Cholesterol and Its Oxidation Derivatives Content in Market Dairy Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Material
2.2. Analytical Methods
2.2.1. Reagents
2.2.2. Determination of Cholesterol and Its Oxidized Derivatives
2.2.3. Fat Content Determination
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schade, D.S.; Shey, L.; Eaton, R.P. Cholesterol Review: A Metabolically Important Molecule. Endocr. Pract. 2020, 26, 1514–1523. [Google Scholar] [CrossRef]
- Corliss, J. How It’s Made: Cholesterol Production in Your Body. Available online: https://www.health.harvard.edu/heart-health/how-its-made-cholesterol-production-in-your-body (accessed on 13 February 2024).
- Mayengbam, S.S.; Singh, A.; Pillai, A.D.; Bhat, M.K. Influence of Cholesterol on Cancer Progression and Therapy. Transl. Oncol. 2021, 14, 101043. [Google Scholar] [CrossRef]
- Vicente, S.J.V.; Sampaio, G.R.; Ferrari, C.K.B.; Torres, E.A.F.S. Oxidation of Cholesterol in Foods and Its Importance for Human Health. Food Rev. Int. 2012, 28, 47–70. [Google Scholar] [CrossRef]
- Poli, G.; Leoni, V.; Biasi, F.; Canzoneri, F.; Risso, D.; Menta, R. Oxysterols: From Redox Bench to Industry. Redox Biol. 2022, 49, 102220. [Google Scholar] [CrossRef]
- Canzoneri, F.; Leoni, V.; Rosso, G.; Risso, D.; Menta, R.; Poli, G. Oxysterols as Reliable Markers of Quality and Safety in Cholesterol Containing Food Ingredients and Products. Front. Nutr. 2022, 9, 853460. [Google Scholar] [CrossRef]
- Kulig, W.; Cwiklik, L.; Jurkiewicz, P.; Rog, T.; Vattulainen, I. Cholesterol Oxidation Products and Their Biological Importance. Chem. Phys. Lipids 2016, 199, 144–160. [Google Scholar] [CrossRef]
- Otaegui-Arrazola, A.; Menéndez-Carreño, M.; Ansorena, D.; Astiasarán, I. Oxysterols: A World to Explore. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2010, 48, 3289–3303. [Google Scholar] [CrossRef] [PubMed]
- Poli, G.; Biasi, F.; Leonarduzzi, G. Oxysterols in the Pathogenesis of Major Chronic Diseases. Redox Biol. 2013, 1, 125–130. [Google Scholar] [CrossRef]
- Zmysłowski, A.; Szterk, A. Oxysterols as a Biomarker in Diseases. Clin. Chim. Acta Int. J. Clin. Chem. 2019, 491, 103–113. [Google Scholar] [CrossRef]
- Zerbinati, C.; Iuliano, L. Cholesterol and Related Sterols Autoxidation. Free Radic. Biol. Med. 2017, 111, 151–155. [Google Scholar] [CrossRef]
- Derewiaka, D. The Influence of the Food Matrix on the Content of Cholesterol and Oxysterols during Simulated Process of Digestion. Eur. J. Lipid Sci. Technol. 2024, 126, 2200175. [Google Scholar] [CrossRef]
- Brown, A.J.; Jessup, W. Oxysterols: Sources, Cellular Storage and Metabolism, and New Insights into Their Roles in Cholesterol Homeostasis. Mol. Asp. Med. 2009, 30, 111–122. [Google Scholar] [CrossRef]
- Maldonado-Pereira, L.; Schweiss, M.; Barnaba, C.; Medina-Meza, I.G. The Role of Cholesterol Oxidation Products in Food Toxicity. Food Chem. Toxicol. 2018, 118, 908–939. [Google Scholar] [CrossRef] [PubMed]
- Tunick, M.H.; Van Hekken, D.L. Dairy Products and Health: Recent Insights. J. Agric. Food Chem. 2015, 63, 9381–9388. [Google Scholar] [CrossRef]
- Bhupathi, V.; Mazariegos, M.; Cruz Rodriguez, J.B.; Deoker, A. Dairy Intake and Risk of Cardiovascular Disease. Curr. Cardiol. Rep. 2020, 22, 11. [Google Scholar] [CrossRef]
- Fontecha, J.; Calvo, M.V.; Juarez, M.; Gil, A.; Martínez-Vizcaino, V. Milk and Dairy Product Consumption and Cardiovascular Diseases: An Overview of Systematic Reviews and Meta-Analyses. Adv. Nutr. 2019, 10, S164–S189. [Google Scholar] [CrossRef]
- Shingla, K.; Mehta, B. Cholesterol and Its Oxidation Products: Occurrence and Analysis in Milk and Milk Products. Int. J. Health Anim. Sci. Food Saf. 2018, 5, 13–39. [Google Scholar] [CrossRef]
- Sieber, R. Oxidised Cholesterol in Milk and Dairy Products. Int. Dairy J. 2005, 15, 191–206. [Google Scholar] [CrossRef]
- Czerwonka, M.; Białek, A.; Bobrowska-Korczak, B. A Novel Method for the Determination of Squalene, Cholesterol and Their Oxidation Products in Food of Animal Origin by GC-TOF/MS. Int. J. Mol. Sci. 2024, 25, 2807. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Kliem, K.E.; Givens, D.I. Dairy Products in the Food Chain: Their Impact on Health. Annu. Rev. Food Sci. Technol. 2011, 2, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Farkye, N.Y. Cheese Technology. Int. J. Dairy Technol. 2004, 57, 91–98. [Google Scholar] [CrossRef]
- Hur, S.J.; Park, G.B.; Joo, S.T. Formation of Cholesterol Oxidation Products (COPs) in Animal Products. Food Control 2007, 18, 939–947. [Google Scholar] [CrossRef]
- Rodriguez-Estrada, M.T.; Garcia-Llatas, G.; Lagarda, M.J. 7-Ketocholesterol as Marker of Cholesterol Oxidation in Model and Food Systems: When and How. Biochem. Biophys. Res. Commun. 2014, 446, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Pikul, J.; Rudzińska, M.; Teichert, J.; Lasik, A.; Danków, R.; Przybylski, R. Cholesterol Oxidation during Storage of UHT-Treated Bovine and Caprine Milk. Int. Dairy J. 2013, 30, 29–32. [Google Scholar] [CrossRef]
- Lercker, G.; Rodriguez-Estrada, M.T. Cholesterol Oxidation: Presence of 7-Ketocholesterol in Different Food Products. J. Food Compos. Anal. 2000, 13, 625–631. [Google Scholar] [CrossRef]
- Derewiaka, D.; Obiedziński, M. Oznaczenie Zawartości Steroli Oraz Produktów Utleniania Steroli w Wybranych Jogurtach Owocowych. Bromatol. Chem. Toksykol. 2009, 42, 64–568. [Google Scholar]
- Schmarr, H.-G.; Gross, H.B.; Shibamoto, T. Analysis of Polar Cholesterol Oxidation Products: Evaluation of a New Method Involving Transesterification, Solid Phase Extraction, and Gas Chromatography. J. Agric. Food Chem. 1996, 44, 512–517. [Google Scholar] [CrossRef]
- Nielsen, J.H.; Olsen, C.E.; Duedahl, C.; Skibsted, L.H. Isolation and Quantification of Cholesterol Oxides in Dairy Products by Selected Ion Monitoring Mass Spectrometry. J. Dairy Res. 1995, 62, 101–113. [Google Scholar] [CrossRef]
- Sander, B.D.; Addis, P.B.; Park, S.W.; Smith, D.E. Quantification of Cholesterol Oxidation Products in a Variety of Foods. J. Food Prot. 1989, 52, 109–114. [Google Scholar] [CrossRef]
- Białek, A.; Białek, M.; Lepionka, T.; Czerwonka, M.; Czauderna, M. Chemometric Analysis of Fatty Acids Profile of Ripening Chesses. Molecules 2020, 25, 1814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Dong, X.; Huang, Z.; Li, X.; Zhao, Y.; Wang, Y.; Zhu, H.; Fang, A.; Giovannucci, E.L. Cheese Consumption and Multiple Health Outcomes: An Umbrella Review and Updated Meta-Analysis of Prospective Studies. Adv. Nutr. 2023, 14, 1170–1186. [Google Scholar] [CrossRef] [PubMed]
- Luu, W.; Sharpe, L.J.; Capell-Hattam, I.; Gelissen, I.C.; Brown, A.J. Oxysterols: Old Tale, New Twists. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 447–467. [Google Scholar] [CrossRef] [PubMed]
- Brzeska, M.; Szymczyk, K.; Szterk, A. Current Knowledge about Oxysterols: A Review. J. Food Sci. 2016, 81, R2299–R2308. [Google Scholar] [CrossRef]
- Cais-Sokolińska, D.; Rudzińska, M. Short Communication: Cholesterol Oxidation Products in Traditional Buttermilk. J. Dairy Sci. 2018, 101, 3829–3834. [Google Scholar] [CrossRef]

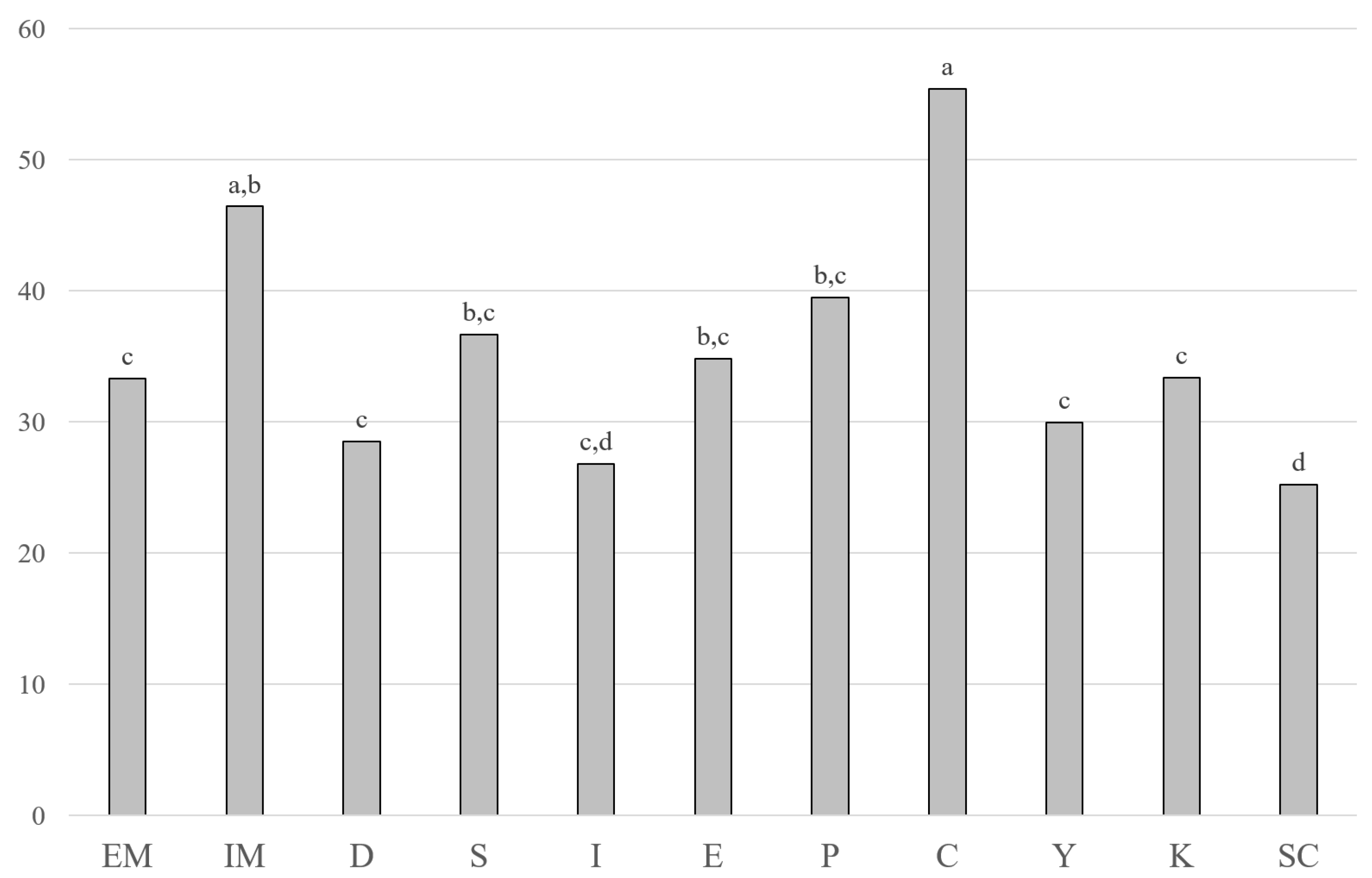
| Group Name | Abbreviation | Number of Samples |
|---|---|---|
| Cheeses with exterior mold (Camembert, Brie) | EM | 12 |
| Cheeses with internal mold (Blue, Gorgonzola) | IM | 12 |
| Dutch-type semi-hard cheeses (Gouda, Edam) | D | 12 |
| Swiss-type semi-hard cheeses (Emmental) | S | 12 |
| Italian-type very hard cheeses (Parmesan) | I | 12 |
| English-type hard cheeses (Cheddar) | E | 12 |
| Processed cheese | P | 12 |
| Curd cheeses/cottage cheese | C | 12 |
| Yoghurts | Y | 12 |
| Kefirs | K | 12 |
| Sour cream | SC | 12 |
| (n = 12 per Group) | (SD) | EM | IM | D | S | I | E | P | C | Y | K | SC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fat | [%] | 27.5 b | 30.2 a,b | 26.8 b | 26.8 b | 28.8 b | 33.5 a | 18.8 c | 9.4 | 3.5 d | 1.8 d | 18.7 c |
| (4.4) | (3.9) | (1.4) | (1.0) | (1.5) | (1.2) | (6.5) | (2.2) | (1.8) | (0.3) | (1.6) | ||
| CH | [mg kg−1] | 579 a,b | 639 a | 506 b,c | 499 c | 623 a | 625 a | 350 d | 308 d | 64 e | 38.4 e | 329 d |
| (42) | (99) | (77) | (48) | (75) | (61) | (64) | (61) | (34) | (9.1) | (49) | ||
| 7K | 3.03 a,b | 5.12 A | 2.52 b,c,A | 3.18 a,b,A | 2.58 b,c,A | 3.72 a,A | 2.57 b,c,A | 1.70 c,d,A | 0.35 e,A | 0.21 e,A | 1.54 d,A | |
| (0.34) | (1.13) | (0.78) | (1.08) | (0.32) | (1.19) | (0.46) | (0.27) | (0.13) | (0.04) | (0.16) | ||
| 7αOH | 1.35 a,A | 1.40 a,B,C | 1.10 a–c,B,C | 1.16 a–c,C | 0.99 a–c,B,C | 1.29 a,b,B,C | 0.64 a–d,B | 0.39 c,d,B | 0.16 d,B | 0.08 d,B | 0.51 b–d,B | |
| (0.16) | (1.16) * | (0.79) * | (0.75) * | (0.35) * | (0.80) * | (0.57) * | (0.41) * | (0.06) | (0.03) * | (0.32) * | ||
| 7βOH | 0.53 d,e | 0.97 a,C | 0.52 d,e,C | 0.73 b,c,C | 0.61 c,d,C | 0.88 a,b,C | 0.53 d,e,B | 0.39 e,B | 0.09 f,B | 0.07 f,B | 0.39 e,B | |
| (0.05) | (0.22) | (0.07) | (0.18) | (0.05) | (0.26) | (0.21) | (0.05) | (0.03) | (0.03) | (0.06) | ||
| 5,6αE | 1.61 a–c,A | 2.19 a,B | 1.43 b–d,B | 2.10 a,b,B | 1.24 c–e,B | 2.18 a,B | 0.67 e,B | 1.08 c–e | nd | nd | 0.84 d,e | |
| (0.78) * | (0.63) | (0.16) | (0.66) | (0.80) * | (0.41) | (0.73) * | (0.13) | nd | nd | (0.40) * | ||
| 5,6βE | 2.42 b | 4.15 a,A | 2.07 b,c,A | 2.71 b,A,B | 2.27 b,c,A | 3.58 a,A | 2.16 b,c,A | 1.50 c,A | 0.35 d,A | 0.21 d,A | 1.42 c,A | |
| (0.26) | (0.91) | (0.15) | (0.90) | (0.28) | (1.61) | (0.40) | (0.15) | (0.12) | (0.07) * | (0.19) | ||
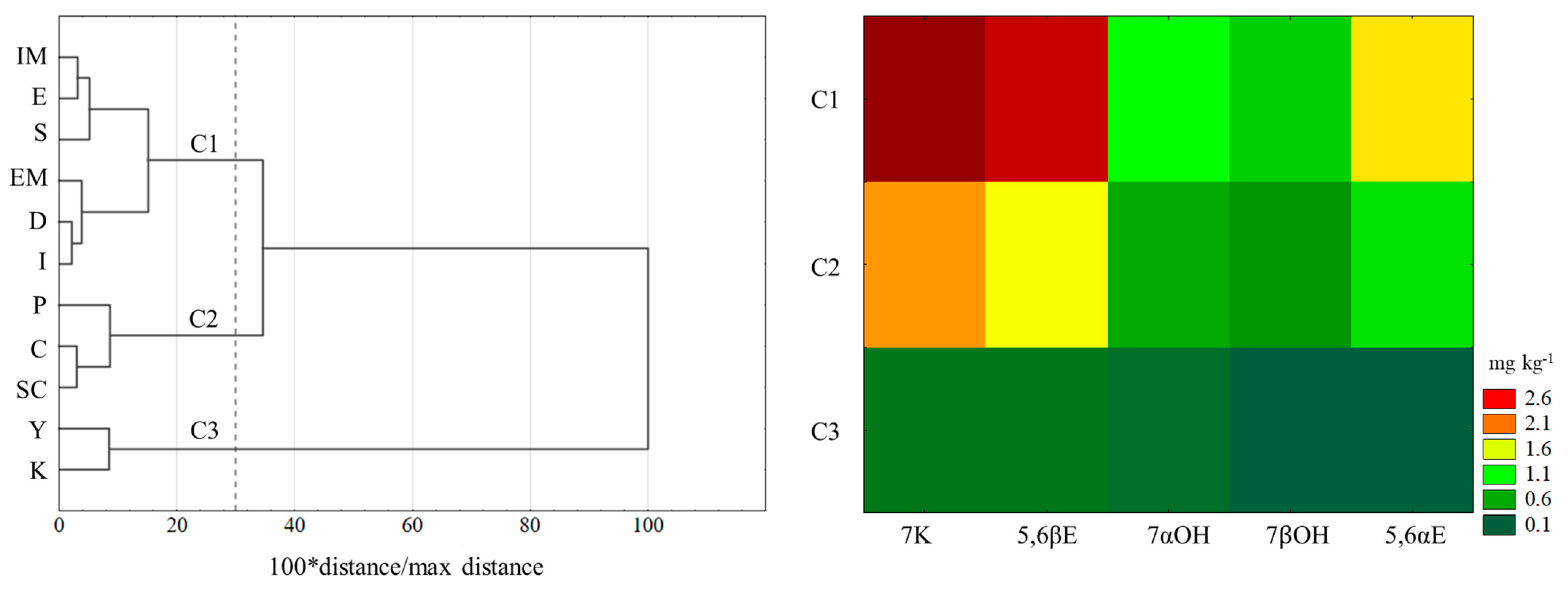
| ∑ COPs | 8.94 c,d | 13.82 a | 7.65 c,d | 9.88 b,c | 7.68 c,d | 11.65 a,b | 6.56 d,e | 5.06 e | 0.94 f | 0.57 f | 4.70 e | |
| (1.43) | (2.46) | (1.66) | (3.34) | (1.28) | (3.48) | (1.36) | (0.82) | (0.30) | (0.11) | (0.50) | ||
| COPs/CH | [%] | 1.54 b,c | 2.26 a | 1.51 b,c | 2.02 a,b | 1.26 c | 1.88 a,b | 1.93 a,b | 1.66 a–c | 1.38 c | 1.55 b,c | 1.46 b,c |
| (0.22) | (0.71) | (0.22) | (0.78) | (0.29) | (0.58) | (0.52) | (0.17) | (0.57) | (0.36) | (0.31) |
| 7K | 7αOH | 7βOH | 5,6αE | 5,6βE | ∑ COPs | |
|---|---|---|---|---|---|---|
| Fat | 0.779 | 0.548 | 0.810 | 0.723 | 0.777 | 0.810 |
| CH | 0.768 | 0.594 | 0.786 | 0.735 | 0.749 | 0.807 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czerwonka, M.; Gielecińska, A.; Białek, A.; Białek, M.; Bobrowska-Korczak, B. Cholesterol and Its Oxidation Derivatives Content in Market Dairy Products. Nutrients 2024, 16, 1371. https://doi.org/10.3390/nu16091371
Czerwonka M, Gielecińska A, Białek A, Białek M, Bobrowska-Korczak B. Cholesterol and Its Oxidation Derivatives Content in Market Dairy Products. Nutrients. 2024; 16(9):1371. https://doi.org/10.3390/nu16091371
Chicago/Turabian StyleCzerwonka, Małgorzata, Anna Gielecińska, Agnieszka Białek, Małgorzata Białek, and Barbara Bobrowska-Korczak. 2024. "Cholesterol and Its Oxidation Derivatives Content in Market Dairy Products" Nutrients 16, no. 9: 1371. https://doi.org/10.3390/nu16091371
APA StyleCzerwonka, M., Gielecińska, A., Białek, A., Białek, M., & Bobrowska-Korczak, B. (2024). Cholesterol and Its Oxidation Derivatives Content in Market Dairy Products. Nutrients, 16(9), 1371. https://doi.org/10.3390/nu16091371








