Long-Term Improvement in Cardiorespiratory Fitness Ameliorates Insulin Sensitivity beyond Changes in Visceral/Ectopic Fat among Men with Visceral Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Lifestyle Intervention
2.3. Nutritional Counseling
2.4. Physical Activity Counseling
2.5. Anthropometric Measurements
2.6. Computed Tomography
2.7. Cardiorespiratory Fitness
2.8. Oral Glucose Tolerance Test
2.9. Plasma Lipoprotein/Lipid Profile
2.10. Diet Assessment
2.11. Physical Activity Level
2.12. Statistical Analyses
3. Results
4. Discussion
4.1. Relative Deterioration in the CMR Profile
4.2. Further Improvement in Insulin Resistance
4.3. Physical Activity and CRF during the Maintenance Period
4.4. Increase in IMF
4.5. CRF Improvement during the Maintenance Period
4.6. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lloyd-Jones, D.M.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F.; et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 2010, 121, 586–613. [Google Scholar] [CrossRef]
- Prince, S.A.; Adamo, K.B.; Hamel, M.E.; Hardt, J.; Connor Gorber, S.; Tremblay, M. A comparison of direct versus self-report measures for assessing physical activity in adults: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2008, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Blair, S.N.; Arena, R.; Church, T.S.; Després, J.P.; Franklin, B.A.; Haskell, W.L.; Kaminsky, L.A.; Levine, B.D.; Lavie, C.J.; et al. Importance of assessing cardiorespiratory fitness in clinical practice: A case for fitness as a clinical vital sign: A scientific statement from the American Heart Association. Circulation 2016, 134, e653–e699. [Google Scholar] [CrossRef] [PubMed]
- Blair, S.N. Physical inactivity: The biggest public health problem of the 21st century. Br. J. Sports Med. 2009, 43, 1–2. [Google Scholar] [PubMed]
- Wei, M.; Kampert, J.B.; Barlow, C.E.; Nichaman, M.Z.; Gibbons, L.W.; Paffenbarger, R.S., Jr.; Blair, S.N. Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. JAMA 1999, 282, 1547–1553. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Gibbons, L.W.; Mitchell, T.L.; Kampert, J.B.; Lee, C.D.; Blair, S.N. The association between cardiorespiratory fitness and impaired fasting glucose and type 2 diabetes mellitus in men. Ann. Intern. Med. 1999, 130, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Després, J.P. Body fat distribution and risk of cardiovascular disease: An update. Circulation 2012, 126, 1301–1313. [Google Scholar] [CrossRef]
- Chartrand, D.J.; Murphy-Després, A.; Alméras, N.; Lemieux, I.; Larose, E.; Després, J.P. Overweight, obesity, and CVD risk: A focus on visceral/ectopic fat. Curr. Atheroscler. Rep. 2022, 24, 185–195. [Google Scholar] [CrossRef]
- Després, J.P.; Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Wolf, D. Skeletal muscle lipid accumulation in obesity, insulin resistance, and type 2 diabetes. Pediatr. Diabetes 2004, 5, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Brons, C.; Grunnet, L.G. MECHANISMS IN ENDOCRINOLOGY: Skeletal muscle lipotoxicity in insulin resistance and type 2 diabetes: A causal mechanism or an innocent bystander? Eur. J. Endocrinol. 2017, 176, R67–R78. [Google Scholar] [CrossRef] [PubMed]
- Snel, M.; Jonker, J.T.; Schoones, J.; Lamb, H.; de Roos, A.; Pijl, H.; Smit, J.W.; Meinders, A.E.; Jazet, I.M. Ectopic fat and insulin resistance: Pathophysiology and effect of diet and lifestyle interventions. Int. J. Endocrinol. 2012, 2012, 983814. [Google Scholar] [CrossRef] [PubMed]
- Shaw, C.S.; Clark, J.; Wagenmakers, A.J. The effect of exercise and nutrition on intramuscular fat metabolism and insulin sensitivity. Annu. Rev. Nutr. 2010, 30, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Maltais, A.; Lemieux, I.; Alméras, N.; Tremblay, A.; Bergeron, J.; Poirier, P.; Després, J.P. One-Year Lifestyle Intervention, Muscle Lipids, and Cardiometabolic Risk. Med. Sci. Sports Exerc. 2019, 51, 2156–2165. [Google Scholar] [CrossRef]
- Borel, A.L.; Nazare, J.A.; Baillot, A.; Alméras, N.; Tremblay, A.; Bergeron, J.; Poirier, P.; Després, J.P. Cardiometabolic risk improvement in response to a 3-yr lifestyle modification program in men: Contribution of improved cardiorespiratory fitness vs. weight loss. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E273–E281. [Google Scholar] [CrossRef]
- Lemieux, S.; Prud’homme, D.; Bouchard, C.; Tremblay, A.; Després, J.P. A single threshold value of waist girth identifies normal-weight and overweight subjects with excess visceral adipose tissue. Am. J. Clin. Nutr. 1996, 64, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, I.; Pascot, A.; Couillard, C.; Lamarche, B.; Tchernof, A.; Alméras, N.; Bergeron, J.; Gaudet, D.; Tremblay, G.; Prud’homme, D.; et al. Hypertriglyceridemic waist. A marker of the atherogenic metabolic triad (hyperinsulinemia, hyperapolipoprotein B, small, dense LDL) in men? Circulation 2000, 102, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Després, J.P.; Carpentier, A.C.; Tchernof, A.; Neeland, I.J.; Poirier, P. Management of obesity in cardiovascular practice: JACC Focus Seminar. J. Am. Coll. Cardiol. 2021, 78, 513–531. [Google Scholar] [CrossRef]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.C.; Chumlea, W.C.; Roche, A.F. Stature, recumbent length, and weight. In Anthropometric Standardization Reference Manual; Lohman, T.G., Roche, A.F., Martorell, R., Eds.; Human Kinetics Books: Champaign, IL, USA, 1988; pp. 3–8. [Google Scholar]
- National Heart, Lung, and Blood Institute. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes. Res. 1998, 6 (Suppl. 2), 51S–209S. [Google Scholar]
- Paré, A.; Dumont, M.; Lemieux, I.; Brochu, M.; Alméras, N.; Lemieux, S.; Prud’homme, D.; Després, J.P. Is the relationship between adipose tissue and waist girth altered by weight loss in obese men? Obes. Res. 2001, 9, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Kelley, D.E.; Thaete, F.L.; He, J.; Ross, R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J. Appl. Physiol. 2000, 89, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Després, J.P.; Ross, R.; Boka, G.; Alméras, N.; Lemieux, I. Effect of rimonabant on the high-triglyceride/ low-HDL-cholesterol dyslipidemia, intraabdominal adiposity, and liver fat: The ADAGIO-Lipids trial. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Maltais, A.; Alméras, N.; Lemieux, I.; Tremblay, A.; Bergeron, J.; Poirier, P.; Després, J.P. Trunk muscle quality assessed by computed tomography: Association with adiposity indices and glucose tolerance in men. Metabolism 2018, 85, 205–212. [Google Scholar] [CrossRef]
- Borel, A.L.; Nazare, J.A.; Smith, J.; Alméras, N.; Tremblay, A.; Bergeron, J.; Poirier, P.; Després, J.P. Improvement in insulin sensitivity following a 1-year lifestyle intervention program in viscerally obese men: Contribution of abdominal adiposity. Metabolism 2012, 61, 262–272. [Google Scholar] [CrossRef]
- Bouchard, C.; Lortie, G.; Simoneau, J.A.; Leblanc, C.; Thériault, G.; Tremblay, A. Submaximal power output in adopted and biological siblings. Ann. Hum. Biol. 1984, 11, 303–309. [Google Scholar] [CrossRef]
- Desbuquois, B.; Aurbach, G.D. Use of polyethylene glycol to separate free and antibody-bound peptide hormones in radioimmunoassays. J. Clin. Endocrinol. Metab. 1971, 33, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Richterich, R.; Dauwalder, H. Determination of plasma glucose by hexokinase-glucose-6-phosphate dehydrogenase method. Schweiz. Med. Wochenschr. 1971, 101, 615–618. [Google Scholar]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Burstein, M.; Samaille, J. On a rapid determination of the cholesterol bound to the serum alpha- and beta-lipoproteins. Clin. Chim. Acta 1960, 5, 609. [Google Scholar] [CrossRef] [PubMed]
- Havel, R.J.; Eder, H.; Bragdon, H.F. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Investig. 1955, 34, 1345–1353. [Google Scholar] [CrossRef]
- Allain, C.C.; Poon, L.S.; Chan, C.S.G.; Richmond, W.; Fu, P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Fossati, P.; Prencipe, L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin. Chem. 1982, 28, 2077–2080. [Google Scholar] [CrossRef] [PubMed]
- Nazare, J.A.; Smith, J.; Borel, A.L.; Alméras, N.; Tremblay, A.; Bergeron, J.; Poirier, P.; Després, J.P. Changes in both global diet quality and physical activity level synergistically reduce visceral adiposity in men with features of metabolic syndrome. J. Nutr. 2013, 143, 1074–1083. [Google Scholar] [CrossRef]
- Bouchard, C.; Tremblay, A.; Leblanc, C.; Lortie, G.; Savard, R.; Thériault, G. A method to assess energy expenditure in children and adults. Am. J. Clin. Nutr. 1983, 37, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Riebe, D., Ehrman, J.K., Liguori, G., Magal, M., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2018. [Google Scholar]
- Look AHEAD Research Group; Wing, R. R. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: Four-year results of the Look AHEAD trial. Arch. Intern. Med. 2010, 170, 1566–1575. [Google Scholar] [CrossRef]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef]
- Tuomilehto, J.; Lindstrom, J.; Eriksson, J.G.; Valle, T.T.; Hamalainen, H.; Ilanne-Parikka, P.; Keinanen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef]
- Borel, A.L.; Nazare, J.A.; Smith, J.; Alméras, N.; Tremblay, A.; Bergeron, J.; Poirier, P.; Després, J.P. Visceral and not subcutaneous abdominal adiposity reduction drives the benefits of a 1-year lifestyle modification program. Obesity 2012, 20, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Borel, A.L.; Boulet, G.; Nazare, J.A.; Smith, J.; Alméras, N.; Tremblay, A.; Bergeron, J.; Poirier, P.; Carpentier, A.C.; Després, J.P. Improved plasma FFA/insulin homeostasis is independently associated with improved glucose tolerance after a 1-year lifestyle intervention in viscerally obese men. Diabetes Care 2013, 36, 3254–3261. [Google Scholar] [CrossRef] [PubMed]
- Look AHEAD Research Group; Wing, R. R.; Bolin, P.; Brancati, F.L.; Bray, G.A.; Clark, J.M.; Coday, M.; Crow, R.S.; Curtis, J.M.; Egan, C.M.; et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N. Engl. J. Med. 2013, 369, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, J.; Louheranta, A.; Mannelin, M.; Rastas, M.; Salminen, V.; Eriksson, J.; Uusitupa, M.; Tuomilehto, J.; Finnish Diabetes Prevention Study, G. The Finnish Diabetes Prevention Study (DPS): Lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 2003, 26, 3230–3236. [Google Scholar] [CrossRef] [PubMed]
- Artinian, N.T.; Fletcher, G.F.; Mozaffarian, D.; Kris-Etherton, P.; Van Horn, L.; Lichtenstein, A.H.; Kumanyika, S.; Kraus, W.E.; Fleg, J.L.; Redeker, N.S.; et al. Interventions to promote physical activity and dietary lifestyle changes for cardiovascular risk factor reduction in adults: A scientific statement from the American Heart Association. Circulation 2010, 122, 406–441. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.L.; Andersen, R.E.; Jakicic, J.M. Lifestyle physical activity interventions. History, short- and long-term effects, and recommendations. Am. J. Prev. Med. 1998, 15, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Thériault, R.; Watkins, S.C.; Kelley, D.E. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism 2000, 49, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; He, J.; Watkins, S.; Kelley, D.E. Skeletal muscle lipid content and insulin resistance: Evidence for a paradox in endurance-trained athletes. J. Clin. Endocrinol. Metab. 2001, 86, 5755–5761. [Google Scholar] [CrossRef]
- Dubé, J.J.; Amati, F.; Stefanovic-Racic, M.; Toledo, F.G.; Sauers, S.E.; Goodpaster, B.H. Exercise-induced alterations in intramyocellular lipids and insulin resistance: The athlete’s paradox revisited. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E882–E888. [Google Scholar] [CrossRef]
- Chartrand, D.J.; Larose, E.; Poirier, P.; Mathieu, P.; Alméras, N.; Pibarot, P.; Lamarche, B.; Rhéaume, C.; Després, J.P. Visceral adiposity and liver fat as mediators of the association between cardiorespiratory fitness and plasma glucose-insulin homeostasis. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E548–E556. [Google Scholar] [CrossRef]
- Bouassida, A.; Chamari, K.; Zaouali, M.; Feki, Y.; Zbidi, A.; Tabka, Z. Review on leptin and adiponectin responses and adaptations to acute and chronic exercise. Br. J. Sports Med. 2010, 44, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Turer, A.T.; Scherer, P.E. Adiponectin: Mechanistic insights and clinical implications. Diabetologia 2012, 55, 2319–2326. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; McAuley, P.; Lavie, C.J.; Després, J.P.; Arena, R.; Kokkinos, P. Physical activity and cardiorespiratory fitness as major markers of cardiovascular risk: Their independent and interwoven importance to health status. Prog. Cardiovasc. Dis. 2015, 57, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Rohatgi, A.; Ayers, C.R.; Willis, B.L.; Haskell, W.L.; Khera, A.; Drazner, M.H.; de Lemos, J.A.; Berry, J.D. Cardiorespiratory fitness and classification of risk of cardiovascular disease mortality. Circulation 2011, 123, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, S.; Prud’homme, D.; Bouchard, C.; Tremblay, A.; Després, J.P. Sex differences in the relation of visceral adipose tissue accumulation to total body fatness. Am. J. Clin. Nutr. 1993, 58, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, S.; Després, J.P.; Moorjani, S.; Nadeau, A.; Thériault, G.; Prud’homme, D.; Tremblay, A.; Bouchard, C.; Lupien, P.J. Are gender differences in cardiovascular disease risk factors explained by the level of visceral adipose tissue? Diabetologia 1994, 37, 757–764. [Google Scholar] [CrossRef]
- Neeland, I.J.; Ross, R.; Després, J.P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef]
- Shephard, R.J. Limits to the measurement of habitual physical activity by questionnaires. Br. J. Sports Med. 2003, 37, 197–206. [Google Scholar] [CrossRef]
| Variables | Baseline | Year 1 | Year 2 | Year 3 |
|---|---|---|---|---|
| Mean annual consultations | 15 ± 2 | 10 ± 2 b | 10 ± 3 b | |
| Physical activity level | ||||
| Pedometer (steps/day) | 7808 ± 2782 | 9682 ± 2966 a | 9235 ± 2871 a | 9077 ± 2893 a |
| MPA (min/day) | 14.2 ± 41.5 | 19.0 ± 49.9 | 12.3 ± 29.4 | 10.7 ± 34.6 |
| VPA (min/day) | 3.0 ± 8.7 | 17.3 ± 21.8 a | 18.3 ± 29.4 a | 19.3 ± 34.3 a |
| MVPA (min/day) | 17.2 ± 41.5 | 36.3 ± 50.3 a | 30.6 ± 37.5 a | 30.0 ± 49.5 a |
| Yearly physical activity | ||||
| % active weeks | - | 80.4 ± 19.4 | 74.2 ± 23.3 | 66.2 ± 26.4 b,c |
| % inactive weeks | - | 12.8 ± 15.8 | 18.5 ± 22.1 | 21.9 ± 22.9 b |
| % forced break * | - | 1.8 ± 7.2 | 4.8 ± 9.2 b | 6.4 ± 14.5 b |
| % unknown weeks | - | 5.0 ± 11.1 | 2.5 ± 4.9 | 3.5 ± 6.9 |
| Mean PA sessions/active week | - | 3.4 ± 1.3 | 3.5 ± 1.8 | 3.3 ± 1.4 |
| Mean PA min/active week | - | 172.8 ± 79.2 | 154.8 ± 94.5 | 158.2 ± 82.8 |
| Mean annual PA sessions/week | - | 2.8 ± 1.4 | 2.5 ± 1.5 | 2.2 ± 1.5 b |
| Mean annual PA min/week | - | 144.5 ± 80.0 | 116.4 ± 90.8 b | 107.1 ± 73.2 b |
| Energy intake and diet quality | ||||
| Total energy intake (kcal/day) | 3026 ± 660 | 2518 ± 537 a | 2533 ± 547 a | 2427 ± 518 a |
| Protein intake (% of energy intake) | 16.2 ± 3.0 | 19.0 ± 2.7 a | 18.3 ± 3.5 a | 18.1 ± 3.0 a |
| Fat intake (% of energy intake) | 34.4 ± 6.1 | 30.5 ± 5.4 a | 31.0 ± 5.4 a | 31.2 ± 4.7 a |
| Carbohydrate intake (% of energy intake) | 45.1 ± 6.9 | 47.3 ± 6.1 a | 46.8 ± 6.4 | 47.1 ± 6.0 |
| DASH-derived diet quality score | 35.5 ± 10.4 | 52.0 ± 13.7 a | - | 50.0 ± 12.6 a |
| Variables | Baseline | Year 1 | Year 3 | % Change Baseline—Year 1 | % Change Year 1—Year 3 |
|---|---|---|---|---|---|
| Age (years) | 49.0 ± 8.2 | 50.1 ± 8.2 | 52.1 ± 8.2 | ||
| Anthropometry | |||||
| Body weight (kg) | 93.9 ± 11.8 | 86.9 ± 12.1 a | 90.1 ± 12.1 a,b | −7.5 | 3.8 |
| Body mass index (kg/m2) | 30.9 ± 3.1 | 28.6 ± 3.2 a | 29.5 ± 3.4 a,b | −7.4 | 3.4 |
| Waist circumference (cm) | 107.8 ± 9.3 | 98.9 ± 10.2 a | 102.5 ± 10.0 a,b | −8.3 | 3.6 |
| Lipoprotein/lipid profile | |||||
| Total cholesterol (mmol/L) | 5.11 ± 0.77 | 5.05 ± 0.72 | 5.03 ± 0.74 | −0.1 | 0.6 |
| LDL cholesterol (mmol/L) | 3.09 ± 0.65 | 3.21 ± 0.69 | 3.13 ± 0.67 | 6.0 | −0.7 |
| HDL cholesterol (mmol/L) | 0.96 ± 0.18 | 1.09 ± 0.20 a | 1.08 ± 0.23 a | 15.5 | −0.4 |
| Cholesterol/HDL cholesterol | 5.45 ± 0.94 | 4.73 ± 0.85 a | 4.79 ± 0.96 a | −12.5 | 2.0 |
| Triglycerides (mmol/L) | 2.49 ± 0.93 | 1.87 ± 0.69 a | 2.01 ± 0.84 a | −21.6 | 12.2 |
| Apolipoprotein B (g/L) | 1.08 ± 0.17 | 1.04 ± 0.18 a | 1.02 ± 0.17 a | −3.6 | −0.6 |
| Apolipoprotein A1 (g/L) | 1.14 ± 0.15 | 1.31 ± 0.16 a | 1.24 ± 0.17 a,b | 15.6 | −4.4 |
| Plasma glucose-insulin homeostasis | |||||
| Glucose at 120 min (mmol/L) | 7.7 ± 1.6 | 6.7 ± 1.7 a | 7.3 ± 1.8 b | −11.3 | 12.8 |
| AUC glucose (mmol/L × 180 min × 10−2) | 14.6 ± 2.3 | 13.5 ± 2.3 a | 14.3 ± 2.1 b | −7.4 | 8.2 |
| AUC insulin (pmol/L × 180 min × 10−3) | 172 ± 79 | 104 ± 54 a | 94 ± 50 a | −34.1 | −1.3 |
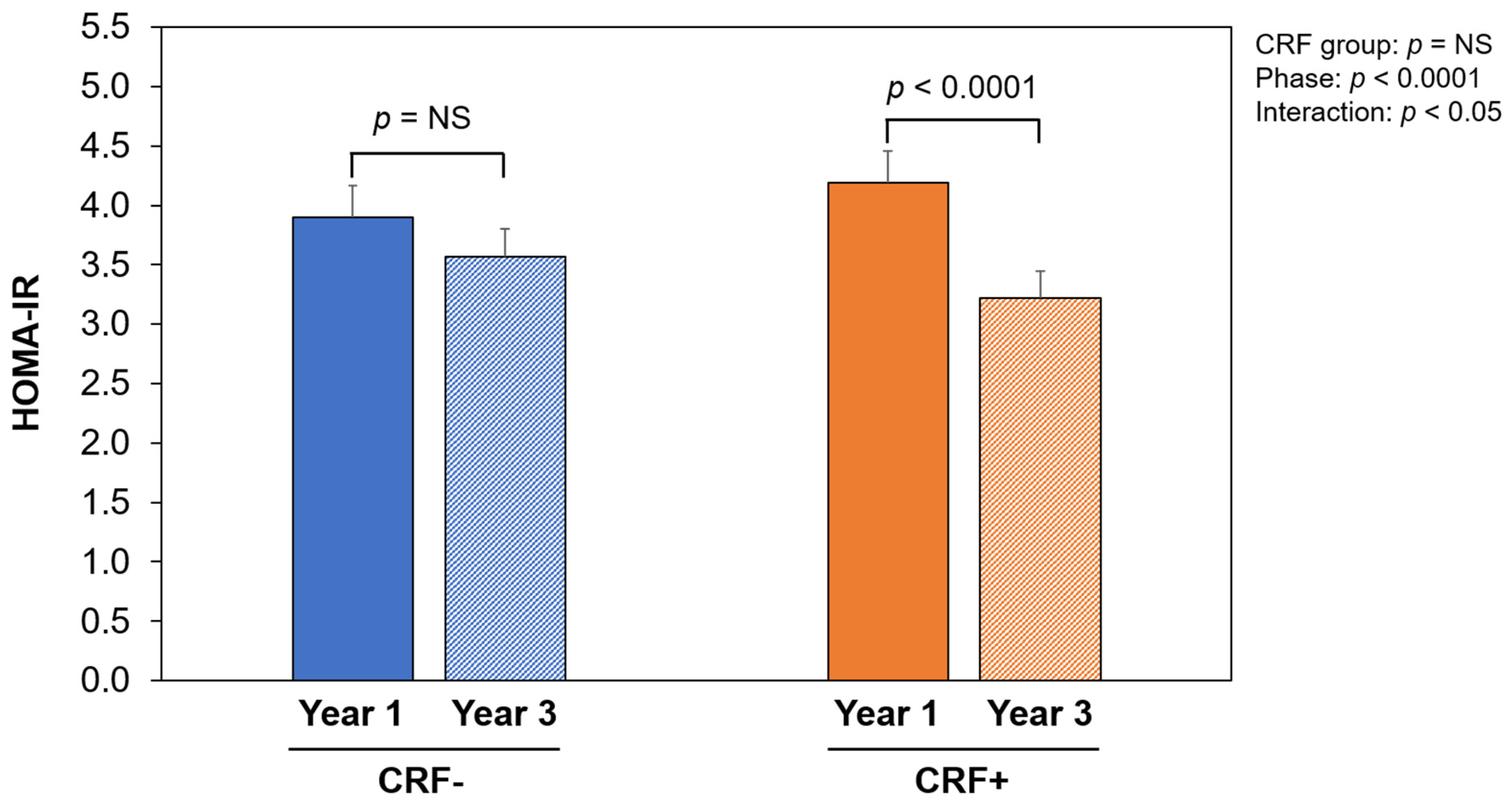
| HOMA-IR | 6.0 ± 3.1 | 4.0 ± 1.7 a | 3.5 ± 1.4 a,b | −19.4 | −5.9 |
| Cardiorespiratory fitness | |||||
| Exercise output at 150 beats/min (METs) | 7.6 ± 1.4 | 9.0 ± 1.6 a | 8.6 ± 1.6 a,b | 19.7 | −3.5 |
| Abdominal adipose tissue | |||||
| L2-L3 VAT (cm2) | 308.3 ± 75.5 | 231.5 ± 88.4 a | 273.1 ± 85.6 a,b | −25.5 | 23.2 |
| L2-L3 SAT (cm2) | 198.2 ± 76.5 | 156.2 ± 67.0 a | 179.1 ± 74.1 a,b | −20.7 | 16.0 |
| L4-L5 VAT (cm2) | 253.8 ± 72.7 | 179.2 ± 79.6 a | 211.5 ± 86.0 a,b | −30.2 | 21.9 |
| L4-L5 SAT (cm2) | 301.7 ± 95.3 | 242.6 ± 91.9 a | 273.3 ± 95.1 a,b | −19.8 | 14.2 |
| VAT volume (cm3) | 1955 ± 490 | 1440 ± 572 a | 1682 ± 547 a,b | −27.1 | 22.1 |
| SAT volume (cm3) | 1725 ± 600 | 1381 ± 529 a | 1575 ± 578 a,b | −19.6 | 14.7 |
| LAM areas | |||||
| Psoas (cm2) | 7.8 ± 2.5 | 6.3 ± 2.5 a | 7.4 ± 2.6 b | −19.4 | 24.6 |
| Core (cm2) | 42.9 ± 11.0 | 37.0 ± 11.2 a | 41.5 ± 11.4 b | −14.1 | 13.9 |
| Mid-thigh (cm2) | 64.4 ± 16.0 | 58.4 ± 18.2 a | 64.2 ± 19.9 b | −9.7 | 10.8 |
| Variables | CRF− (n = 36) | CRF+ (n = 36) | Group p Value | Phase p Value | Interaction p Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Year 1 | Year 3 | Delta | Year 1 | Year 3 | Delta | ||||
| Anthropometry | |||||||||
| Body mass index (kg/m2) | 28.7 ± 3.0 | 30.0 ± 3.5 | 1.3 ± 1.1 a | 28.2 ± 3.8 | 28.7 ± 3.6 | 0.6 ± 0.9 a | NS | <0.0001 | <0.005 |
| Waist circumference (cm) | 98.4 ± 9.9 | 103.4 ± 10.4 | 5.0 ± 3.7 a | 98.2 ± 11.3 | 100.2 ± 10.3 | 2.0 ± 3.1 a | NS | <0.0001 | <0.0005 |
| Plasma glucose-insulin homeostasis | |||||||||
| Glucose at 120 min (mmol/L) | 6.79 ± 1.82 | 7.15 ± 1.80 | 0.36 ± 1.63 | 6.59 ± 1.60 | 7.17 ± 1.79 | 0.58 ± 1.81 | NS | <0.05 | NS |
| AUC glucose (mmol/L × 180 min × 10−2) | 14.3 ± 2.3 | 14.8 ± 2.2 | 0.6 ± 1.4 | 12.7 ± 2.5 | 13.5 ± 1.9 | 0.8 ± 1.2 | <0.05 | <0.0005 | NS |
| AUC insulin (pmol/L × 180 min × 10−3) | 119.5 ± 60.9 | 115.6 ± 62.2 | −3.9 ± 51.0 | 100.6 ± 53.8 | 77.2 ± 31.4 | −23.3 ± 43.5 | <0.05 | <0.05 | NS |
| Computed tomography | |||||||||
| VAT volume (cm3) | 1359 ± 490 | 1693 ± 543 | 334 ± 265 a | 1363 ± 482 | 1549 ± 487 | 186 ± 194 a | NS | <0.0001 | <0.05 |
| Psoas LAM area (cm2) | 5.5 ± 2.1 | 7.3 ± 2.4 | 1.8 ± 1.8 a | 6.6 ± 2.5 * | 7.4 ± 2.7 | 0.8 ± 1.6 a | NS | <0.0001 | <0.05 |
| Core LAM area (cm2) | 35.1 ± 9.4 | 40.6 ± 10.7 | 5.5 ± 5.4 | 36.6 ± 11.6 | 40.5 ± 11.6 | 3.9 ± 3.7 | NS | <0.0001 | NS |
| Mid-thigh LAM area (cm2) | 53.2 ± 13.7 | 61.0 ± 15.6 | 7.7 ± 6.5 a | 60.5 ± 19.8 | 64.6 ± 20.2 | 4.1 ± 7.0 a | NS | <0.0001 | <0.05 |
| Physical activity | |||||||||
| Pedometer (steps/day) | 10,553 ± 2479 | 9050 ± 2870 | −1503 ± 1838 a | 9577 ± 3552 | 9320 ± 3132 | −257 ± 2449 | NS | <0.005 | <0.05 |
| Mean annual sessions/week | 2.7 ± 1.3 | 1.9 ± 1.2 | −0.8 ± 1.4 | 2.9 ± 1.4 | 2.5 ± 1.5 | −0.5 ± 1.7 | NS | <0.005 | NS |
| Mean annual PA min/week | 143.8 ± 72.0 | 88.5 ± 56.1 | −55.3 ± 78.8 | 144.0 ± 75.6 | 117.2 ± 77.0 | −26.8 ± 92.1 | NS | <0.0005 | NS |
| MPA (min/day) | 23.1 ± 51.5 | 6.3 ± 15.1 | −16.8 ± 48.8 | 9.2 ± 24.7 | 13.0 ± 52.9 | 3.8 ± 56.3 | NS | NS | NS |
| VPA (min/day) | 18.6 ± 24.1 | 15.0 ± 20.9 | −3.6 ± 26.5 | 15.6 ± 20.2 | 28.2 ± 48.2 | 12.6 ± 52.6 | NS | NS | NS |
| MVPA (min/day) | 41.7 ± 53.1 | 21.3 ± 29.4 | −20.4 ± 50.2 | 24.8 ± 27.2 | 41.2 ± 67.7 | 16.4 ± 75.6 | NS | NS | <0.05 |
| Cardiorespiratory fitness | |||||||||
| Exercise output at 150 beats/min (METs) | 9.5 ± 1.5 | 8.3 ± 1.3 | −1.2 ± 0.6 a | 8.6 ± 1.8 * | 8.9 ± 1.8 | 0.4 ± 0.9 a | NS | <0.0001 | <0.0001 |
| Energy intake and diet quality | |||||||||
| Total energy intake (kcal/day) | 2608 ± 524 | 2368 ± 425 | −240 ± 482 | 2540 ± 480 | 2483 ± 510 | −56 ± 548 | NS | <0.05 | NS |
| DASH-derived diet quality score | 53.2 ± 14.5 | 49.3 ± 13.9 | −3.8 ± 13.5 | 53.3 ± 13.1 | 54.9 ± 9.8 | 1.7 ± 10.5 | NS | NS | NS |
| Model | |||||
|---|---|---|---|---|---|
| Dependent Variables | Independent Variables | Total R² × 100 | Partial R² × 100 | p | Standardized β |
| Δ Glucose 120 min | 5.6 | ||||
| Δ VAT volume | 5.6 | <0.05 | 0.23572 | ||
| Δ AUC glucose | 13.6 | ||||
| Δ VAT volume | 9.4 | <0.05 | 0.31338 | ||
| Δ Energy intake | - | NS | −0.20663 | ||
| Δ AUC insulin | 29.8 | ||||
| Δ VAT volume | 18.8 | <0.005 | 0.28388 | ||
| Δ Energy intake | 6.2 | <0.05 | −0.26920 | ||
| Δ Core LAM area | - | NS | 0.27179 | ||
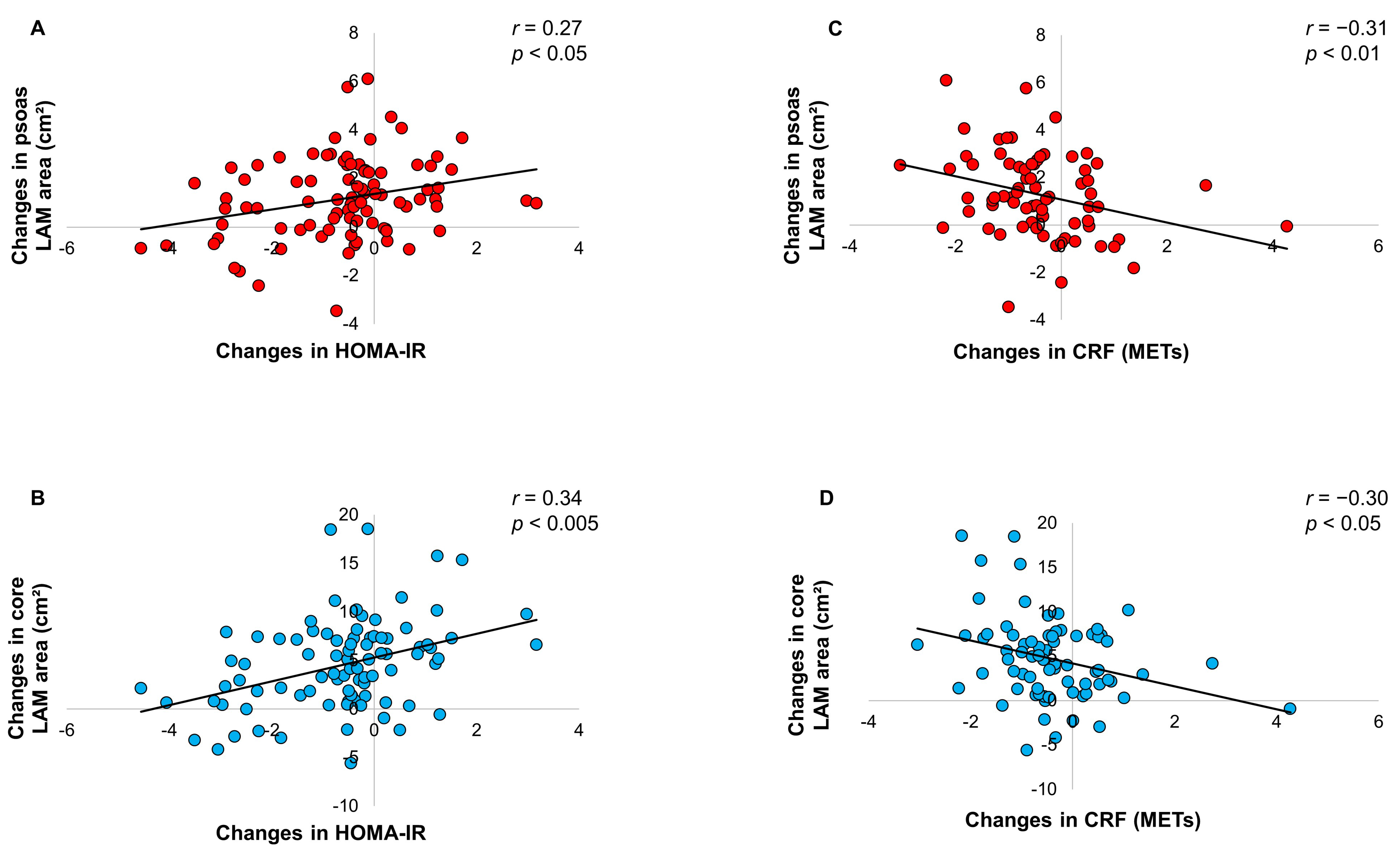
| Δ HOMA-IR | 18.3 | ||||
| Δ Core LAM area | 11.4 | <0.05 | 0.32159 | ||
| Δ PA min/week | 6.8 | <0.05 | −0.26190 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murphy-Després, A.; Chartrand, D.J.; Lemieux, I.; Tremblay, A.; Bergeron, J.; Poirier, P.; Alméras, N.; Després, J.-P. Long-Term Improvement in Cardiorespiratory Fitness Ameliorates Insulin Sensitivity beyond Changes in Visceral/Ectopic Fat among Men with Visceral Obesity. Nutrients 2024, 16, 1377. https://doi.org/10.3390/nu16091377
Murphy-Després A, Chartrand DJ, Lemieux I, Tremblay A, Bergeron J, Poirier P, Alméras N, Després J-P. Long-Term Improvement in Cardiorespiratory Fitness Ameliorates Insulin Sensitivity beyond Changes in Visceral/Ectopic Fat among Men with Visceral Obesity. Nutrients. 2024; 16(9):1377. https://doi.org/10.3390/nu16091377
Chicago/Turabian StyleMurphy-Després, Adrien, Dominic J. Chartrand, Isabelle Lemieux, Angelo Tremblay, Jean Bergeron, Paul Poirier, Natalie Alméras, and Jean-Pierre Després. 2024. "Long-Term Improvement in Cardiorespiratory Fitness Ameliorates Insulin Sensitivity beyond Changes in Visceral/Ectopic Fat among Men with Visceral Obesity" Nutrients 16, no. 9: 1377. https://doi.org/10.3390/nu16091377
APA StyleMurphy-Després, A., Chartrand, D. J., Lemieux, I., Tremblay, A., Bergeron, J., Poirier, P., Alméras, N., & Després, J.-P. (2024). Long-Term Improvement in Cardiorespiratory Fitness Ameliorates Insulin Sensitivity beyond Changes in Visceral/Ectopic Fat among Men with Visceral Obesity. Nutrients, 16(9), 1377. https://doi.org/10.3390/nu16091377










