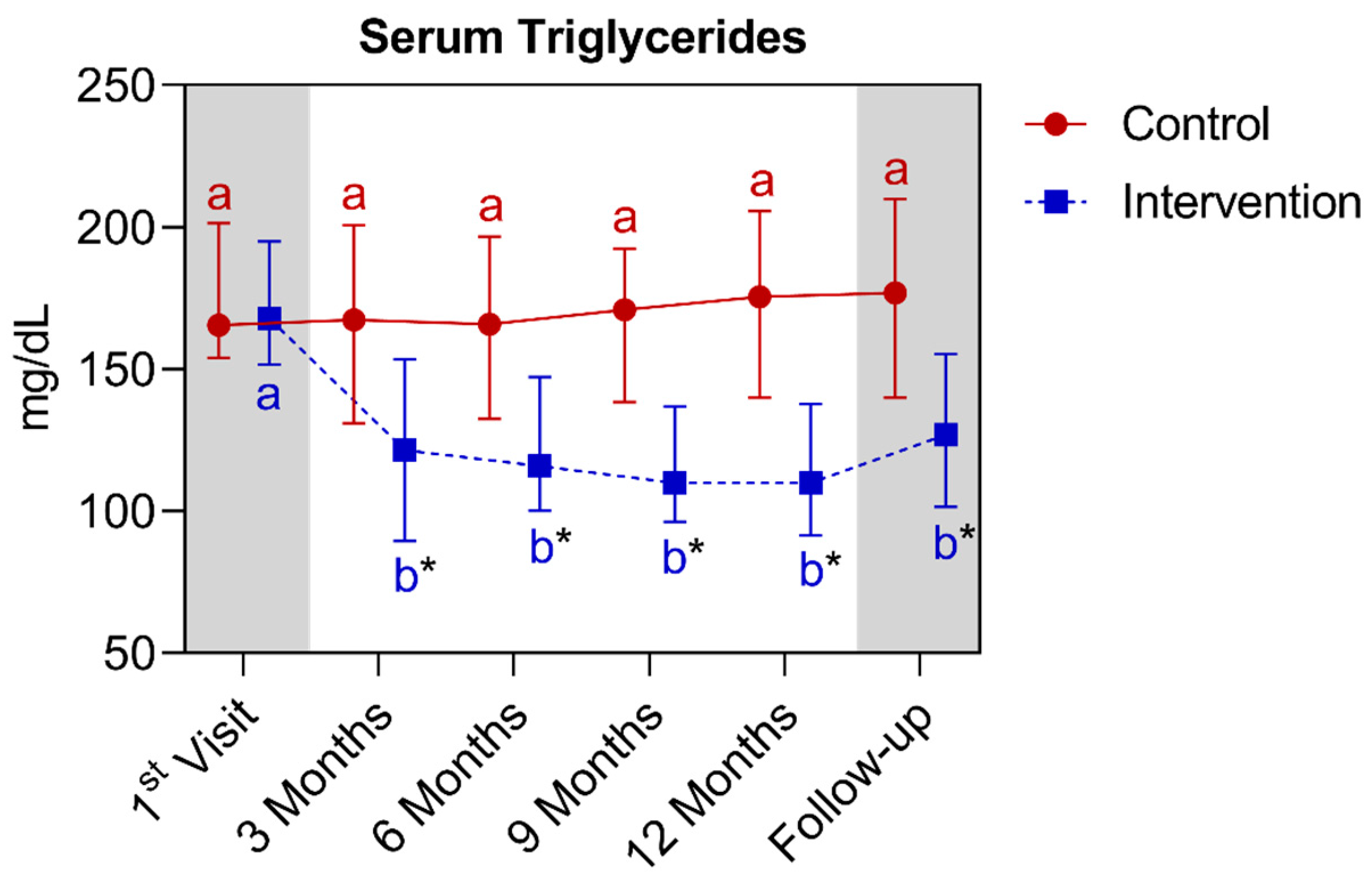
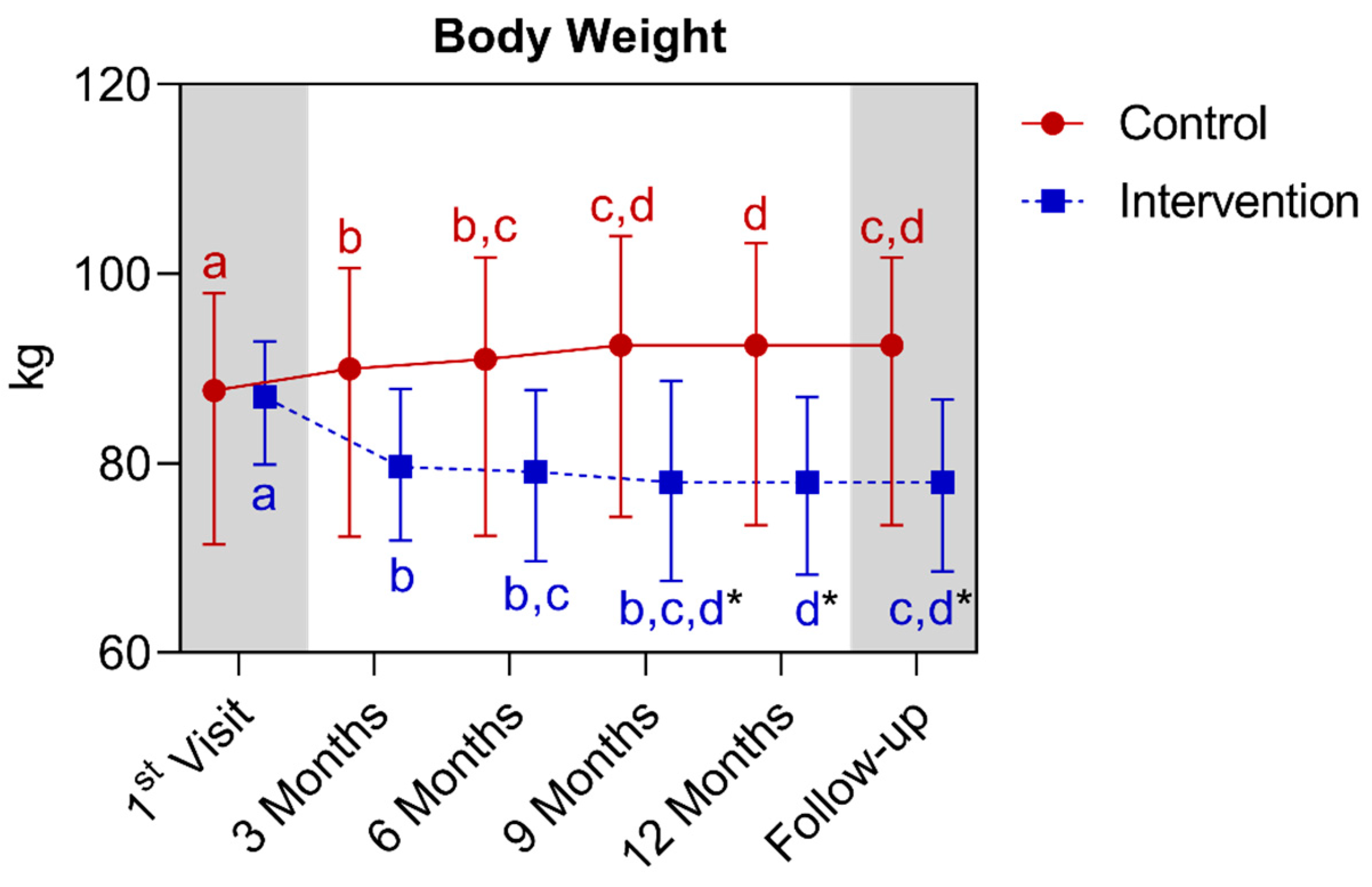
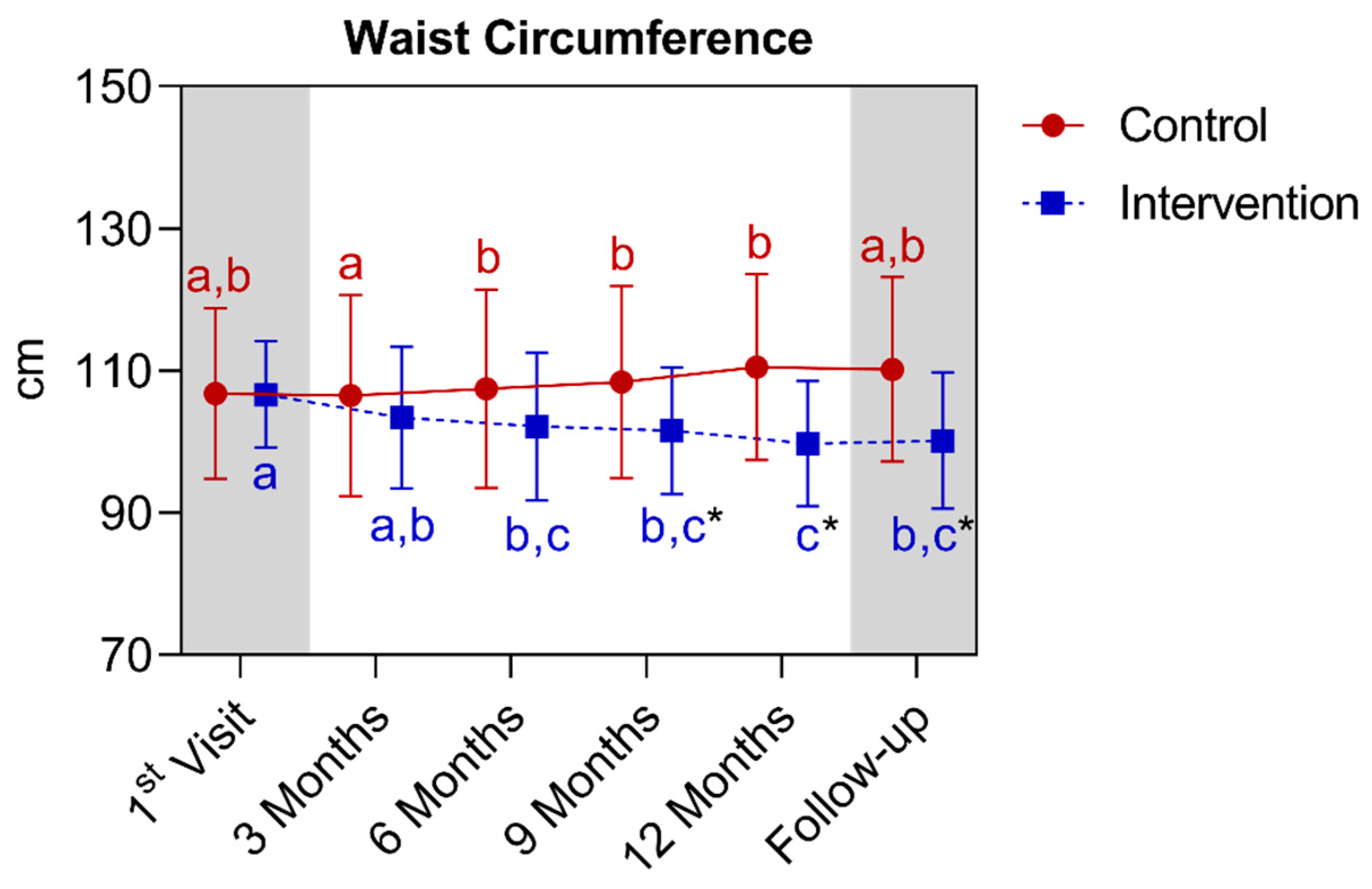
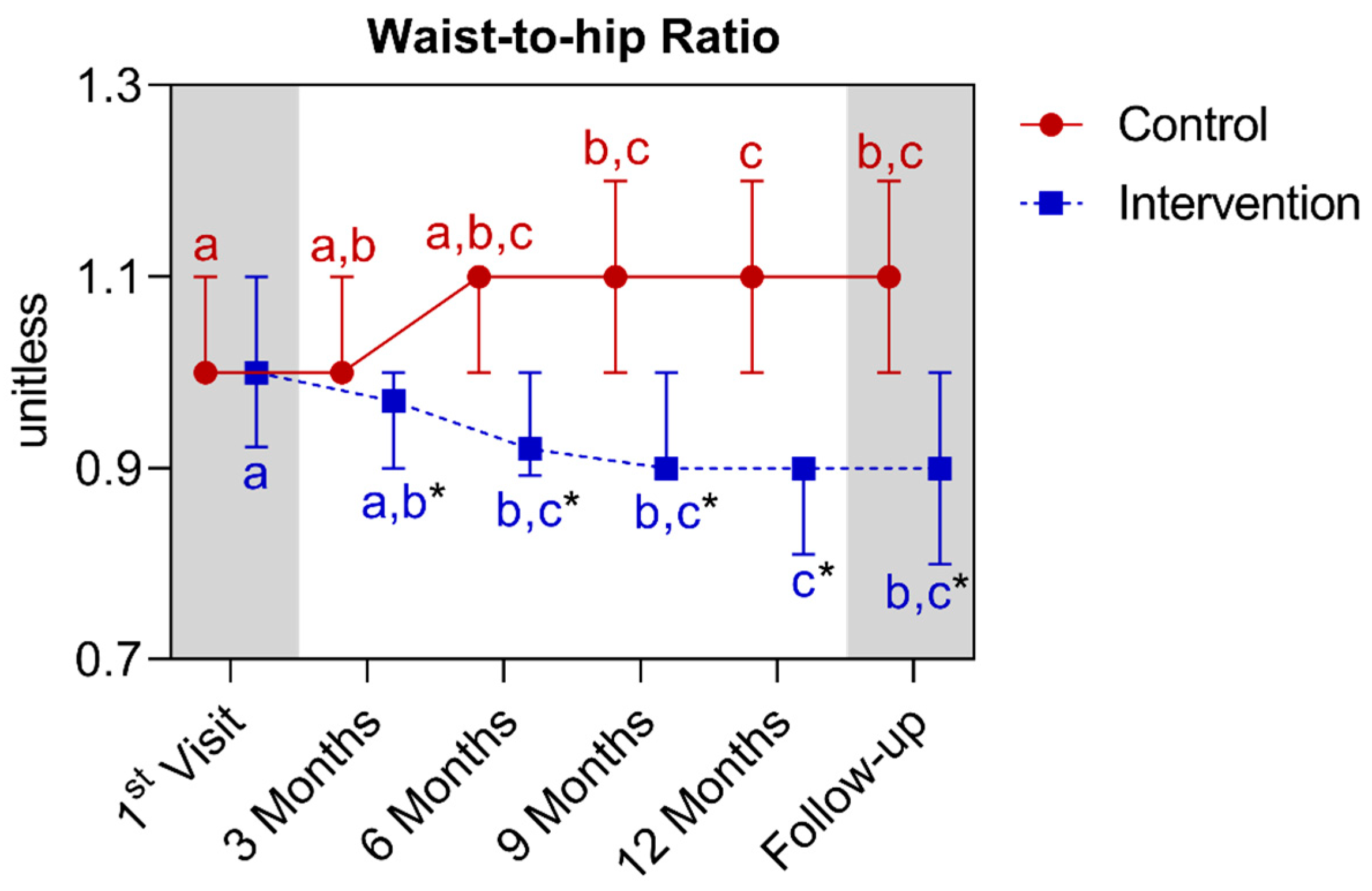
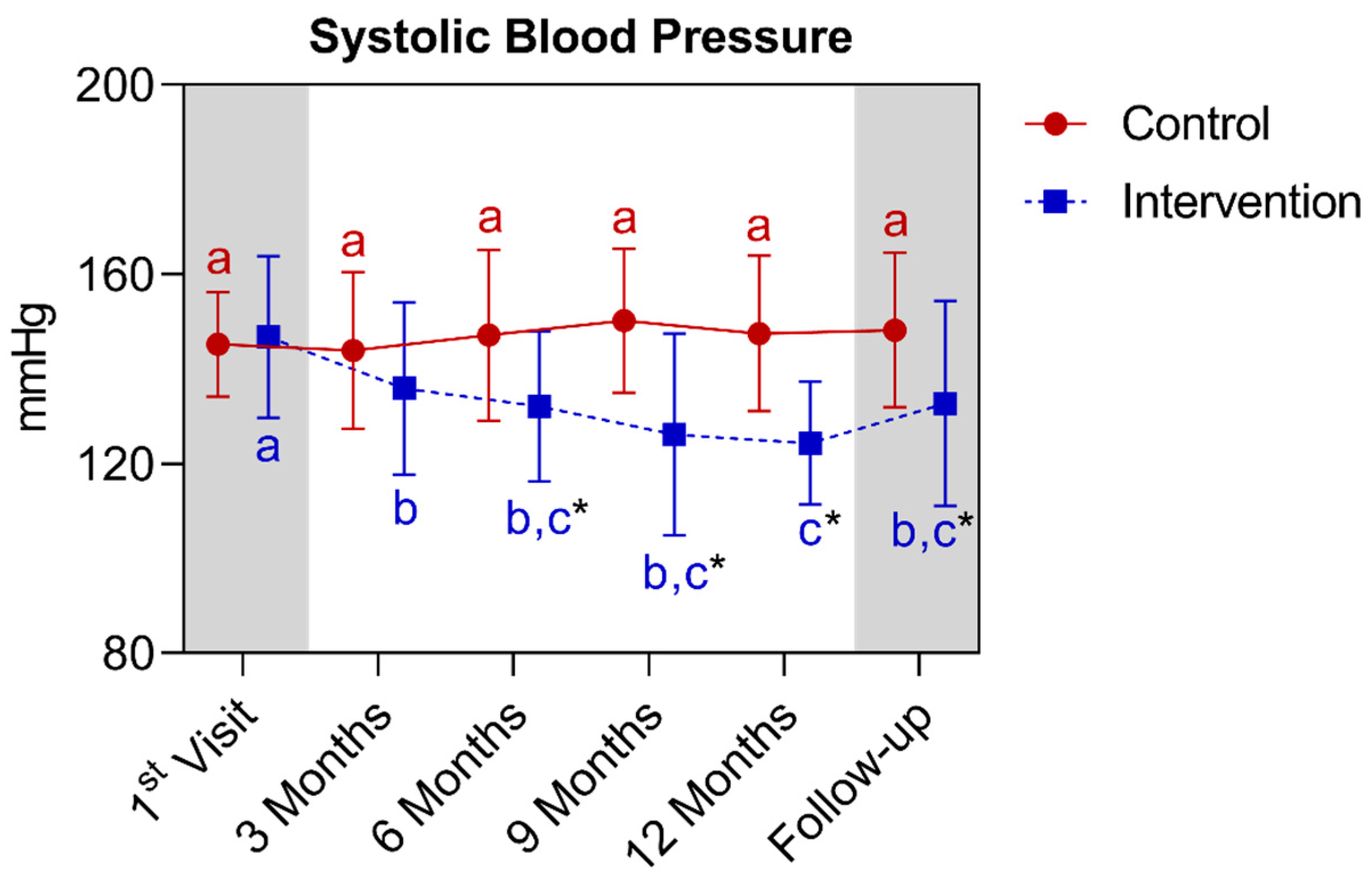
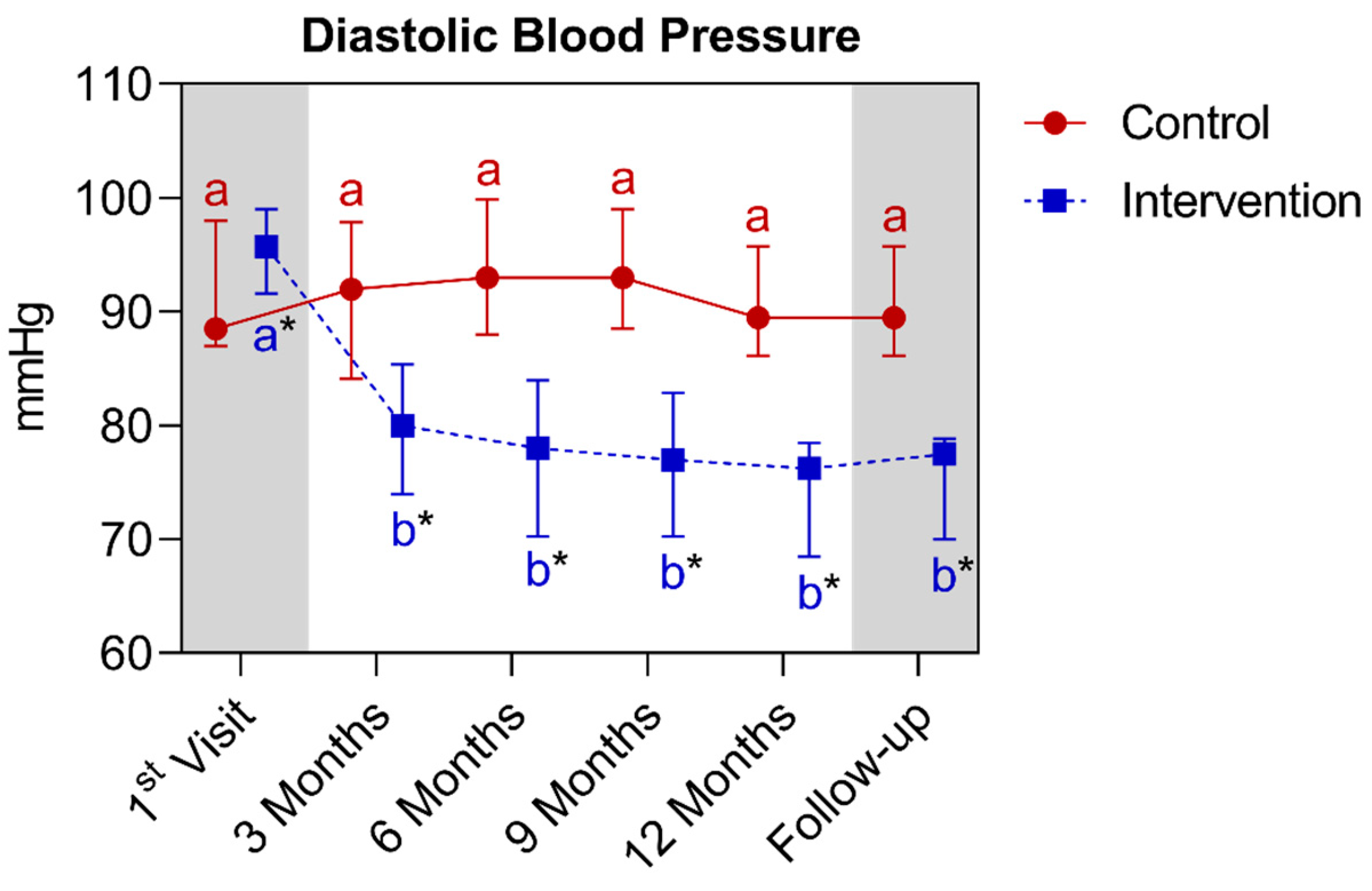
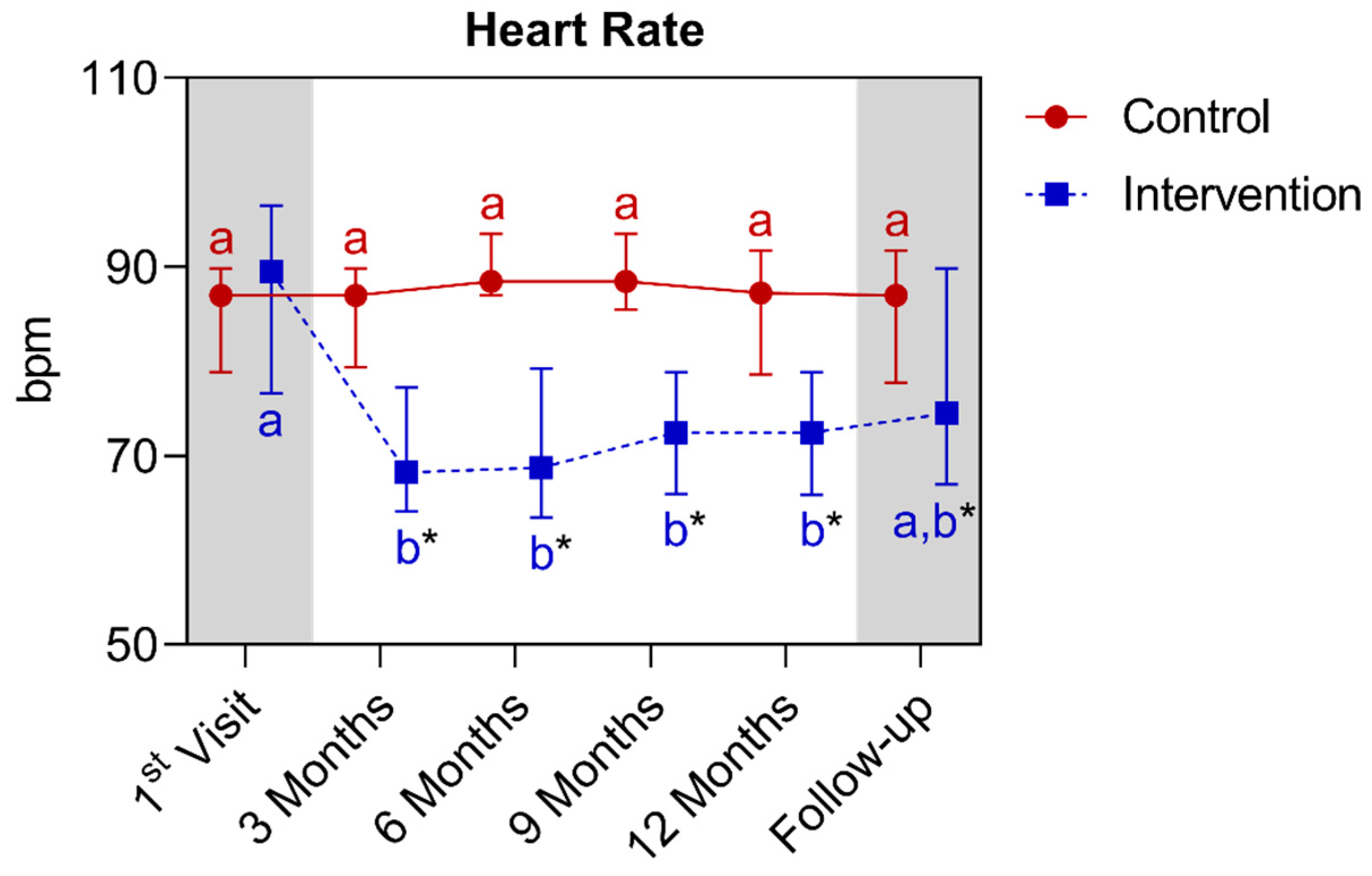
4.1.4. Longitudinal Comparison of the Intervention Group with the Control Group for Anthropometric, Biochemical, and Cardiovascular Parameters over 12 Months, Followed by 3 Months of Follow-Up (15th Month)
4.2. Qualitative Results
4.2.1. Diet and Eating Behavior
4.2.2. Regional Traditions, Culture, and Religion
4.2.3. Physical Activity
4.2.4. Medications
4.2.5. Follow-Up
5. Discussion
5.1. Sociodemographic and Qualitative Data
5.2. General Lifestyle Data, Anthropometric, Biochemical, and Cardiovascular Markers
5.3. Body Weight, Blood Glucose, and Blood Pressure
5.4. COVID-19 Pandemic
5.5. Follow-Up
5.6. Qualitative Aspects of Diet and Eating Behavior
5.7. Positive Points
- -
- This study followed an interventionist approach and included a control group. The control group was composed of a sample that closely resembled the intervention group in most parameters during the baseline period.
- -
- A follow-up was conducted, which is extremely relevant to assess the stability of eating behaviors post-intervention.
- -
- There was a control of variables that could interfere with the results, such as medications and physical exercises.
- -
- A completely individualized dietary plan, standardized to the Brazilian food culture (considering preferences and glycemic targets), was crafted and delivered at the time of the first consultation.
- -
- A broad repertoire of food options and variability was provided, allowing for greater palatability and adherence, thus avoiding monotony, especially for patients with taste aversion and difficulty swallowing (>65 years).
- -
- Participants’ nutritional management was adapted to their socioeconomic reality, enabling effective and accessible strategies. This aspect is interesting not only for the Brazilian public health system but also for other countries facing the dichotomy between rising obesity and diabetes rates, alongside high levels of food insecurity and malnutrition.
- -
- There was constant contact with patients through phone and/or text messages or mobile video calls, maximizing the clarification of doubts.
- -
- The data collection period was relatively long, that is, 3 years of follow-up, to fill out a 15-month spreadsheet, concurrent with the challenging presence of the global COVID-19 pandemic and lockdown.
- -
- This work evaluated other qualitative aspects, which are not yet so elucidated in the literature, such as COVID-19 infection and its consequences for people with T2D.
- -
- This study used a representative sample of a large part of the Brazilian population, both at the socioeconomic level (Classes C and D) and in racial aspects (white, brown, and black).
- -
- This study provided a good literature review and update on the topic.
5.8. Limitations and Future Perspectives
- -
- The COVID-19 pandemic reduced the flow of consultations during the second lockdown.
- -
- The level of physical activity and patient engagement during the pandemic was also null.
- -
- A shortage of fresh and minimally processed foods was observed in São José do Rio Preto city, SP, Brazil, especially in the first year of the pandemic (March 2020 to 2021).
- -
- There was no random allocation or blinding of all those involved in the study (patients and researchers) to the distribution of groups.
- -
- There was also little interaction with the patients in the control group, as they did not undergo nutritional assessment.
- -
- The sample size was relatively small (n = 84). Unfortunately, it is very difficult to conduct longitudinal studies without reductions in sample size. This research, unfortunately, had some dropouts (patients stopped going to the clinic appointments) and deaths.
- -
- The majority of participants in the intervention group were women, and the baseline DBP level was also slightly higher in this group compared to the control. However, it is noted that this difference was reversed by the 3rd month, extending until the 15th month. For future studies, greater attention is emphasized on obtaining groups of patients with proportional sex quantities and verifying the absence of differences between all parameters in the baseline period.
- -
- Despite a large portion of the parameters showing similarity (with no statistical difference at the start of the study), the groups were not technically completely matched. Therefore, it cannot be stated that this is a clinical trial, but rather an experimental study. This is a common occurrence in experimental studies and does not necessarily invalidate the results, but it is an important factor to consider when interpreting them.
- -
- The data analysis was not conducted by separating genders because the aim of this research was not to analyze the effect of the intervention on sex. Instead, it aimed to analyze the effect of the intervention on anthropometric, biochemical, and cardiovascular markers in individuals of both sexes and as many patients as possible. This was especially the case since a convenience sample was used, i.e., a sample of available patients interested in participating who were from the Hypertension and Endocrinology Outpatient Clinic. Separating the data analysis by gender would reduce the sample size to the point of altering the robustness of the results. In addition, the number of paired individuals was also similar (n = 40 control/n = 44 intervention). Dividing by gender would harm the analysis, decreasing the quality of the statistical power.
- -
- There was a reversal of roles in seeking treatment, meaning the researcher approached the patients and invited them to participate in the study. This reversal of the search for treatment can hinder the patient’s interest and their appreciation of the intervention. In most successful clinical practice cases, the search is the opposite; the patient takes the initiative to seek out the professional and wants to change their lifestyle. The act of seeking help, the “action,” is the first step towards therapeutic optimization and is fundamental to establishing new eating habits.
- -
- In future studies, it may be interesting to assess body masses (lean mass and body fat). Unfortunately, it was not possible to measure them, as the hypertension clinic did not have specific equipment for in-depth anthropometric assessment (a bioimpedance measure and an adipometer, for example). Moreover, most patients had abdominal obesity, which makes it difficult to measure skin folds. Another important point is that the participants were also using diuretics, which negatively interfere with hydration levels and, consequently, the accuracy of bioimpedance, for example.
- -
- Additionally, it may be interesting to include the assessment of neck circumference and the waist (cm)/height (cm) ratio, as these are emerging measures [2] that have shown interesting results in patients with T2D and high cardiovascular risks [3], and being that these measures are simple and economically accessible for measurement at the ambulatory level of public health systems [23].
- -
- Future studies may be able to increase the information derived from basic anthropometrics, with the use of allometric indices for waist circumference, A Body Shape Index (ABSI), and for hip circumference, the Hip Index (HI). For practical purposes, these indices are mutually independent of the BMI and can therefore be readily combined statistically [98,99].
6. Conclusions
7. Take-Home Message
- -
- Personalized nutrition is a gold standard for the management of patients with type 2 diabetes mellitus (T2D). A single, universal strategy will never be the solution for the long-term control and prevention of this disease.
- -
- It is possible to prescribe meal plans that combine taste and health, within contexts that involve socioeconomic vulnerabilities.
- -
- The Mediterranean and DASH diets offer a wide food repertoire of foods and in recent years, they have been showing good results in adherence, in the longitudinal improvement of health parameters, and in their adaptation to other food cultures worldwide.
- -
- Weight loss may not follow a straight, decreasing line, as it involves various complexities and challenges. Adjustments to the meal plan and constant monitoring are necessary to optimize long-term results.
- -
- T2D is a complex disease, which affects different people, countries, and cultures. It does not have a specific color, address, or socioeconomic situation defined to it, that is, it can affect any individual, deserving constant attention from all public health systems.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; Available online: http://www.diabetesatlas.org (accessed on 20 January 2022).
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 8. Association Obesity and Weight Management for the Prevention and Treatment of Type 2 Diabetes: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46 (Suppl. S1), S128–S139. [Google Scholar] [CrossRef] [PubMed]
- Marx, N.; Federici, M.; Schütt, K.; Müller-Wieland, D.; Ajjan, R.A.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B.; et al. ESC Scientific Document Group. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur. Heart J. 2023, 44, 4043–4140. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Fact Sheet: Obesity and Overweight; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 20 November 2021).
- World Health Organization (WHO). Proposed Policy Priorities for Preventing Obesity and Diabetes in the Eastern Mediterranean Region; Technical Report Series; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Factor Collaboration (NCD-RisC). Worldwide trends in underweight and obesity from 1990 to 2022: A pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024, 403, 1027–1050. [Google Scholar] [CrossRef]
- Ministry of Health; Secretariat of Health Surveillance and Environment; Department of Epidemiological Analysis and Surveillance of Non-Communicable Diseases. Vigitel Brazil 2023: Surveillance of Risk Factors and Protection for Chronic Diseases by Telephone Survey: Estimates on Frequency and Sociodemographic Distribution of Risk Factors and Protection for Chronic Diseases in the Capitals of the 26 Brazilian States and in the Federal District in 2023 [Electronic Resource]; Ministry of Health: Brasília, Brazil, 2023; 131p. Available online: http://bvsms.saude.gov.br/bvs/publicacoes/vigitel_brasil_2023.pdf (accessed on 20 November 2023).
- Berger, S.E.; Huggins, G.S.; McCaffery, J.M.; Jacques, P.F.; Lichtenstein, A.H. Change in Cardiometabolic Risk Factors Associated With Magnitude of Weight Regain 3 Years After a 1-Year Intensive Lifestyle Intervention in Type 2 Diabetes Mellitus: The Look AHEAD Trial. J. Am. Heart Assoc. 2019, 8, e010951. [Google Scholar] [CrossRef] [PubMed]
- Carbone, S.; Dixon, D.L.; Buckley, L.F.; Abbate, A. Glucose-Lowering Therapies for Cardiovascular Risk Reduction in Type 2 Diabetes Mellitus: State-of-the-Art Review. Mayo Clin. Proc. 2018, 93, 1629–1647. [Google Scholar] [CrossRef] [PubMed]
- Minari, T.P.; Tácito, L.H.B.; Yugar, L.B.T.; Ferreira-Melo, S.E.; Manzano, C.F.; Pires, A.C.; Moreno, H.; Vilela-Martin, J.F.; Cosenso-Martin, L.N.; Yugar-Toledo, J.C. Nutritional Strategies for the Management of Type 2 Diabetes Mellitus: A Narrative Review. Nutrients 2023, 15, 5096. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Das, S.R.; Hilliard, M.E.; Isaacs, D.; et al. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46 (Suppl. S1), S158–S190. [Google Scholar] [CrossRef] [PubMed]
- Izar, M.C.O.; Lottenberg, A.M.; Giraldez, V.Z.R.; Santos Filho, R.D.D.; Machado, R.M.; Bertolami, A.; Assad, M.H.V.; Saraiva, J.F.K.; Faludi, A.A.; Moreira, A.S.B.; et al. Position Statement on Fat Consumption and Cardiovascular Health—2021. Arq. Bras. Cardiol. 2021, 116, 160–212. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/AphA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A eporto of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, 1269–1324. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 1. Improving Care and Promoting Health in Populations: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46 (Suppl. S1), S10–S18. [Google Scholar] [CrossRef]
- Koebnick, C.; Imperatore, G.; Jensen, E.T.; Stafford, J.M.; Shah, A.S.; Mottl, A.K.; Bell, R.A.; Dabelea, D.; Liese, A.D.; Marcovina, S.M.; et al. Progression to hypertension in youth and epor adults with type 1 or type 2 diabetes: The SEARCH for Diabetes in Youth Study. J. Clin. Hypertens. 2020, 22, S41–S48, Diabetes Care 2023, 46, 1716–1717. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. American Diabetes Association. Addendum. 3. Prevention or Delay of Type 2 Diabetes and Associated Comorbidities: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46 (Suppl. S1), 1716–1717. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 5. Facilitating Positive Health Behaviors and Well-being to Improve Health Outcomes: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46 (Suppl. S1), S68–S96. [Google Scholar] [CrossRef] [PubMed]
- Belalcazar, L.M.; Ballantyne, C.M. Looking Back at Look AHEAD Through the Lens of Recent Diabetes Outcome Trials. Circulation 2017, 135, 720–723. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.E.; Huggins, G.S.; McCaffery, J.M.; Lichtenstein, A.H. Comparison among criteria to define successful weight-loss maintainers and regainers in the Action for Health in Diabetes (Look AHEAD) and Diabetes Prevention Program trials. Am. J. Clin. Nutr. 2017, 106, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Kökten, T.; Hansmannel, F.; Ndiaye, N.C.; Heba, A.C.; Quilliot, D.; Dreumont, N.; Arnone, D.; Peyrin-Biroulet, L. Calorie Restriction as a New Treatment of Inflammatory Diseases. Adv. Nutr. 2021, 12, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Wing, R.R.; Bolin, P.; Brancati, F.L.; Bray, G.A.; Clark, J.M.; Coday, M.; Crow, R.S.; Curtis, J.M.; Egan, C.M.; Espeland, M.A.; et al. Look AHEAD Research Group; Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N. Engl. J. Med. 2013, 369, 145–154. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Li, Y.; Luo, B.; Lin, Z.; Chen, K.; Liu, Y. Dietary patterns and cardiometabolic health: Clinical evidence and mechanism. Med. Comm. 2023, 4, e212. [Google Scholar] [CrossRef]
- Ramos, S.; Campos, L.F.; Strufaldi, D.R.B.M.; Gomes, D.L.; Guimarães, D.B.; Souto, D.L.; Marques, M.; Sousa, S.S.S.; Campos, T.F. Official Guideline of the Brazilian Society of Diabetes SBD; Brazilian Society of Diabetes: São Paulo, SP, Brazil, 2023; ISBN 978-85-5722-906-8. [Google Scholar] [CrossRef]
- Brazilian Diabetes Society. Carbohydrate Counting Manual; Department of Nutrition of the Brazilian Diabetes Society (SBD): Rio De Janeiro, Brazil, 2021; Available online: https://diabetes.org.br/wp-content/uploads/2021/05/manual-de-contagem-de-carbo.pdf (accessed on 20 September 2023).
- Monteiro, C.A. Food Guide for the Brazilian Population. 2014. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/guia_alimentar_para_a_pop_brasiliera_miolo_internet.pdf (accessed on 20 October 2023).
- Ivers, N.M.; Jiang, M.; Alloo, J.; Singer, A.; Ngui, D.; Casey, C.G.; Yu, C.H. Diabetes Canadian 2018 clinical practice guidelines: Key messages for family physicians caring for patients living with type 2 diabetes. Can. Fam. Physician 2019, 65, 14–24. [Google Scholar]
- Ramesh, G.; Wood, A.C.; Allison, M.A.; Rich, S.S.; Jensen, E.T.; Chen, Y.I.; Rotter, J.I.; Bertoni, A.G.; Goodarzi, M.O. Associations between adherence to the dietary approaches to stop hypertension (DASH) diet and six glucose homeostasis traits in the Microbiome and Insulin Longitudinal Evaluation Study (MILES). Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1418–1426. [Google Scholar] [CrossRef]
- Pavlidou, E.; Papadopoulou, S.K.; Mentzelou, M.; Dakanalis, A.; Vorvolakos, T.; Antasouras, G.; Spanoudaki, M.; Pandi, A.L.; Serdari, A.; Chrysafi, M.; et al. Association of Mediterranean Diet Adherence with Sociodemographic, Anthropometric, and Lifestyle Factors during the COVID-19 Pandemic: A Cross-Sectional Study in Greece. Nutrients 2023, 15, 4123. [Google Scholar] [CrossRef] [PubMed]
- Andreo-López, M.C.; Contreras-Bolívar, V.; Muñoz-Torres, M.; García-Fontana, B.; García-Fontana, C. Influence of the Mediterranean Diet on Healthy Aging. Int. J. Mol. Sci. 2023, 24, 4491. [Google Scholar] [CrossRef] [PubMed]
- Itsiopoulos, C.; Brazionis, L.; Kaimakamis, M.; Cameron, M.; Best, J.D.; O’Dea, K.; Rowley, K. Can the Mediterranean diet lower HbA1c in type 2 diabetes? Results from a randomized cross-over study. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 740–747. [Google Scholar] [CrossRef]
- Elhayany, A.; Lustman, A.; Abel, R.; Attal-Singer, J.; Vinker, S. A low carbohydrate Mediterranean diet improves cardiovascular risk factors and diabetes control among overweight patients with type 2 diabetes mellitus: A 1-year prospective randomized intervention study. Diabetes Obes. Metab. 2010, 12, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61 (Suppl. S6), 1402S–1406S. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Mora, J.J.; García-Vigara, A.; Sánchez-Sánchez, M.L.; García-Pérez, M.Á.; Tarín, J.; Cano, A. The Mediterranean diet: A historical perspective on food for health. Maturitas 2020, 132, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Liu, W.J.; Lee, C.L. Associations of Adherence to the DASH Diet and the Mediterranean Diet With All-Cause Mortality in Subjects With Various Glucose Regulation States. Front. Nutr. 2022, 9, 828792. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Bulló, M.; Estruch, R.; Ros, E.; Covas, M.I.; Ibarrola-Jurado, N.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; et al. Prevention of diabetes with Mediterranean diets: A subgroup analysis of a randomized trial. Ann. Intern. Med. 2014, 160, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for The Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R., 3rd; Simons-Morton, D.G.; et al. DASH-Sodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef]
- Azadbakht, L.; Fard, N.R.; Karimi, M.; Baghaei, M.H.; Surkan, P.J.; Rahimi, M.; Esmaillzadeh, A.; Willett, W.C. Effects of the Dietary Approaches to Stop Hypertension (DASH) eating plan on cardiovascular risks among type 2 diabetic patients: A randomized crossover clinical trial. Diabetes Care 2011, 34, 55–57. [Google Scholar] [CrossRef]
- Drehmer, M.; Odegaard, A.O.; Schmidt, M.I.; Duncan, B.B.; Cardoso, L.O.; Matos, S.M.A.; Molina, M.D.C.B.; Barreto, S.M.; Pereira, M.A. Brazilian dietary patterns and the dietary approaches to stop hypertension (DASH) diet-relationship with metabolic syndrome and newly diagnosed diabetes in the ELSA-Brasil study. Diabetol. Metab. Syndr. 2017, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef]
- Gregg, E.W.; Jakicic, J.M.; Blackburn, G.; Bloomquist, P.; Bray, G.A.; Clark, J.M.; Curtis, J.M.; Egan, C.; Evans, M.; Foreyt, J.; et al. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: A post-hoc analysis of the Look AHEAD clinical trial. Lancet Diabetes Endocrinol. 2016, 4, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R. Management of hyperglycemia in type 2 diabetes, 2015: A patient-centered approach: Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015, 38, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Powers, M.A.; Bardsley, J.; Cypress, M.; Duker, P.; Funnell, M.M.; Fischl, A.H.; Fischl, A.H.; Maryniuk, M.D.; Siminerio, L.; Vivian, E. Diabetes Self-Management Education and Support in Type 2 Diabetes: A Joint Position Statement of the American Diabetes Association, the American Association of Diabetes Educators, and the Academy of Nutrition and Dietetics. J. Acad. Nutr. Diet. 2015, 115, 1323–1334. [Google Scholar] [CrossRef]
- SBD—Brazilian Diabetes Society. Brazilian Diabetes Society Guidelines 2019–2020. Clannad, 2019; 419p. Available online: https://www.saude.ba.gov.br/wp-content/uploads/2020/02/Diretrizes-Sociedade-Brasileira-de-Diabetes-2019-2020.pdf (accessed on 20 September 2023).
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef]
- Shukla, A.P.; Dickison, M.; Coughlin, N.; Karan, A.; Mauer, E.; Truong, W.; Casper, A.; Emiliano, A.B.; Kumar, R.B.; Saunders, K.H.; et al. The impact of food order on postprandial glycaemic excursions in prediabetes. Diabetes Obes. Metab. 2019, 21, 377–381. [Google Scholar] [CrossRef]
- Shukla, A.P.; Andono, J.; Touhamy, S.H.; Casper, A.; Iliescu, R.G.; Mauer, E.; Mauer, E.; Shan Zhu, Y.; Ludwig, D.S.; Aronne, L.J. Carbohydrate-last meal pattern lowers postprandial glucose and insulin excursions in type 2 diabetes. BMJ Open Diabetes Res. Care 2017, 5, e000440. [Google Scholar] [CrossRef] [PubMed]
- Mita, T.; Osonoi, Y.; Osonoi, T.; Saito, M.; Nakayama, S.; Someya, Y.; Ishida, H.; Gosho, M.; Watada, H. Breakfast skipping is associated with persistently increased arterial stiffness in patients with type 2 diabetes. BMJ Open Diabetes Res. Care 2020, 8, e001162. [Google Scholar] [CrossRef] [PubMed]
- Mason, I.C.; Qian, J.; Adler, G.K.; Scheer, F.A.J.L. Impact of circadian disruption on glucose metabolism: Implications for type 2 diabetes. Diabetologia 2020, 63, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Borror, A.; Zieff, G.; Battaglini, C.; Stoner, L. The Effects of Postprandial Exercise on Glucose Control in Individuals with Type 2 Diabetes: A Systematic Review. Sports. Med. 2018, 48, 1479–1491. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hu, Y.; Yan, R.; Li, H.; Zhang, D.; Li, F.; Su, X.; Ma, J. Twenty Minute Moderate-Intensity Post-Dinner Exercise Reduces the Postprandial Glucose Response in Chinese Patients with Type 2 Diabetes. Med. Sci. Monit. 2018, 24, 7170–7177. [Google Scholar] [CrossRef] [PubMed]
- Mason, A.E.; Saslow, L.; Moran, P.J.; Kim, S.; Wali, P.K.; Abousleiman, H.; Hartman, A.; Richler, R.; Schleicher, S.; Hartogensis, W.; et al. Examining the Effects of Mindful Eating Training on Adherence to a Carbohydrate-Restricted Diet in Patients With Type 2 Diabetes (the DELISH Study): Protocol for a Randomized Controlled Trial. JMIR Res. Protoc. 2019, 8, e11002. [Google Scholar] [CrossRef] [PubMed]
- Minari, T.P.; Araújo-Filho, G.M.d.; Tácito, L.H.B.; Yugar, L.B.T.; Rubio, T.d.A.; Pires, A.C.; Vilela-Martin, J.F.; Cosenso-Martin, L.N.; Fattori, A.; Yugar-Toledo, J.C.; et al. Effects of Mindful Eating in Patients with Obesity and Binge Eating Disorder. Nutrients 2024, 16, 884. [Google Scholar] [CrossRef]
- Kumar, A.; Alam, S.; Bano, S.; Prakash, R.; Jain, V. Association of eating disorders with glycaemic control and insulin resistance in patients of type 2 diabetes mellitus. Int. J. Biochem. Mol. Biol. 2023, 14, 40–50. [Google Scholar] [PubMed]
- Wang, D.D.; Toledo, E.; Hruby, A.; Rosner, B.A.; Willett, W.C.; Sun, Q.; Razquin, C.; Zheng, Y.; Ruiz-Canela, M.; Guasch-Ferré, M.; et al. Plasma Ceramides, Mediterranean Diet, and Incident Cardiovascular Disease in the PREDIMED Trial (Prevención con Dieta Mediterránea). Circulation 2017, 135, 2028–2040. [Google Scholar] [CrossRef]
- Valls-Pedret, C.; Sala-Vila, A.; Serra-Mir, M.; Corella, D.; de la Torre, R.; Martínez-González, M.Á.; Martínez-Lapiscina, E.H.; Fitó, M.; Pérez-Heras, A.; Salas-Salvadó, J.; et al. Mediterranean Diet and Age-Related Cognitive Decline: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1094–1103. [Google Scholar] [CrossRef]
- van den Brink, A.C.; Brouwer-Brolsma, E.M.; Berendsen, A.A.M.; van de Rest, O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diets Are Associated with Less Cognitive Decline and a Lower Risk of Alzheimer’s Disease-A Review. Adv. Nutr. 2019, 10, 1040–1065. [Google Scholar] [CrossRef] [PubMed]
- Mirabelli, M.; Chiefari, E.; Arcidiacono, B.; Corigliano, D.M.; Brunetti, F.S.; Maggisano, V.; Russo, D.; Foti, D.P.; Brunetti, A. Mediterranean Diet Nutrients to Turn the Tide against Insulin Resistance and Related Diseases. Nutrients 2020, 12, 1066. [Google Scholar] [CrossRef] [PubMed]
- Appel, L.J.; Champagne, C.M.; Harsha, D.W.; Cooper, L.S.; Obarzanek, E.; Elmer, P.J.; Stevens, V.J.; Vollmer, W.M.; Lin, P.H.; Svetkey, L.P.; et al. Writing Group of the PREMIER Collaborative Research Group. Effects of comprehensive lifestyle modification on blood pressure control: Main results of the PREMIER clinical trial. JAMA 2003, 289, 2083–2093. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.K.; Lewis, C.E.; Varady, K.A.; Su, Y.R.; Madhur, M.S.; Lackland, D.T.; Reis, J.P.; Wang, T.J.; Lloyd-Jones, D.M.; Allen, N.B. Effect of Dietary Sodium on Blood Pressure: A Crossover Trial. JAMA 2023, 330, 2258–2266. [Google Scholar] [CrossRef] [PubMed]
- Filippou, C.D.; Tsioufis, C.P.; Thomopoulos, C.G.; Mihas, C.C.; Dimitriadis, K.S.; Sotiropoulou, L.I.; Chrysochoou, C.A.; Nihoyannopoulos, P.I.; Tousoulis, D.M. Dietary Approaches to Stop Hypertension (DASH) Diet and Blood Pressure Reduction in Adults with and without Hypertension: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2020, 11, 1150–1160. [Google Scholar] [CrossRef]
- Lima, L.S.; Araujo, M.A.; Ornelas, G.C.; Logrado, M.H. Validation of a Nutritional Screening Tool. Acta Med. Port 2012, 25, 10–14. [Google Scholar] [PubMed]
- Durco, E.S. Treatment Protocol for Young Adult Patient with Type 2 Diabetes Mellitus; Federal University of Minas Gerais, Faculty of Medicine, Collective Health Education Center: Belo Horizonte, Brazil, 2009; p. 82f. Available online: https://repositorio.ufmg.br/bitstream/1843/BUBD9CDFDR/1/monografia_ednaldo_silva_durco.pdf (accessed on 20 August 2020)Monograph (Specialization in Basic Family Health Care).
- Junqueira, S.A.E. Socio-Demographic and Clinical Profile of Psychiatric Patients Treated in Day Hospital. Ph.D. Thesis, University of São Paulo, Ribeirão Preto, Brazil, 2009. Available online: http://www.teses.usp.br/teses/disponiveis/17/17148/tde-29062009-121226/pt-br.php (accessed on 20 August 2020).
- Mifflin, M.D.; St Jeor, S.T.; Hill, L.A.; Scott, B.J.; Daugherty, J.Á.; Koh, Y.O. A new predictive equation for resting energy expenditure in healthy individuals. Am. J. Clin. Nutr. 1990, 51, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Frankenfield, D.C.; Rowe, W.A.; Smith, J.S.; Cooney, R.N. Validation of several established equations for resting metabolic rate in obese and nonobese people. J. Am. Diet. Assoc. 2003, 103, 1152–1159. [Google Scholar] [CrossRef]
- Frankenfield, D.; Roth-Yousey, L.; Compher, C. Comparison of predictive equations for resting metabolic rate in healthy nonobese and obese adults: A systematic review. J. Am. Diet. Assoc. 2005, 105, 775–789. [Google Scholar] [CrossRef]
- Fisberg, R.M.; Slater Villar, B.; Marchioni, D.M.L.; Martini, L.A. Inquéritos Alimentares: Métodos e Bases Científicos; USP Repositories: Barueri, SP, Brazil, 2005; 334p, ISBN 85-204-1638-1. [Google Scholar]
- Stewart, A.; Marfell-Jones, M.; Olds, T.; Ridder, H. International Standards for Anthropometric Assessment; Hutt, L., Ed.; International Society for the Advancement of Kinanthropometry—ISAK: Lower Hutt, New Zealand, 2011. [Google Scholar]
- Barroso, W.K.S.; Rodrigues, C.I.S.; Bortolotto, L.A.; Mota-Gomes, M.A.; Brandão, A.A.; Feitosa, A.D.M.; Machado, C.A.; Poli-de-Figueiredo, C.E.; Amodeo, C.; Júnior, D.M.; et al. Brazilian Guidelines for Arterial Hypertension—2020. Arq. Bras. Cardiol. 2021, 116, 516–658. Available online: http://departamentos.cardiol.br/sbc-dha/profissional/pdf/Diretriz-HAS-2020.pdf (accessed on 20 January 2022). [CrossRef]
- Colberg, S.R.; Sigal, R.J.; Fernhall, B.; Regensteiner, J.G.; Blissmer, B.J.; Rubin, R.R.; Chasan-Taber, L.; Albright, A.L.; Braun, B. Exercise and type 2 diabetes: The American College of Sports Medicine and the American Diabetes Association: Joint position statement. Diabetes Care 2010, 33, e147–e167. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metada-ta-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; Duda, S.N. REDCap Consortium. The REDCap consortium: Building an international of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Graphpad Prism for Windows, Version 9.0; GraphPad Software: La Jolla, CA, USA, 2015; Available online: www.graphpad.com (accessed on 16 January 2024).
- Nilson, E.A.F.; Ferrari, G.; Louzada, M.L.C.; Levy, R.B.; Monteiro, C.A.; Rezende, L.F.M. Premature Deaths Attributable to the Consumption of Ultraprocessed Foods in Brazil. Am. J. Prev. Med. 2023, 64, 129–136. [Google Scholar] [CrossRef] [PubMed]
- IBGE. Family Budget Survey 2017–2018: Analysis of Personal Food Consumption in Brazil; Nutritional evaluation of the availability of household food in Brazil; IBGE: Rio de Janeiro, Brazil, 2020. Available online: https://biblioteca.ibge.gov.br/visualizacao/livros/liv101742.pdf (accessed on 20 November 2022).
- Gardner, C.D.; Landry, M.J.; Perelman, D.; Petlura, C.; Durand, L.R.; Aronica, L.; Crimarco, A.; Cunanan, K.M.; Chang, A.; Dant, C.C.; et al. Effect of a ketogenic diet versus Mediterranean diet on glycated in individuals with prediabetes and type 2 diabetes mellitus: The interventional Keto-Med randomized crossover trial. Am. J. Clin. Nutr. 2022, 116, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Theodoridis, X.; Chourdakis, M.; Chrysoula, L.; Chroni, V.; Tirodimos, I.; Dipla, K.; kaliagkousi, E.; Triantafyllou, A. Adherence to the DASH Diet and Risk of Hypertension: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 3261. [Google Scholar] [CrossRef]
- Chan, Q.; Wren, G.M.; Lau, C.E.; Ebbels, T.M.D.; Gibson, R.; Loo, R.L.; Aljuraiban, G.S.; Posma, J.M.; Dyer, A.R.; Steffen, L.M.; et al. Blood pressure interactions with the DASH dietary pattern, sodium, and potassium: The International Study of Macro-/Micronutrients and Blood Pressure (INTERMAP). Am. J. Clin. Nutr. 2022, 116, 216–229. [Google Scholar] [CrossRef]
- Gardner, C.D.; Trepanowski, J.F.; Del Gobbo, L.C.; Hauser, M.E.; Rigdon, J.; Ioannidis, J.P.A.; Desai, M.; King, A.C. Effect of Low-Fat vs Low-Carbohydrate Diet on 12-Month Weight Loss in Overweight Adults and the Association With Genotype Insulin Secretion: The DIETFITS Randomized Clinical Trial. JAMA 2018, 319, 667–679. [Google Scholar] [CrossRef]
- Hamdy, O.; Mottalib, A.; Morsi, A.; El-Sayed, N.; Goebel-Fabbri, A.; Arathuzik, G.; Shahar, J.; Kirpitch, A.; Zrebiec, J. Long-term effect of intensive lifestyle intervention on cardiovascular risk factors in patients with diabetes in real-world clinical practice: A 5-year longitudinal study. BMJ Open Diabetes Res. Care 2017, 5, e000259. [Google Scholar] [CrossRef]
- Tomah, S.; Zhang, H.; Al-Badri, M.; Salah, T.; Dhaver, S.; Khater, A.; Asabehji, M.W.; Hamdy, O. Long-term effect of intensive lifestyle intervention on cardiometabolic risk factors and microvascular complications in patients with diabetes in real-world clinical practice: A 10-year longitudinal study. BMJ Open Diabetes Res. Care 2023, 11, e003179. [Google Scholar] [CrossRef]
- Ruiz-Roso, M.B.; Knott-Torcal, C.; Matilla-Escalante, D.C.; Garcimartín, A.; Sampedro-Nuñez, M.A.; Dávalos, A.; Marazuela, M. COVID-19 Lockdown and Changes of the Dietary Pattern and Physical Activity Habits in a Cohort of Patients with Type 2 Diabetes Mellitus. Nutrients 2020, 12, 2327. [Google Scholar] [CrossRef]
- Young, I.E.; Poobalan, A.; Steinbeck, K.; O’Connor, H.T.; Parker, H.M. Distribution of energy intake across the day and weight loss: A systematic review and meta-analysis. Obes. Rev. 2023, 24, e13537. [Google Scholar] [CrossRef] [PubMed]
- Sankar, P.; Ahmed, W.N.; Mariam Koshy, V.; Jacob, R.; Sasidharan, S. Effects of COVID-19 lockdown on type 2 diabetes, lifestyle and psychosocial health: A hospital-based cross-sectional survey from South India. Diabetes Metab. Syndr. 2020, 14, 1815–1819. [Google Scholar] [CrossRef]
- Grabia, M.; Markiewicz-Żukowska, R.; Puścion-Jakubik, A.; Bielecka, J.; Nowakowski, P.; Gromkowska-Kępka, K.; Mielcarek, K.; Socha, K. The Nutritional and Health Effects of the COVID-19 Pandemic on Patients with Diabetes Mellitus. Nutrients 2020, 12, 3013. [Google Scholar] [CrossRef]
- Wrona, M.; Skrypnik, D. New-Onset Diabetes Mellitus, Hypertension, Dyslipidaemia as Sequelae of COVID-19 Infection-Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 13280. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S. Post-COVID dysautonomias: What we know and (mainly) what we don’t know. Nat. Rev. Neurol. 2024, 20, 99–113. [Google Scholar] [CrossRef]
- Van Baak, M.A.; Mariman, E.C.M. Mechanisms of weight regain after weight loss—The role of adipose tissue. Nat. Rev. Endocrinol. 2019, 15, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Busetto, L.; Bettini, S.; Makaronidis, J.; Roberts, C.A.; Halford, J.C.G.; Batterham, R.L. Mechanisms of weight regain. Eur. J. Intern. Med. 2021, 93, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Godoy, M.F. Chaos Theory Applied to Medicine. Ph.D. Thesis, [Free Teaching in Cardiology]—Faculty of Medicine of São José do Rio Preto, São José do Rio Preto, SP, Brazil, 2003; 179p. Available online: http://www.mfgodoy.med.br/caos.pdf (accessed on 20 October 2023).
- Guasch-Ferré, M.; Becerra-Tomás, N.; Ruiz-Canela, M.; Corella, D.; Schröder, H.; Estruch, R.; Ros, E.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. Total and subtypes of dietary fat intake and risk of type 2 diabetes mellitus in the Prevención já Dieta Mediterránea (PREDIMED) study. Am. J. Clin. Nutr. 2017, 105, 723–735. [Google Scholar] [CrossRef]
- Huo, R.; Du, T.; Xu, Y.; Xu, W.; Chen, X.; Sun, K.; Yu, X. Effects of Mediterranean-style diet on glycemic control, weight loss and cardiovascular risk factors among type 2 diabetes individuals: A meta-analysis. Eur. J. Clin. Nutr. 2015, 69, 1200–1208. [Google Scholar] [CrossRef]
- Sugimoto, T.; Arai, H.; Sakurai, T. Já update on cognitive frailty: Its definition, impact, associated factors and underlying mechanisms, and interventions. Geriatr. Gerontol. Int. 2022, 22, 99–109. [Google Scholar] [CrossRef] [PubMed]
- 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 updated AGS for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 2023, 71, 2052–2081. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, N.Y.; Krakauer, J.C. An Anthropometric Risk Index Based on Combining Height, Weight, Waist, and Hip Measurements. J. Obes. 2016, 2016, 8094275. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hewage, N.; Wijesekara, U.; Perera, R. Determining the best method for evaluating obesity and the risk for non-communicable diseases in women of childbearing age by measuring the body mass index, waist circumference, waist-to-hip ratio, waist-to-height ratio, A Body Shape Index, and hip index. Nutrition 2023, 114, 112135. [Google Scholar] [CrossRef] [PubMed]



| Meetings | Objectives | Procedures |
|---|---|---|
| 1st meeting: Initial Interview, evaluation, intervention, and data collection. |
|
|
| Quarterly meetings: Evaluation and data collection. |
| |
| Follow-up |
|
| Control | Intervention | p | |||
|---|---|---|---|---|---|
| Gender (number and percentage) | Male | Female | Male | Female | n/a |
| 20 (50.0%) | 20 (50.0%) | 12 (27.3%) | 32 (72.7%) | ||
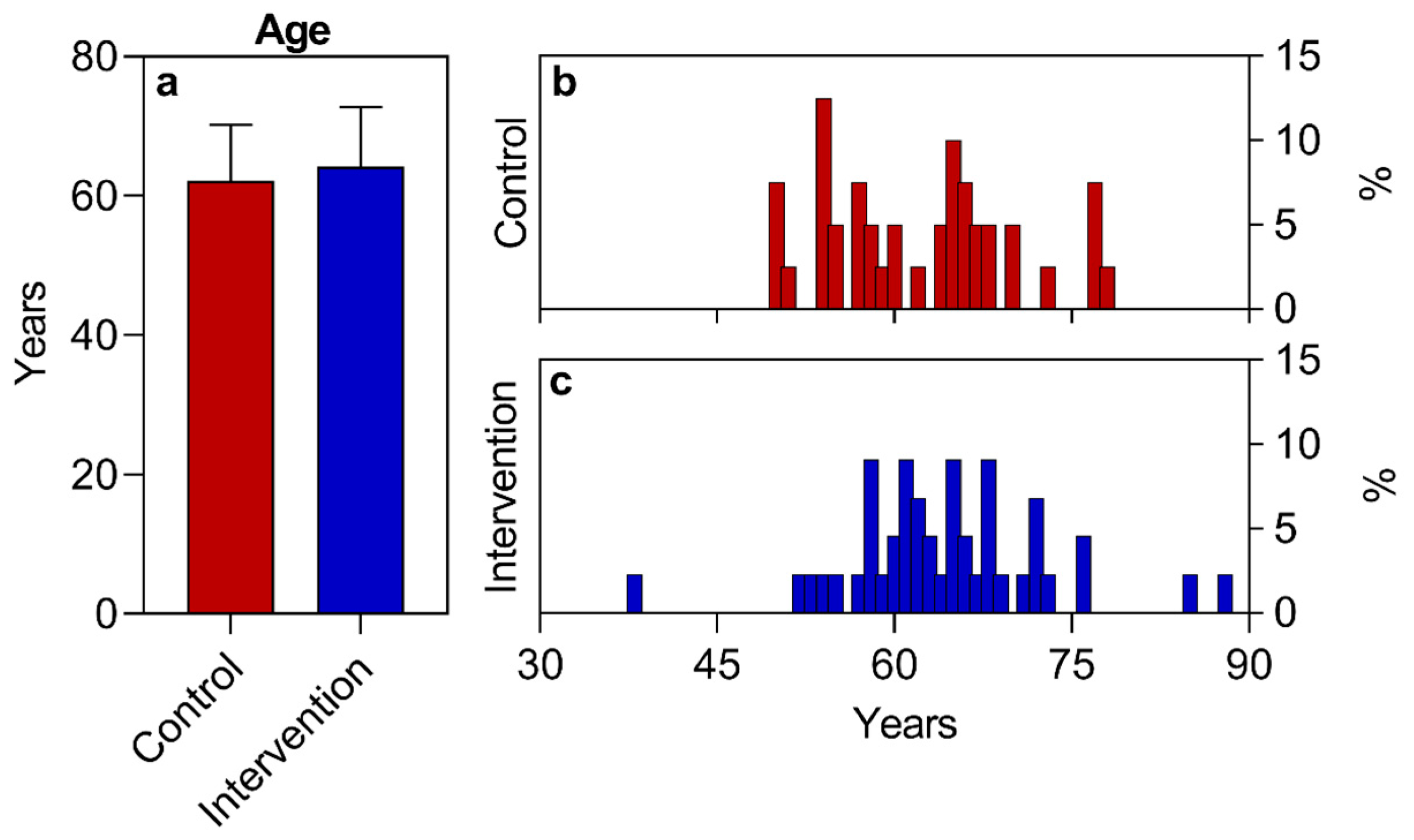
| Age (years) (mean ± SD) | 62.2 ± 8.0 | 64.2 ± 8.6 | 0.2661 | ||
| Race | White Brown Black 10 (25%) 14 (35%) 16 (40%) | White Brown Black 11 (25%) 15 (35%) 18(40%) | n/a | ||
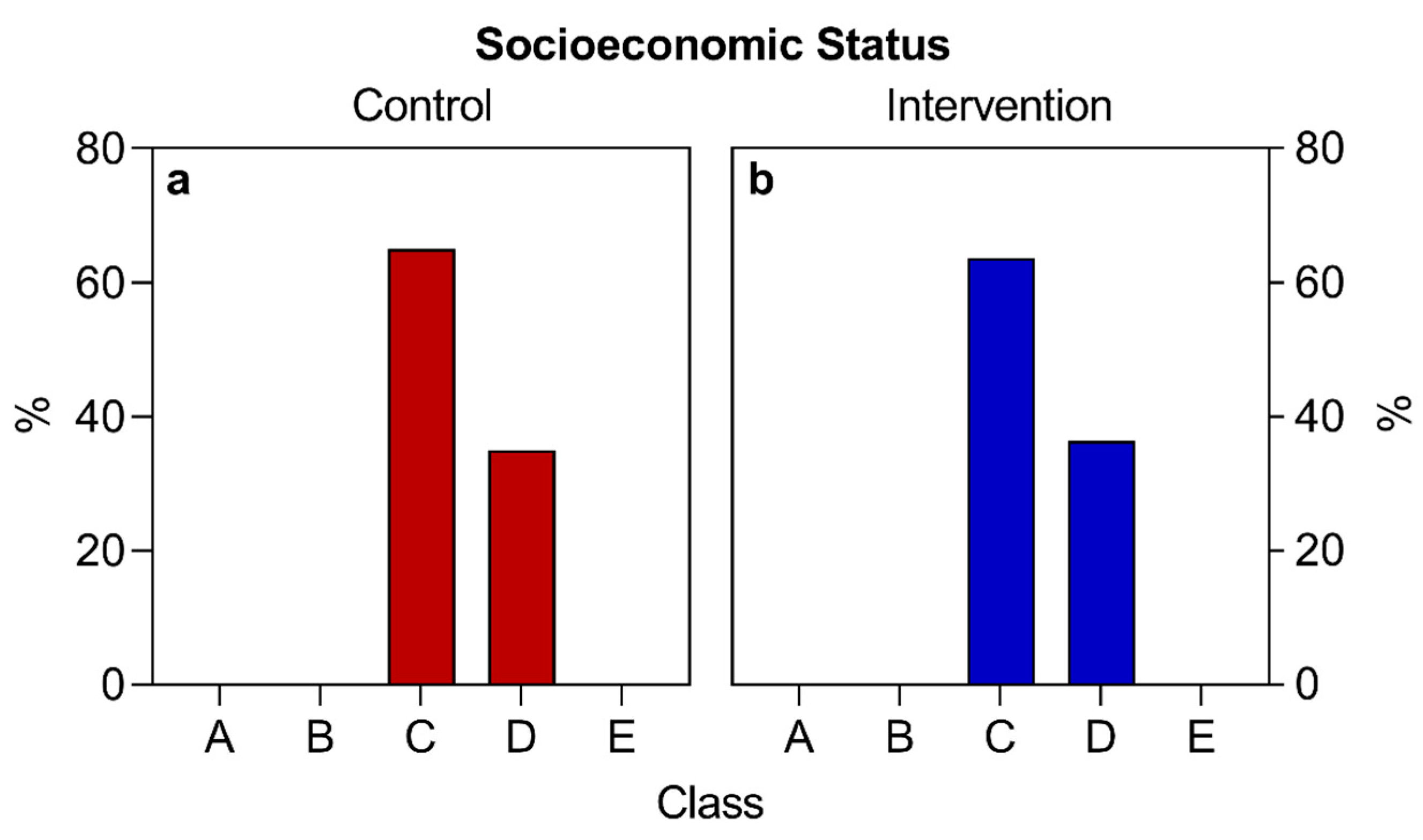
| Socioeconomic status (class) (number and percentage) | C | D | C | D | n/a |
| 26 (65.0%) | 14 (35.0%) | 28 (63.6%) | 16 (36.4%) | ||
| COVID-19 infection | No | Yes | No | Yes | n/a |
| 12 (30.0%) | 28 (70.0%) | 12 (27.3%) | 32 (72.7%) | ||
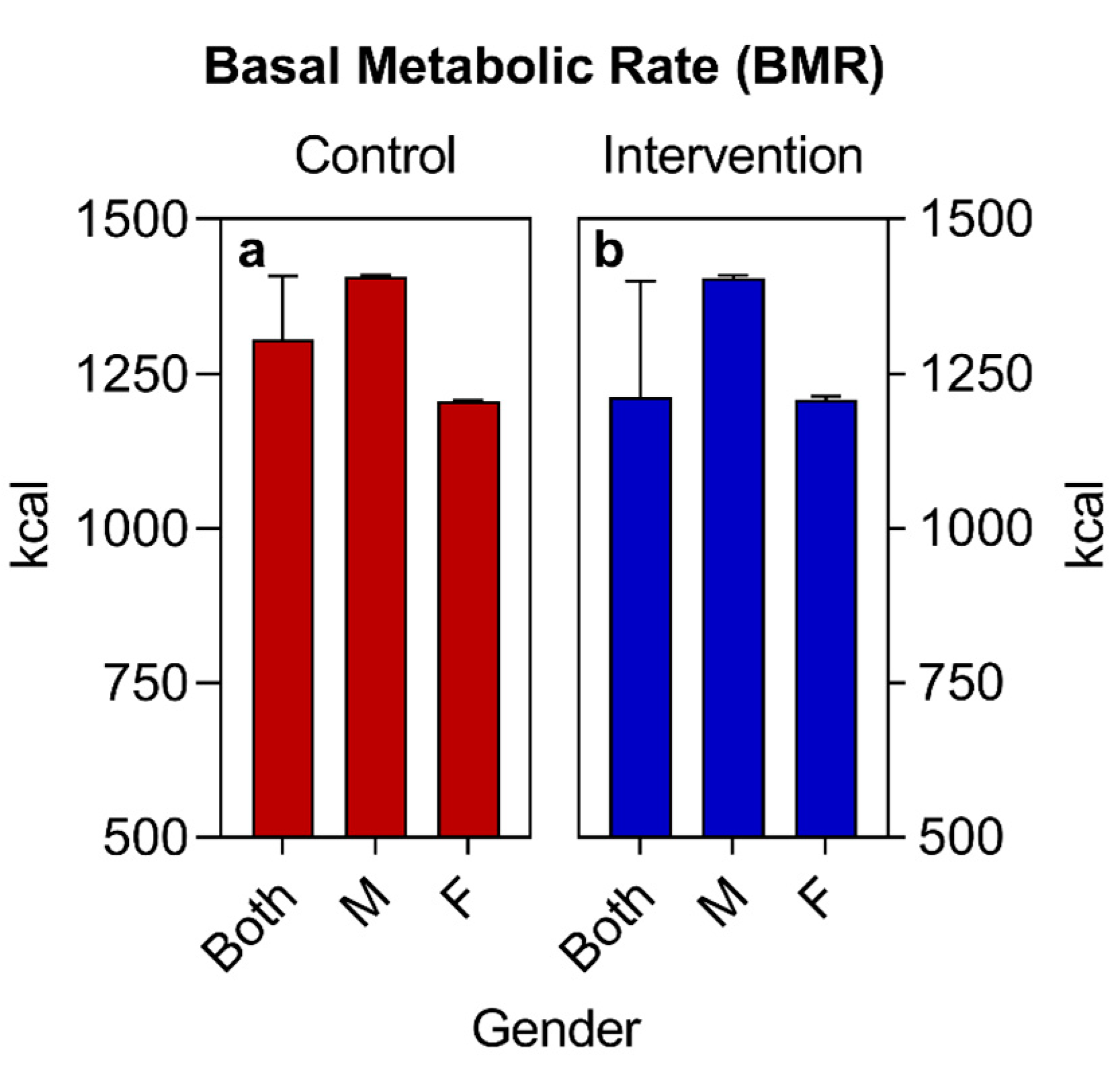
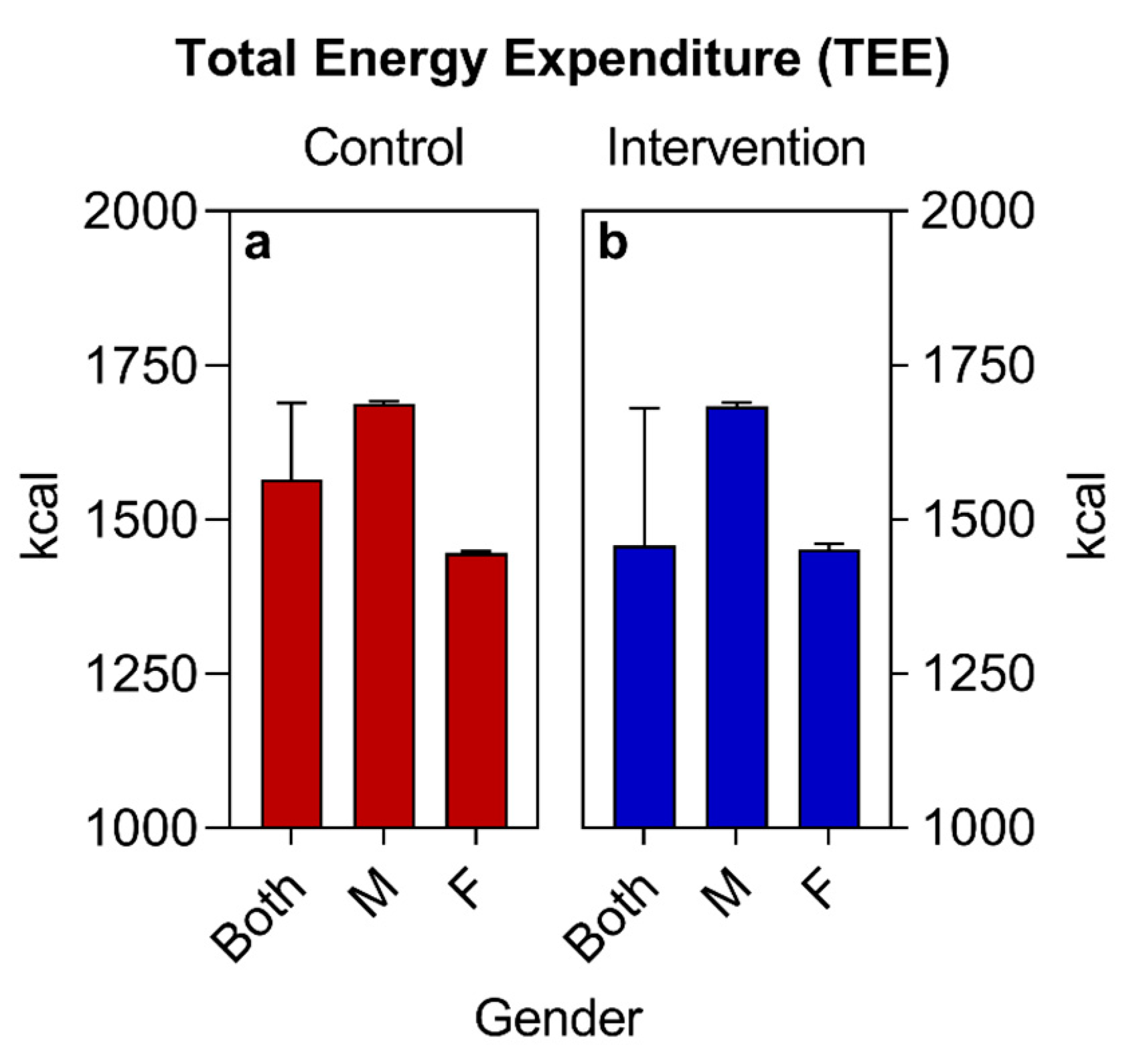
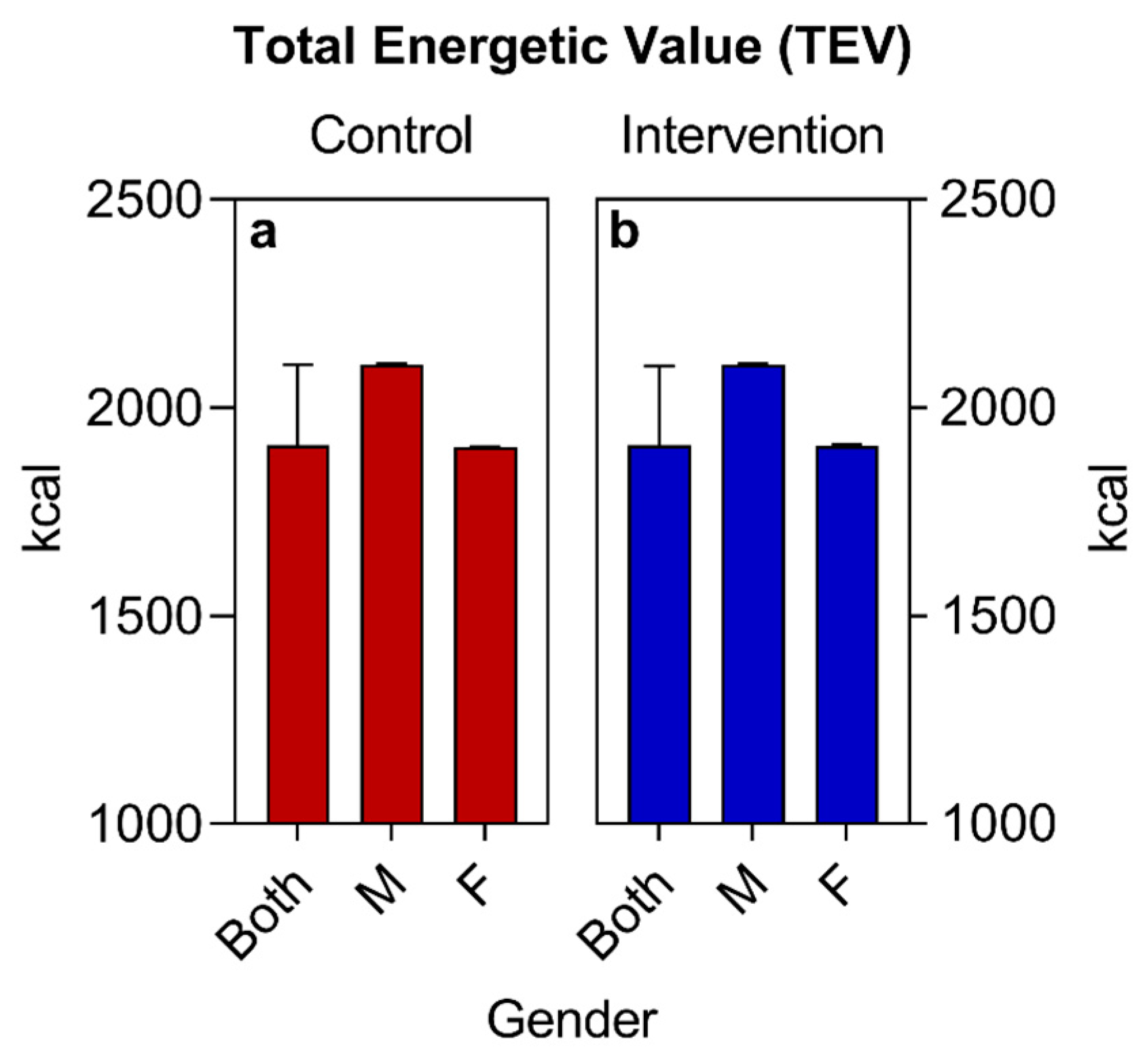
| BMR (kcal) (median ± IQR) | 1306 (1408–1204) | 1212 (1400–1204) | 0.4076 | ||
| TEE (kcal) (median ± IQR) | 1565 (1689–1446) | 1458 (1680–1448) | 0.5424 | ||
| TEV (kcal) (median ± IQR) | 1910 (2103–1904) | 1911 (2100–1902) | 0.2318 | ||
| Control | Intervention | p | |||||
|---|---|---|---|---|---|---|---|
| First Visit | First Visit | ||||||
| Exercise habits (number and percentage) | S 40 (100%) | L 0 (0%) | M/I 0 (0%) | S 44 (100%) | L 0 (0%) | M/I 0 (0%) | n/a |
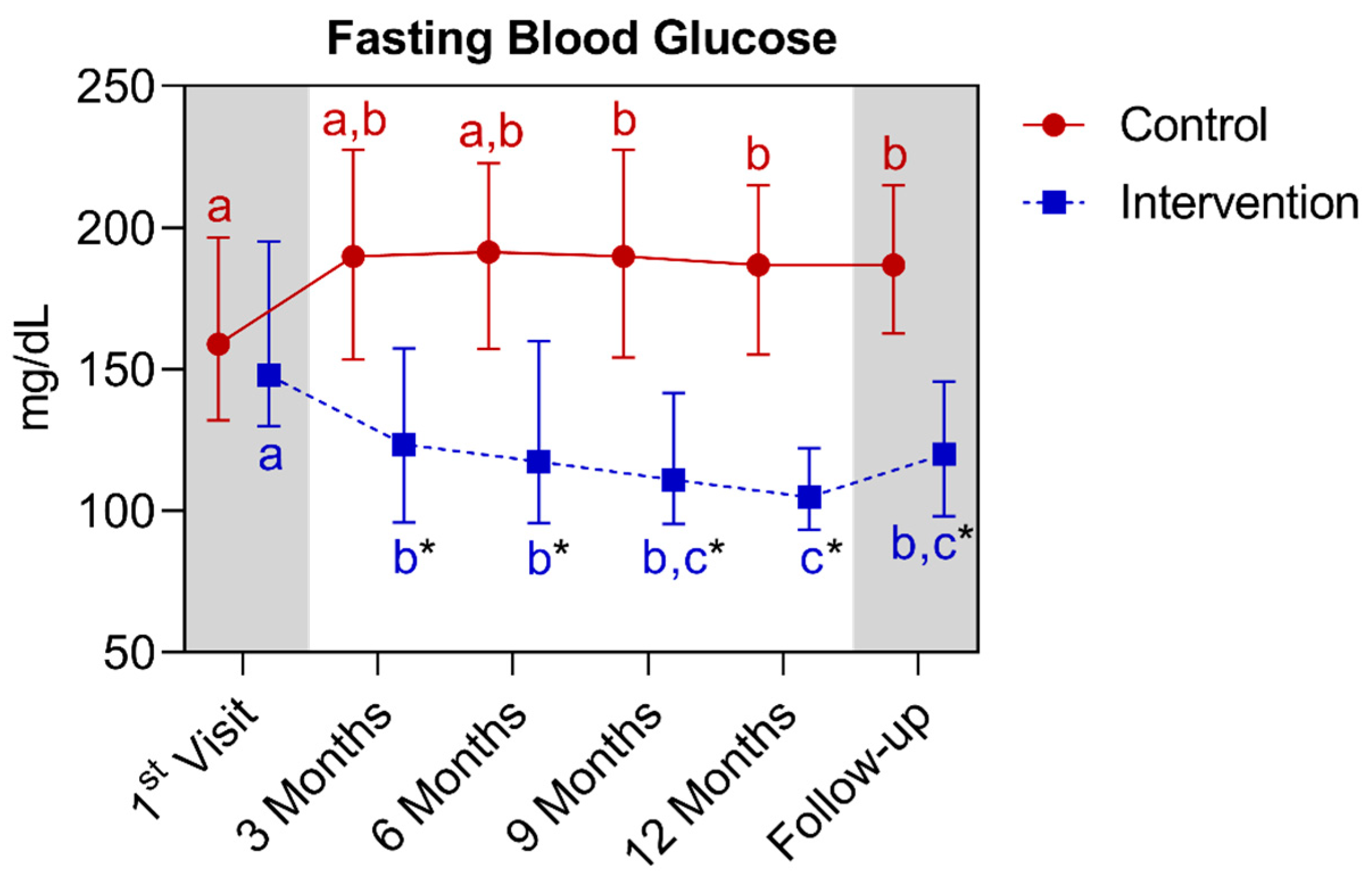
| Fasting blood glucose (mg/dL) (median ± IQR) | 159.0 (196.5–132.0) | 148.0 (195.3–130.0) | >0.9999 | ||||
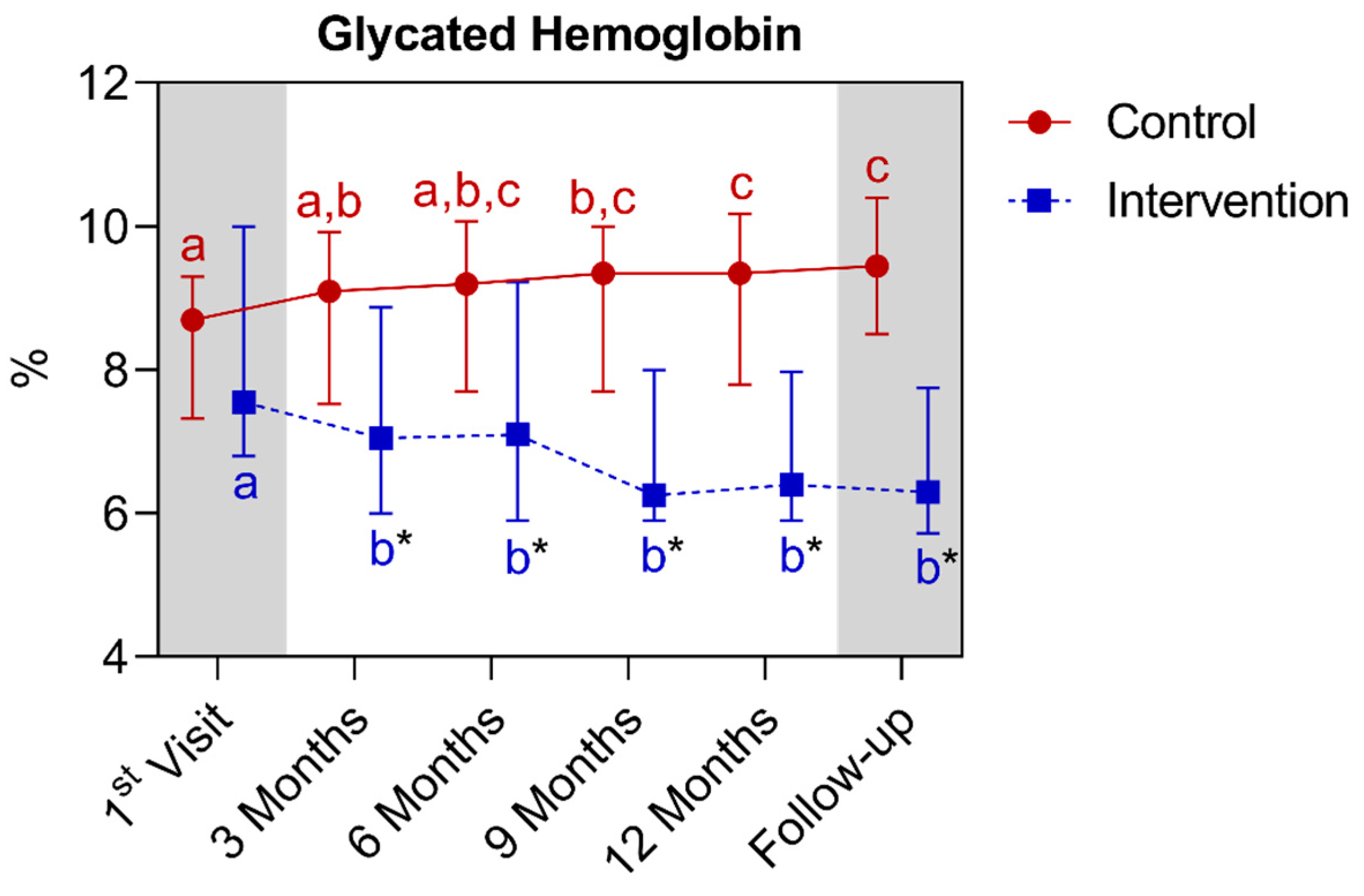
| Glycated hemoglobin (%) (median ± IQR) | 8.7 (9.3–7.3) | 7.5 (10.0–6.8) | >0.9999 | ||||
| Total cholesterol (mg/dL) (median ± IQR) | 171.5 (199.8–142.0) | 159.0 (211.5–129.0) | >0.9999 | ||||
| LDL cholesterol (mg/dL) (mean ± SD) | 102.4 ± 15.1 | 102.3 ± 19.7 | >0.9999 | ||||
| HDL cholesterol (mg/dL) (median ± IQR) | 43.0 (49.5–35.5) | 46.0 (53.0–40.0) | >0.9999 | ||||
| Serum triglycerides (mg/dL) (median ± IQR) | 165.5 (201.5–154.0) | 168.0 (195.0–151.8) | >0.9999 | ||||
| Body weight (kg) (median ± IQR) | 87.7 (98.0–71.5) | 87.0 (92.9–79.9) | >0.9999 | ||||
| BMI (kg/m2) (median ± IQR) | 30.0 (35.5–27.2) | 31.5 (35.7–28.5) | >0.9999 | ||||
| Waist circumference (cm) (mean ± SD) | 106.8 ± 12.0 | 106.7 ± 7.5 | >0.9999 | ||||
| Waist-to-hip ratio (unitless) (median ± IQR) | 1.0 (1.1–1.0) | 1.0 (1.1–0.9) | >0.9999 | ||||
| Systolic blood pressure (mmHg) (mean ± SD) | 145.3 ± 11.0 | 146.8 ± 17.1 | 0.9967 | ||||
| Diastolic blood pressure (mmHg) (median ± IQR) | 88.5 (98.0–87.0) | 95.7 (99.0–91.6) * | 0.0464 | ||||
| Heart rate (bpm) (median ± IQR) | 87.0 (89.9–78.9) | 89.5 (96.5–76.6) | >0.9999 | ||||
| Control | Intervention | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First Visit | Twelfth Month | p | First Visit | Twelfth Month | p | |||||||||
| Exercise habits (number and percentage) | S 40 (100%) | L 0 (0%) | M/I 0 (0%) | S 40 (100%) | L 0 (0%) | M/I 0 (0%) | n/a | S 44 (100%) | L 0 (0%) | M/I 0 (0%) | S 44 (100%) | L 0 (0%) | M/I 0 (0%) | n/a |
| Fasting blood glucose (mg/dL) (median ± IQR) | 159.0 (196.5–132.0) | 187.0 (215.9–155.3) * | 0.0028 | 148.0 (195.3–130.0) | 105.0 (122.3–93.5) * | <0.0001 | ||||||||
| Glycated hemoglobin (%) (median ± IQR) | 8.7 (9.3–7.3) | 9.3 (10.2–7.8) * | <0.0001 | 7.5 (10.0–6.8) | 6.4 (8.0–5.9) * | <0.0001 | ||||||||
| Total cholesterol (mg/dL) (median ± IQR) | 171.5 (199.8–142.0) | 179.5 (195.8–152.5) | 0.2326 | 159.0 (211.5–129.0) | 144.0 (170.0–120.8) | >0.9999 | ||||||||
| LDL cholesterol (mg/dL) (mean ± SD) | 102.4 ± 15.1 | 100.7 ± 16.8 | >0.9999 | 102.3 ± 19.7 | 76.0 ± 24.4 * | <0.0001 | ||||||||
| HDL cholesterol (mg/dL) (median ± IQR) | 43.0 (49.5–35.5) | 39.0 (43.0–34.0) | 0.0818 | 46.0 (53.0–40.0) | 49.0 (60.2–42.0) * | 0.0105 | ||||||||
| Serum triglycerides (mg/dL) (median ± IQR) | 165.5 (201.5–154.0) | 175.5 (205.8–140.0) | >0.9999 | 168.0 (195.0–151.8) | 110.0 (137.8–91.5) * | <0.0001 | ||||||||
| Body weight (kg) (median ± IQR) | 87.7 (98.0–71.5) | 92.5 (103.3–73.5) * | <0.0001 | 87.0 (92.9–79.9) | 78.0 (87.0–68.2) * | <0.0001 | ||||||||
| BMI (kg/m2) (median ± IQR) | 30.0 (35.5–27.2) | 32.0 (37.0–28.0) * | <0.0001 | 31.5 (35.7–28.5) | 29.0 (32.3–26.6) * | <0.0001 | ||||||||
| Waist circumference (cm) (mean ± SD) | 106.8 ± 12.0 | 110.6 ± 13.1 | 0.0987 | 106.7 ± 7.5 | 99.7 ± 8.8 * | <0.0001 | ||||||||
| Waist-to-hip ratio (unitless) (median ± IQR) | 1.0 (1.1–1.0) | 1.0 (1.2–1.0) * | 0.0003 | 1.0 (1.1–0.9) | 0.9 (0.9–0.8) * | <0.0001 | ||||||||
| Systolic blood pressure (mmHg) (mean ± SD) | 145.3 ± 11.0 | 147.6 ± 16.4 | 0.9998 | 146.8 ± 17.1 | 124.4 ± 13.0 * | <0.0001 | ||||||||
| Diastolic blood pressure (mmHg) (median ± IQR) | 88.5 (98.0–87.0) | 89.5 (95.7–86.1) | >0.9999 | 95.7 (99.0–91.6) | 76.2 (78.5–68.5) * | <0.0001 | ||||||||
| Heart rate (bpm) (median ± IQR) | 87.0 (89.9–78.9) | 87.2 (91.7–78.6) | >0.9999 | 89.5 (96.5–76.6) | 72.5 (78.9–65.9) * | 0.0129 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minari, T.P.; Manzano, C.F.; Tácito, L.H.B.; Yugar, L.B.T.; Sedenho-Prado, L.G.; Rubio, T.d.A.; Pires, A.C.; Vilela-Martin, J.F.; Cosenso-Martin, L.N.; Moreno, H.; et al. The Impact of a Nutritional Intervention on Glycemic Control and Cardiovascular Risk Markers in Type 2 Diabetes. Nutrients 2024, 16, 1378. https://doi.org/10.3390/nu16091378
Minari TP, Manzano CF, Tácito LHB, Yugar LBT, Sedenho-Prado LG, Rubio TdA, Pires AC, Vilela-Martin JF, Cosenso-Martin LN, Moreno H, et al. The Impact of a Nutritional Intervention on Glycemic Control and Cardiovascular Risk Markers in Type 2 Diabetes. Nutrients. 2024; 16(9):1378. https://doi.org/10.3390/nu16091378
Chicago/Turabian StyleMinari, Tatiana Palotta, Carolina Freitas Manzano, Lúcia Helena Bonalume Tácito, Louise Buonalumi Tácito Yugar, Luis Gustavo Sedenho-Prado, Tatiane de Azevedo Rubio, Antônio Carlos Pires, José Fernando Vilela-Martin, Luciana Neves Cosenso-Martin, Heitor Moreno, and et al. 2024. "The Impact of a Nutritional Intervention on Glycemic Control and Cardiovascular Risk Markers in Type 2 Diabetes" Nutrients 16, no. 9: 1378. https://doi.org/10.3390/nu16091378
APA StyleMinari, T. P., Manzano, C. F., Tácito, L. H. B., Yugar, L. B. T., Sedenho-Prado, L. G., Rubio, T. d. A., Pires, A. C., Vilela-Martin, J. F., Cosenso-Martin, L. N., Moreno, H., & Yugar-Toledo, J. C. (2024). The Impact of a Nutritional Intervention on Glycemic Control and Cardiovascular Risk Markers in Type 2 Diabetes. Nutrients, 16(9), 1378. https://doi.org/10.3390/nu16091378






