Analysis of the Protective Effects of Rosa roxburghii-Fermented Juice on Lipopolysaccharide-Induced Acute Lung Injury in Mice through Network Pharmacology and Metabolomics
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. UHPLC-ESI-Q-Exactive Plus Orbitrap-MS Analysis
2.3. Animals Experiments
2.4. Lung Histopathology
2.5. Measurement of Lung Wet/Dry Ratio
2.6. Determination of Cytokine Concentration
2.7. Determination of Lung MDA, SOD, GSH, and MPO
2.8. Plasma and Lung Sample Preparation
2.9. Metabolomics Analysis
2.10. Network Pharmacology Analysis
2.11. Comprehensive Analysis of Metabolomics and Network Pharmacology
2.12. Molecular Docking
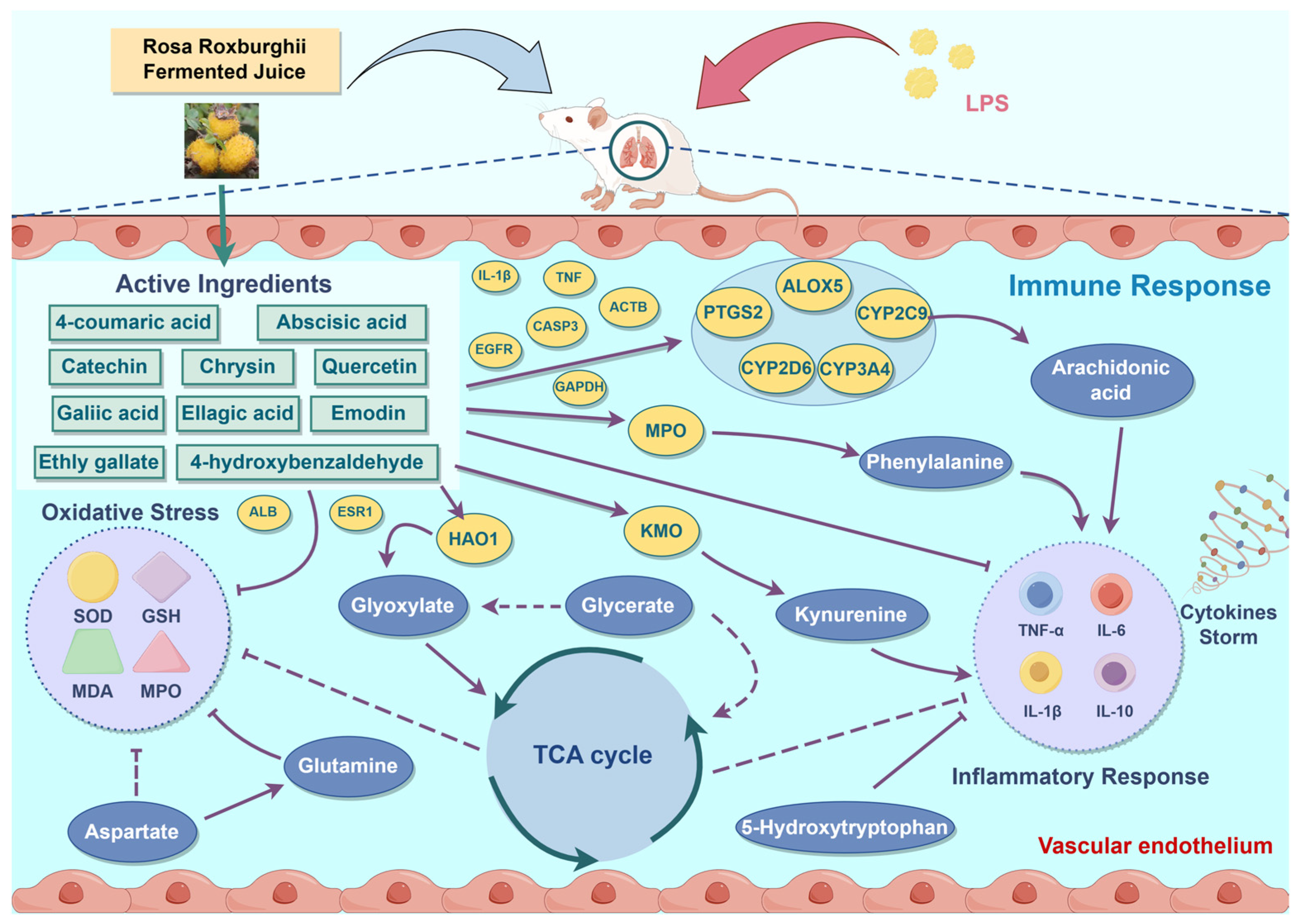
3. Results
3.1. Tentative Identification of RRFJ’s Active Ingredients
3.2. Effects of RRFJ on Histological Change in ALI Mice
3.3. Effects of RRFJ on W/D Ratio in LPS-Treated Mice
3.4. RRFJ Inhibits Inflammatory Response in LPS-Induced ALI
3.5. RRFJ Inhibits Oxidative Stress in LPS Induced ALI
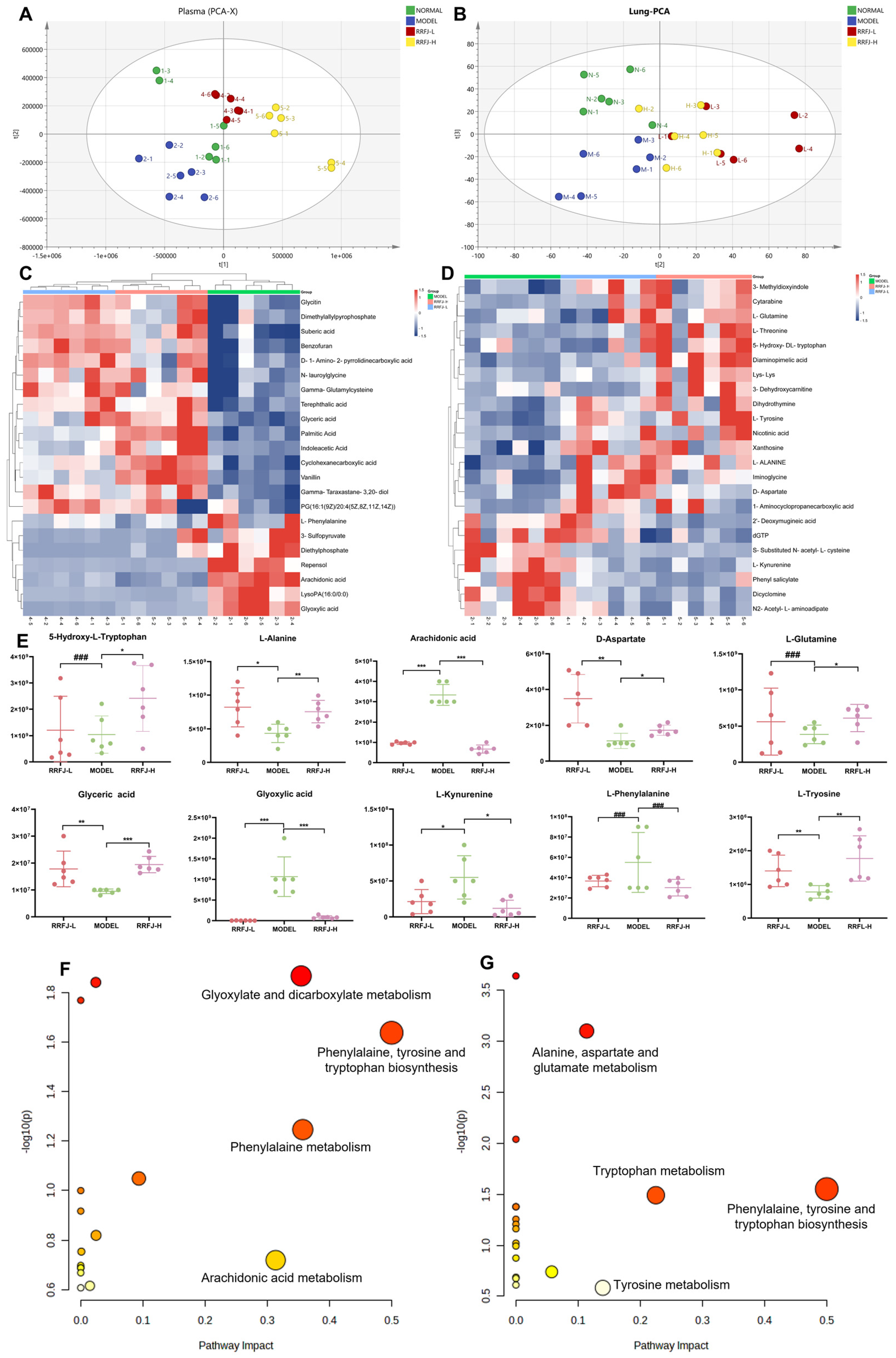
3.6. Metabolomics Analysis in Plasma and Lung Tissue
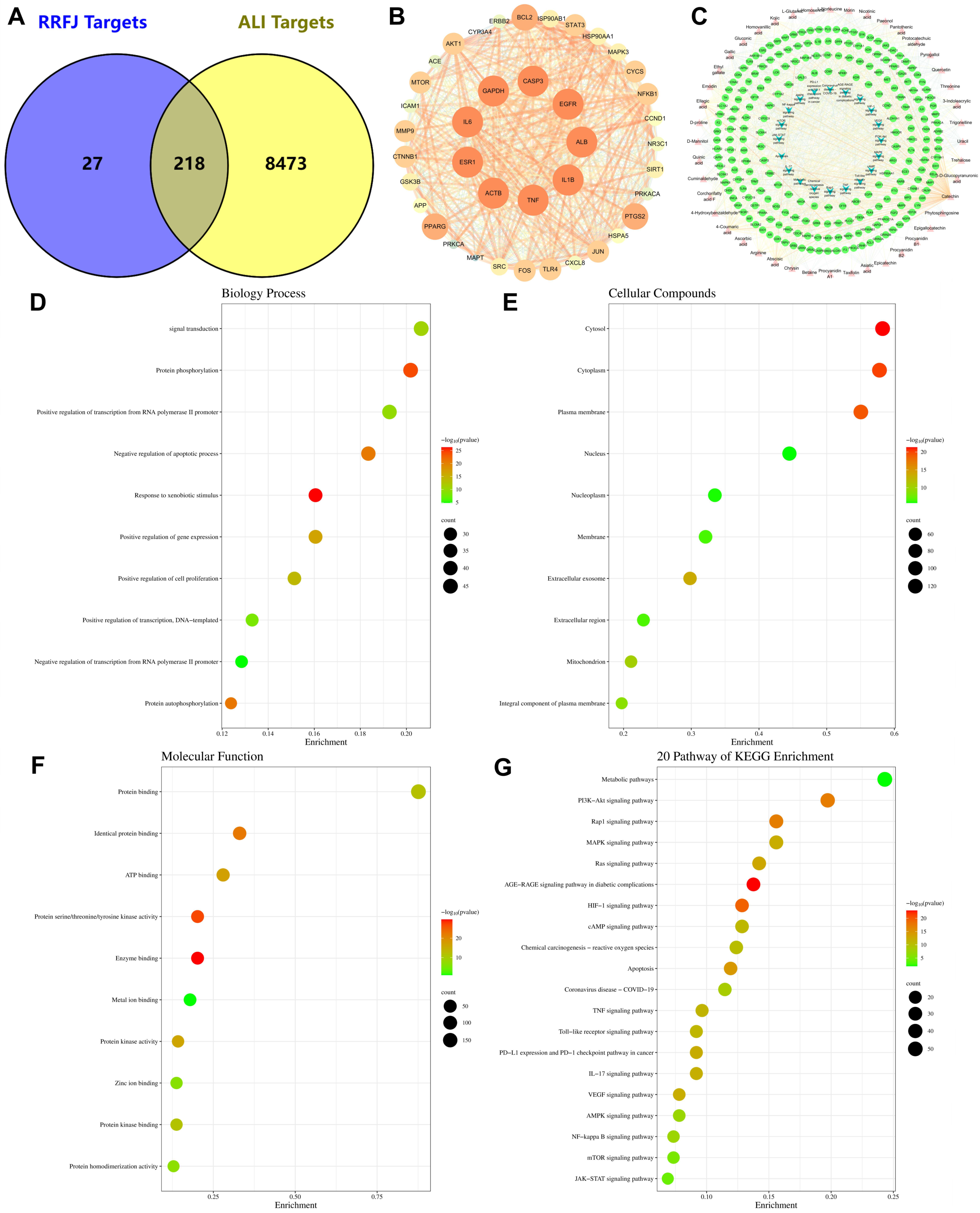
3.7. GO and KEGG Enrichment and Network Analysis of Targets Related to RRFJ Activity on ALI
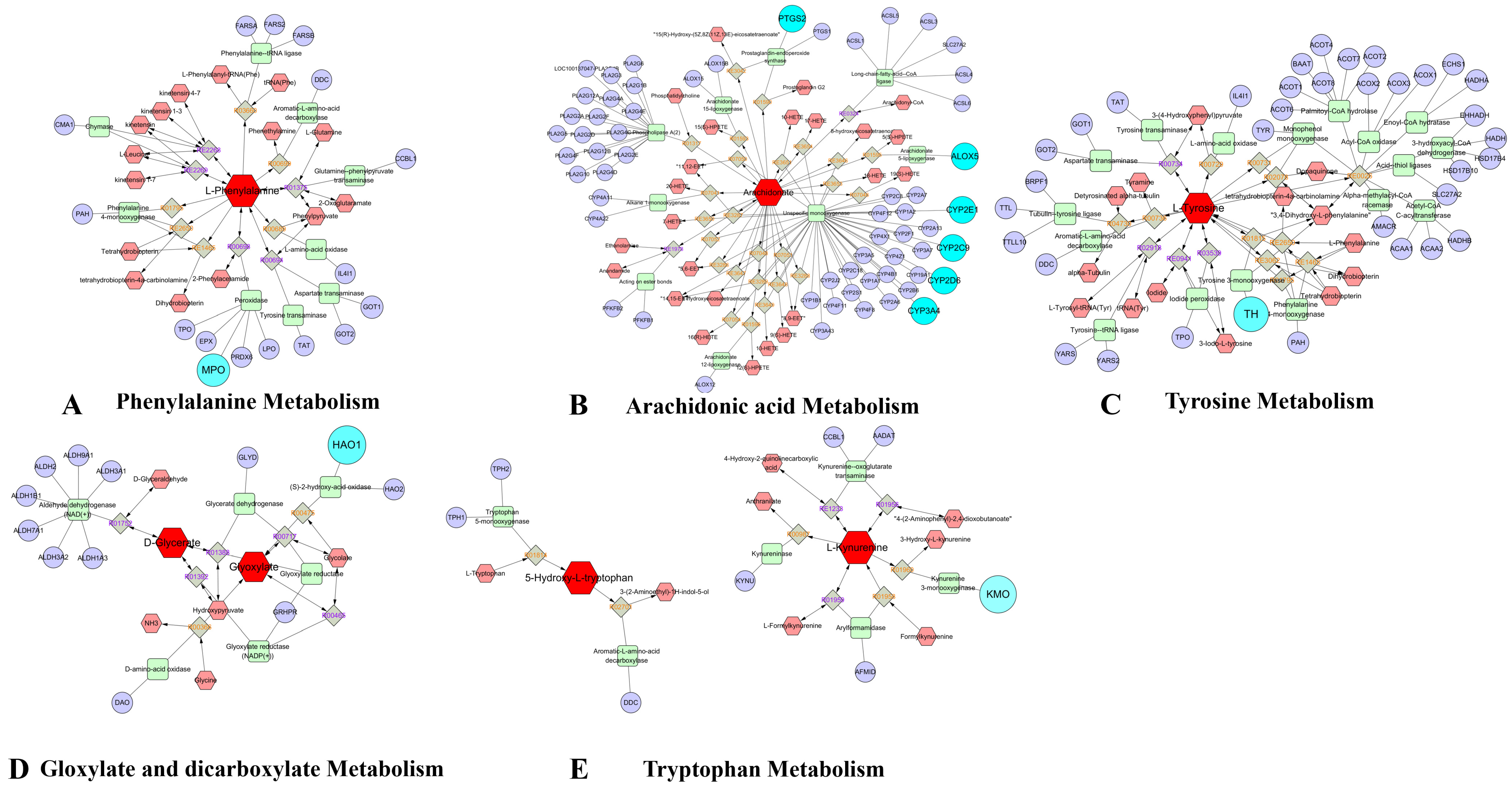
3.8. Comprehensive Analysis of Metabolomics and Network Pharmacology
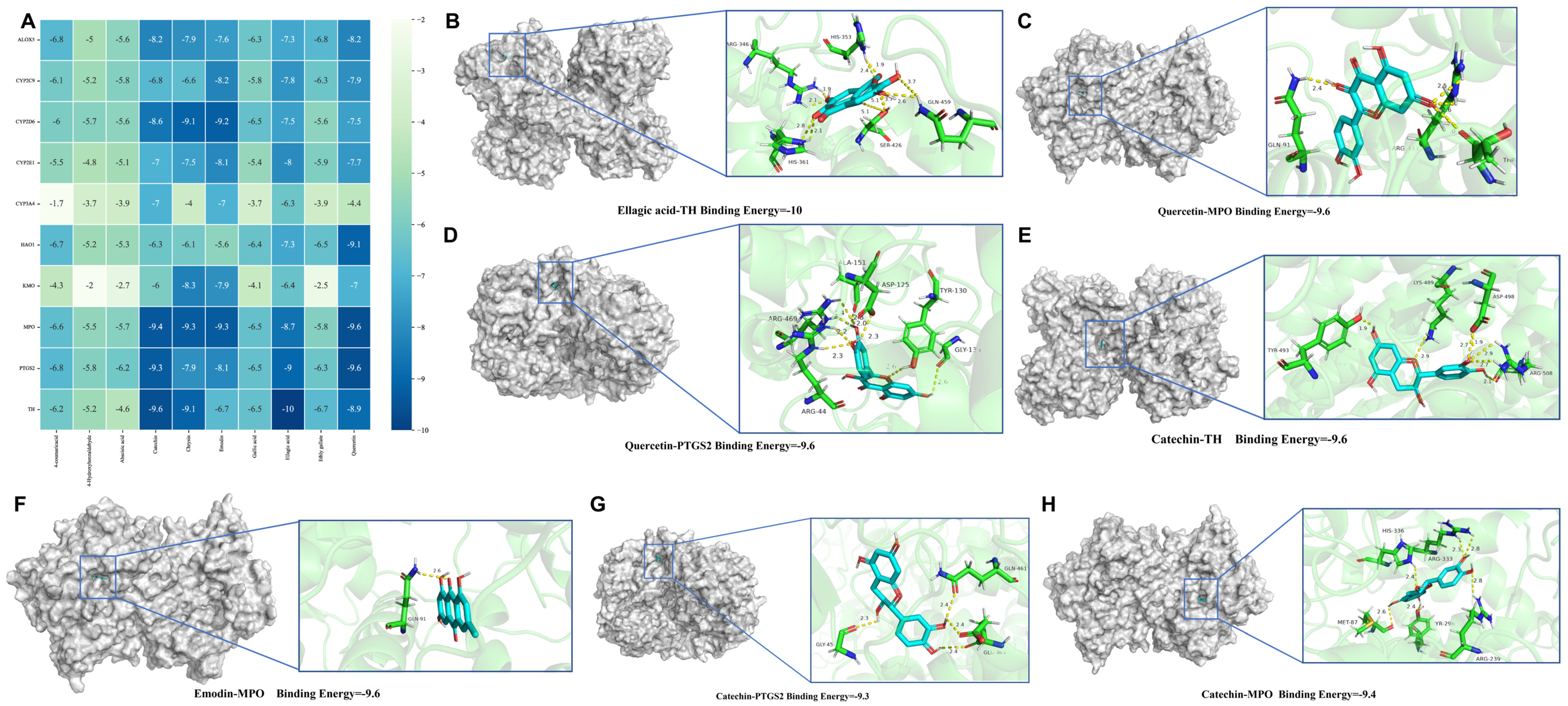
3.9. Molecular Docking Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Chen, X.; Zhang, H.; Xiao, J.; Yang, C.; Chen, W.; Wei, Z.; Chen, X.; Liu, J. 4-Octyl Itaconate Alleviates Lipopolysaccharide-Induced Acute Lung Injury in Mice by Inhibiting Oxidative Stress and Inflammation. Drug Des. Dev. Ther. 2020, 14, 5547–5558. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Fan, K.; Wang, K.; Bian, C. Atractylodin attenuates lipopolysaccharide-induced acute lung injury by inhibiting NLRP3 inflammasome and TLR4 pathways. J. Pharmacol. Sci. 2018, 136, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.R.; Matthay, M.A. Acute Lung Injury: Epidemiology, Pathogenesis, and Treatment. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zeng, Q.; Sun, B.; Wei, S.; Wang, Q.; Zhang, A. Assessing the Role of Nrf2/GPX4-Mediated Oxidative Stress in Arsenic-Induced Liver Damage and the Potential Application Value of Rosa roxburghii Tratt [Rosaceae]. Oxidative Med. Cell. Longev. 2022, 2022, 9865606. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Xu, L.; Zeng, Y.; Gong, F. Effect of gut microbiota on LPS-induced acute lung injury by regulating the TLR4/NF-kB signaling pathway. Int. Immunopharmacol. 2021, 91, 107272. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Lei, J.; Ni, Y.; Zhong, D.; Xie, N.; Ma, J.; Wang, H.; Si, S.; Wu, Y.; Jiang, T. Regulation of CD18 stability by SIGIRR-modulated ubiquitination: New insights into the relationship between innate immune response and acute lung injury. FEBS J. 2023, 290, 2721–2743. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.M.; Hrusch, C.L.; Fei, N.; Barrón, G.M.; Mills, K.A.M.; Hollinger, M.K.; Velez, T.E.; Leone, V.A.; Chang, E.B.; Sperling, A.I. Gut microbiota modulates bleomycin-induced acute lung injury response in mice. Respir. Res. 2022, 23, 337. [Google Scholar] [CrossRef] [PubMed]
- Candelli, M.; Franza, L.; Pignataro, G.; Ojetti, V.; Covino, M.; Piccioni, A.; Gasbarrini, A.; Franceschi, F. Interaction between Lipopolysaccharide and Gut Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 6242. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Li, Y.; Xue, Z.; Shao, R.; Li, L.; Zhu, Y.; Zhang, H.; Yang, J. Xuanfei Baidu Decoction reduces acute lung injury by regulating infiltration of neutrophils and macrophages via PD-1/IL17A pathway. Pharmacol. Res. 2022, 176, 106083. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xiao, K.; Xie, L. Advances in the use of exosomes for the treatment of ALI/ARDS. Front. Immunol. 2022, 13, 971189. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Zhang, S.; Yuan, M.; Zhang, M.; Tang, L.; Wang, P.; Liu, Y.; Xu, C.; Luo, P.; Gao, X. Fermented Rosa Roxburghii Tratt Juice Alleviates High-Fat Diet-Induced Hyperlipidemia in Rats by Modulating Gut Microbiota and Metabolites. Front. Pharmacol. 2022, 13, 883629. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, F.; Liu, X. Effects of Rosa roxburghii Tratt Must on the Growth, Nutrient Composition, and Antioxidant Activity of Pleurotus ostreatus Mycelia. Molecules 2022, 27, 3585. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yan, H.; Zhai, L.; Yang, Z.; Yi, Y. Characterization of the Rosa roxburghii Tratt transcriptome and analysis of MYB genes. PLoS ONE 2019, 14, e0203014. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, W.; Xian, W.; Huang, W.; Yang, R. Free and Bound Phenolic Profiles of Rosa roxburghii Tratt Leaves and Their Antioxidant and Inhibitory Effects on α-Glucosidase. Front. Nutr. 2022, 9, 922496. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhang, S.; Zhang, M.; Wang, P.; Liang, G.; Gao, X. Analysis of protective effects of Rosa Roxburghii Tratt fruit polyphenols on lipopolysaccharide-induced acute lung injury through network pharmacology and metabolomics. Food Sci. Nutr. 2022, 10, 4258–4269. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wei, T.; Zheng, L.; Jiang, F.; Ma, W.; Lu, M.; Wu, X.; An, H. Recent Advances on Main Active Ingredients, Pharmacological Activities of Rosa roxbughii and Its Development and Utilization. Foods 2023, 12, 1051. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Luo, Z.; Deng, T.; Cui, X.; Yang, J.; Pan, X.; Yang, L.; Wang, Y.; Li, L.; Li, L.; et al. Effect on hypoglycemic activity and UPLC–MS/MS profiling of Rosa roxburghii fruit fermented with Chinese traditional distiller’s yeast. Food Sci. Technol. 2022, 42, e41822. [Google Scholar] [CrossRef]
- Bauermeister, A.; Mannochio-Russo, H.; Costa-Lotufo, L.V.; Jarmusch, A.K.; Dorrestein, P.C. Mass spectrometry-based metabolomics in microbiome investigations. Nat. Rev. Microbiol. 2022, 20, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Marchev, A.S.; Vasileva, L.V.; Amirova, K.M.; Savova, M.S.; Balcheva-Sivenova, Z.P.; Georgiev, M.I. Metabolomics and health: From nutritional crops and plant-based pharmaceuticals to profiling of human biofluids. Cell. Mol. Life Sci. 2021, 78, 6487–6503. [Google Scholar] [CrossRef]
- Xu, H.; Xu, S.; Li, L.; Wu, Y.; Mai, S.; Xie, Y.; Tan, Y.; Li, A.; Xue, F.; He, X.; et al. Integrated metabolomics, network pharmacology and biological verification to reveal the mechanisms of Nauclea officinalis treatment of LPS-induced acute lung injury. Chin. Med. 2022, 17, 131. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Wang, X.; Liu, M.; Huang, M.; Shen, Z.; Feng, J.; Yang, H.; Li, Z.; Gao, J.; Ye, X. Exploring the treatment of COVID-19 with Yinqiao powder based on network pharmacology. Phytother. Res. 2021, 35, 2651–2664. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wei, S.; Niu, S.; Ma, X.; Li, H.; Jing, M.; Zhao, Y. Network pharmacology prediction and molecular docking-based strategy to explore the potential mechanism of Huanglian Jiedu Decoction against sepsis. Comput. Biol. Med. 2022, 144, 105389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhu, X.; Bai, H.; Ning, K. Network Pharmacology Databases for Traditional Chinese Medicine: Review and Assessment. Front. Pharmacol. 2019, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.G.; Dos Santos, R.N.; Oliva, G.; Andricopulo, A.D. Molecular Docking and Structure-Based Drug Design Strategies. Molecules 2015, 20, 13384–13421. [Google Scholar] [CrossRef] [PubMed]
- Pinzi, L.; Rastelli, G. Molecular Docking: Shifting Paradigms in Drug Discovery. Int. J. Mol. Sci. 2019, 20, 4331. [Google Scholar] [CrossRef] [PubMed]
- Wahid, M.; Saqib, F.; Qamar, M.; Ziora, Z.M. Antispasmodic activity of the ethanol extract of Citrullus lanatus seeds: Justifying ethnomedicinal use in Pakistan to treat asthma and diarrhea. J. Ethnopharmacol. 2022, 295, 115314. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Q.-X.; Liao, Z.-Z.; Liu, Y.; Ouyang, Y.; Jiang, W.-J.; Tang, M.-T.; Hu, J.-F.; Zhang, W. Anti-inflammatory effect and component analysis of Chaihu Qingwen granules. J. Ethnopharmacol. 2023, 317, 116763. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, F.; Gu, L.; Jiang, Y.; Cai, Z.; Zhao, Y.; Chen, L.; Zhu, Z.; Liu, X. Discovery and validation of COX2 as a target of flavonoids in Apocyni Veneti Folium: Implications for the treatment of liver injury. J. Ethnopharmacol. 2024, 326, 117919. [Google Scholar] [CrossRef] [PubMed]
- Esposito, T.; Pisanti, S.; Martinelli, R.; Celano, R.; Mencherini, T.; Re, T.; Aquino, R.P. Couroupita guianensis bark decoction: From Amazonian medicine to the UHPLC-HRMS chemical profile and its role in inflammation processes and re-epithelialization. J. Ethnopharmacol. 2023, 313, 116579. [Google Scholar] [CrossRef] [PubMed]
- Mokra, D.; Kosutova, P. Biomarkers in acute lung injury. Respir. Physiol. Neurobiol. 2015, 209, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Deng, J.-S.; Huang, W.-C.; Jiang, W.-P.; Huang, G.-J. Attenuation of Lipopolysaccharide-Induced Acute Lung Injury by Hispolon in Mice, Through Regulating the TLR4/PI3K/Akt/mTOR and Keap1/Nrf2/HO-1 Pathways, and Suppressing Oxidative Stress-Mediated ER Stress-Induced Apoptosis and Autophagy. Nutrients 2020, 12, 1742. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Liu, Q.; Wen, Z.; Feng, H.; Deng, X.; Ci, X. Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3β-Nrf2 signal axis. Redox Biol. 2017, 12, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Hu, T.; Gao, H.; Zhai, J.; Gong, J.; Zhang, Y.; Tao, L.; Sun, J.; Li, Z.; Qu, X. Altered metabolic profiles and biomarkers associated with astragaloside IV-mediated protection against cisplatin-induced acute kidney injury in rats: An HPLC-TOF/MS-based untargeted metabolomics study. Biochem. Pharmacol. 2020, 183, 114299. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yu, Y.; Li, R.; Tao, Z.; Zhang, L.; Wang, X.; Qi, X.; Li, Y.; Meng, T.; Qu, H.; et al. Phenylalanine promotes alveolar macrophage pyroptosis via the activation of CaSR in ARDS. Front. Immunol. 2023, 14, 1114129. [Google Scholar] [CrossRef] [PubMed]
- Sorgdrager, F.J.H.; Naudé, P.J.W.; Kema, I.P.; Nollen, E.A.; De Deyn, P.P. Tryptophan Metabolism in Inflammaging: From Biomarker to Therapeutic Target. Front. Immunol. 2019, 10, 2565. [Google Scholar] [CrossRef] [PubMed]
- Bizjak, D.A.; Stangl, M.; Börner, N.; Bösch, F.; Durner, J.; Drunin, G.; Buhl, J.-L.; Abendroth, D. Kynurenine serves as useful biomarker in acute, Long- and Post-COVID-19 diagnostics. Front. Immunol. 2022, 13, 1004545. [Google Scholar] [CrossRef] [PubMed]
- Haq, S.; Grondin, J.A.; Khan, W.I. Tryptophan-derived serotonin-kynurenine balance in immune activation and intestinal inflammation. FASEB J. 2021, 35, e21888. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, M.; Horriatkhah, E.; Mohajery, R. The effect of glutamine supplementation on serum levels of some inflammatory factors, oxidative stress, and appetite in COVID-19 patients: A case–control study. Inflammopharmacology 2021, 29, 1769–1776. [Google Scholar] [CrossRef] [PubMed]
- Toney, M.D. Aspartate aminotransferase: An old dog teaches new tricks. Arch. Biochem. Biophys. 2014, 544, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Tang, Y.; Wang, Q.; Chu, D.; Zhou, J.; Zhou, Y. Yangyinqingfei decoction attenuates PM2.5-induced lung injury by enhancing arachidonic acid metabolism. Front. Pharmacol. 2022, 13, 1056078. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-D.; Zhang, D.; Xiao, C.-L.; Zhou, Y.; Li, X.; Wang, L.; He, Z.; Reilly, J.; Xiao, Z.-Y.; Shu, X. P-Coumaric Acid Reverses Depression-Like Behavior and Memory Deficit Via Inhibiting AGE-RAGE-Mediated Neuroinflammation. Cells 2022, 11, 1594. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, S.; Wu, Y.; Liu, X.; Wang, J.; Han, D. Ellagic Acid Alleviates Diquat-Induced Jejunum Oxidative Stress in C57BL/6 Mice through Activating Nrf2 Mediated Signaling Pathway. Nutrients 2022, 14, 1103. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Marengo, B.; Zuccari, G. Oxidative Stress, Antioxidant Capabilities, and Bioavailability: Ellagic Acid or Urolithins? Antioxidants 2020, 9, 707. [Google Scholar] [CrossRef] [PubMed]
- Stark, A.-K.; Sriskantharajah, S.; Hessel, E.M.; Okkenhaug, K. PI3K inhibitors in inflammation, autoimmunity and cancer. Curr. Opin. Pharmacol. 2015, 23, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Zuehlke, A.D.; Beebe, K.; Neckers, L.; Prince, T. Regulation and function of the human HSP90AA1 gene. Gene 2015, 570, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Hirayama, A.; Matsumoto, T.; Sato, Y.; Kobayashi, T.; Ikeda, S.; Maruyama, M.; Kaneko, M.; Shigeta, M.; Ito, E.; et al. Hao1 Is Not a Pathogenic Factor for Ectopic Ossifications but Functions to Regulate the TCA Cycle In Vivo. Metabolites 2022, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, H.; Du, Q.; Shen, J. Targeting Myeloperoxidase (MPO) Mediated Oxidative Stress and Inflammation for Reducing Brain Ischemia Injury: Potential Application of Natural Compounds. Front. Physiol. 2020, 11, 433. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, S.; Deshmukh, S.S.; Dastidar, S.G. COX inhibitors for airway inflammation. Expert Opin. Ther. Targets 2012, 16, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Nwabufo, C.K.; Hoque, T.; Yip, L.; Khara, M.; Mubareka, S.; Pollanen, M.S.; Bendayan, R. SARS-CoV-2 infection dysregulates the expression of clinically relevant drug metabolizing enzymes in Vero E6 cells and membrane transporters in human lung tissues. Front. Pharmacol. 2023, 14, 1124693. [Google Scholar] [CrossRef] [PubMed]
- Platten, M.; Nollen, E.A.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef]


| NO | Identification Name | Molecular Formular | RT (min) | Observed MS1 (m/z) | MS2 (m/z) |
|---|---|---|---|---|---|
| 1 | L-Threonine | C4H9NO3 | 0.923 | 120.0657 (+1.55) | 102.05528, 84.04484, 74.06068, 56.05026 |
| 2 | Gluconic acid | C6H12O7 | 0.944 | 195.0503 (−3.66) | 159.02901, 129.01617, 75.06745 |
| 3 | β-D-Glucopyranuronic acid | C6H10O7 | 0.946 | 193.0349 (−2.84) | 113.02319, 103.00240, 85.02815, 72.99180 |
| 4 | D-Mannitol | C6H14O6 | 0.947 | 181.6710 (+1.74) | 163.06003, 131.03355, 119.03498, 101.02442, 89.0244, 71.01385 |
| 5 | Betaine | C15H11NO2 | 0.965 | 118.0865 (+1.96) | 59.07378 |
| 6 | Trehalose | C12H22O11 | 0.989 | 341.1093 (−0.17) | 179.05525, 119.03376, 89.02309, 71.01254 |
| 7 | Quinic acid | C7H12O6 | 0.993 | 191.0192 (−3.20) | 173.04477, 127.03871, 85.02814 |
| 8 | Trigonelline | C7H7NO2 | 1.009 | 138.0549 (−0.17) | 110.06035, 94.0656 |
| 9 | L-Glutamic acid | C5H9NO4 | 1.016 | 148.0604 (+0.35) | 130.0500, 102.05538, 84.04497, 56.03430 |
| 10 | D-proline | C5H9NO2 | 1.049 | 124.0394 (+1.07) | 96.04478, 80.05005 |
| 11 | Arginine | C6H14N4O2 | 1.057 | 175.1078 (+0.00) | 130.09749, 98.06047, 70.06585 |
| 12 | Pantothenic acid | C9H17NO5 | 1.902 | 220.1180 (+1.92) | 174.11255, 156.10155 |
| 13 | L-Homoserine | C4H9NO3 | 1.118 | 120.0657 (+1.55) | 102.05528, 84.04486, 74.06068, 56.05026 |
| 14 | Pyrogallol | C6H6O3 | 1.235 | 127.0393 (+1.31) | 109.02876, 81.03349, 53.03942 |
| 15 | Ascorbic acid | C6H14O6 | 1.25 | 175.0583 (−1.65) | 115.00425, 87.00745 |
| 16 | Uracil | C4H4N2O | 1.284 | 113.0239 (+4.08) | 96.00851 |
| 17 | Nicotinic acid | C6H5NO2 | 1.322 | 124.0394 (+1.07) | 96.04478, 80.05005, 70.02950 |
| 18 | Kojic acid | C6H6O4 | 1.697 | 143.0340 (−0.06) | 125.02338, 113.02338, 97.02879, 69.03418 |
| 19 | L-Norleucine | C6H13NO2 | 1.892 | 132.1022 (+0.99) | 86.09695, 69.07059 |
| 20 | Gallic acid | C7H6O5 | 2.074 | 169.0136 (−3.71) | 125.02316, 79.01754 |
| 21 | Epigallocatechin | C15H14O7 | 4.746 | 305.0672 (−0.29) | 261.067605, 125.03291 |
| 22 | 3-Indoleacrylic acid | C11H9NO2 | 6.494 | 188.0705 (−0.31) | 170.06004, 146.06003, 118.06513, 91.05470 |
| 23 | Procyanidin A1 | C30H24O12 | 6.878 | 575.1204 (+0.27) | 423.07266 |
| 24 | Procyanidin B2 | C3OH26O12 | 6.921 | 579.1489 (+1.28) | 427.10135, 409.09106, 291.0858 |
| 25 | Procyanidin B1 | C30H26O12 | 6.922 | 577.1354 (−0.92) | 425.08820, 407.07663, 289.0720, 291.0871 |
| 26 | Emodin | C15H10O5 | 6.957 | 271.0596 (−1.75) | 229.04954, 197.05971, 173.05971, 145.06490, 131.04893 |
| 27 | 4-Hydroxybenzaldehyde | C7H6O2 | 7.144 | 123.0440 (−1.72) | 95.04955, 77.03918 |
| 28 | Catechin | C15H14O6 | 7.165 | 290.07458 (−1.06) | 245.0448, 205.04993, 203.07065, 179.03412, 151.03908 |
| 29 | Homovanillic acid | C9H16O4 | 7.268 | 181.0500 (−3.36) | 166.02579, 137.05962, 122.03733, 107.04891 |
| 30 | p-Coumaric acid | C9H8O3 | 7.307 | 165.0544 (−0.35) | 147.04381, 119.04916, 91.05460, 65.03925 |
| 31 | Epicatechin | C15H14O6 | 7.795 | 291.0858 (−2.46) | 245.04413, 161.05949, 139.03885, 123.0446 |
| 32 | Chrysin | C15H14O7 | 7.953 | 255.0643 (−3.30) | 181.06543, 153.06988, 68.9910 |
| 33 | Protocatechualdehyde | C7H6O3 | 7.99 | 139.0388 (−2.76) | 111.04431, 93.03349, 83.04969 |
| 34 | Cuminaldehyde | C10H12O | 8.545 | 149.0961 (−0.46) | 134.07307, 105.07024, 79.05423 |
| 35 | Ellagic acid | C7H6O | 8.815 | 301.0002 (+1.31) | 283.99600, 257.00867, 229.01373 |
| 36 | Ethyl gallate | C9H10O5 | 8.896 | 197.0449 (−2.62) | 169.01338, 125.02332, 78.95766 |
| 37 | Taxifolin | C15H12O7 | 9.27 | 303.0514 (+0.98) | 285.04044, 259.06107, 153.08145, 125.02322 |
| 38 | Paeonol | C9H10O3 | 10.593 | 167.0128 (−1.59) | 149.96600, 121.06496, 84.96029 |
| 39 | Abscisic acid | C15H20O4 | 11.015 | 263.1290 (+0.06) | 245.13214, 204.11478, 161.09610, 111.04515 |
| 40 | Quercetin | C15H10O7 | 11.945 | 301.0358 (+0.51) | 151.00368, 121.02823 |
| 41 | Morin | C15H20O7 | 11.924 | 302.0425 (+0.51) | 229.01344, 151.00255 |
| 42 | Asiatic acid | C36H48O5 | 13.222 | 471.3464 (−2.13) | 453.33633, 407.33084, 203.17943, 107.08553 |
| 43 | Corchorifatty acid F | C18H32O5 | 13.556 | 327.2178 (+0.79) | 229.14453, 209.11832, 185.11831, 127.11284, 97.06589, 85.02950 |
| 44 | Phytosphingosine | C18H39NO3 | 16.997 | 318.2997 (−2.22) | 300.28851, 256.26318, 85.10162 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Zhang, S.; Sun, X.; Meng, D.; Lai, C.; Zhang, M.; Wang, P.; Huang, X.; Gao, X. Analysis of the Protective Effects of Rosa roxburghii-Fermented Juice on Lipopolysaccharide-Induced Acute Lung Injury in Mice through Network Pharmacology and Metabolomics. Nutrients 2024, 16, 1376. https://doi.org/10.3390/nu16091376
Chen Z, Zhang S, Sun X, Meng D, Lai C, Zhang M, Wang P, Huang X, Gao X. Analysis of the Protective Effects of Rosa roxburghii-Fermented Juice on Lipopolysaccharide-Induced Acute Lung Injury in Mice through Network Pharmacology and Metabolomics. Nutrients. 2024; 16(9):1376. https://doi.org/10.3390/nu16091376
Chicago/Turabian StyleChen, Zhiyu, Shuo Zhang, Xiaodong Sun, Duo Meng, Chencen Lai, Min Zhang, Pengjiao Wang, Xuncai Huang, and Xiuli Gao. 2024. "Analysis of the Protective Effects of Rosa roxburghii-Fermented Juice on Lipopolysaccharide-Induced Acute Lung Injury in Mice through Network Pharmacology and Metabolomics" Nutrients 16, no. 9: 1376. https://doi.org/10.3390/nu16091376
APA StyleChen, Z., Zhang, S., Sun, X., Meng, D., Lai, C., Zhang, M., Wang, P., Huang, X., & Gao, X. (2024). Analysis of the Protective Effects of Rosa roxburghii-Fermented Juice on Lipopolysaccharide-Induced Acute Lung Injury in Mice through Network Pharmacology and Metabolomics. Nutrients, 16(9), 1376. https://doi.org/10.3390/nu16091376






