Synergistic Inhibition of Synbiotic Cultures among Lactobacilli and Plant Extracts against Vaginal Discharge Causing Candida albicans
Abstract
1. Introduction
2. Materials and Methods
2.1. Lactic Acid Bacteria
2.2. Microbial Indicators
2.3. Screening of Anticandidal Activity
2.4. Antibacterial Activity of the Selected Anticandidal Probiotic Isolate
2.5. Determination of Anticandidal Activity
2.6. Preparations of Plant Powder Extracts
2.7. Preparation of the Cell-Free Supernatant of Synbiotic Cultures
2.8. Determination of Minimal Inhibitory Concentration (MIC)
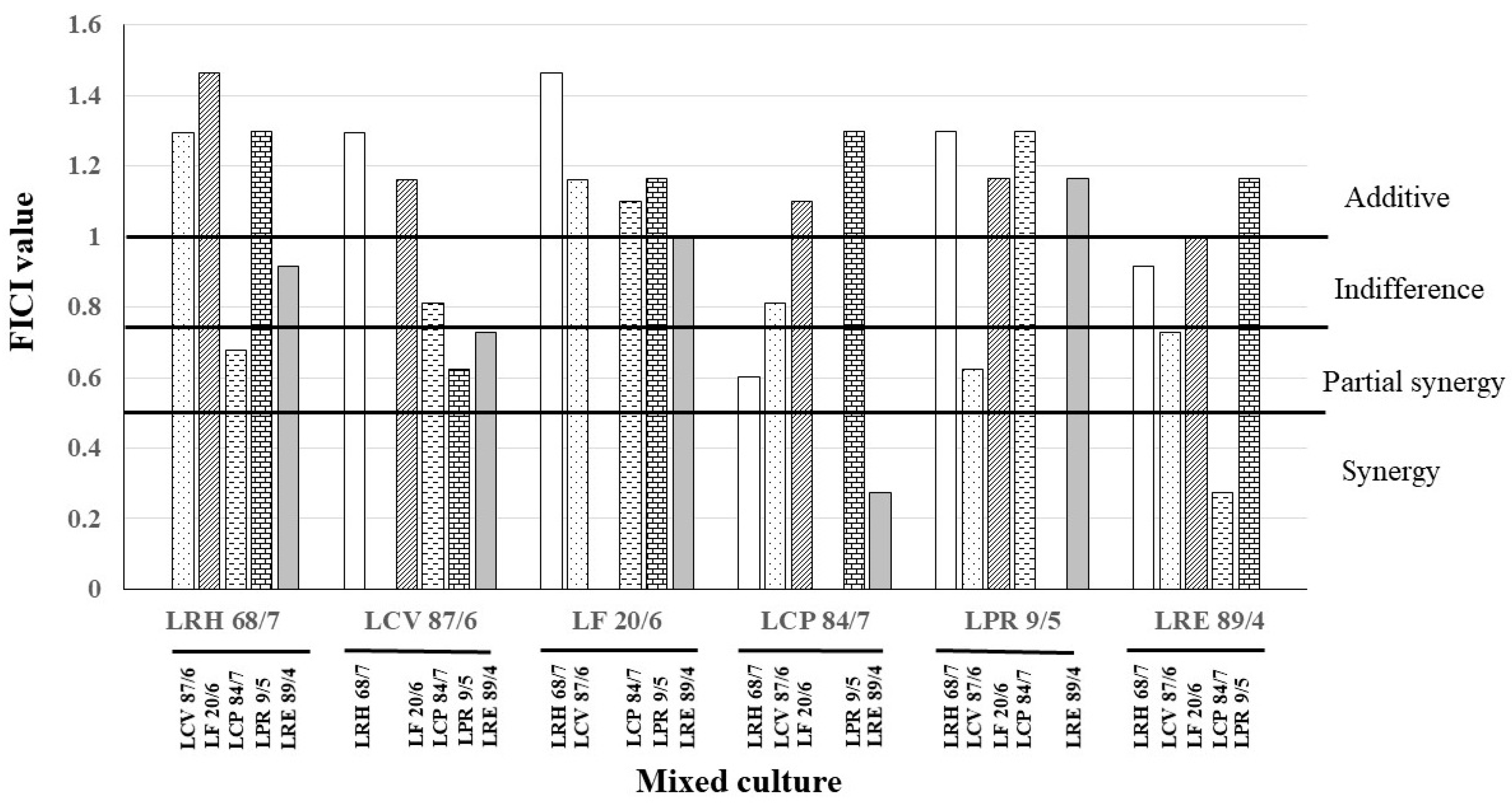
2.9. Fractional Inhibitory Concentration Index of Anticandidal Activity of LAB Culture Combination
- FICI ≤ 0.50 denoting synergism;
- 0.50 <FICI ≤ 0.75 denoting partial synergy;
- 0.75 < FICI ≤ 1 denoting an additive effect;
- 1 < FICI ≤ 4 denoting indifference or no interaction;
- and FICI > 4 denoting antagonism.
2.10. Biofilm Preparation
2.11. Inhibition of Candidal Viability and Biofilm Formation Detected by Fluorescence Microscopy
2.12. Characterization of Inhibitory Substances
2.13. One-Dimensional Polyacrylamide Electrophoresis
2.14. Partial Purification of Potent Bacteriocins
2.15. Statistical Analysis
3. Results
3.1. Screening of Anticandidal Activity
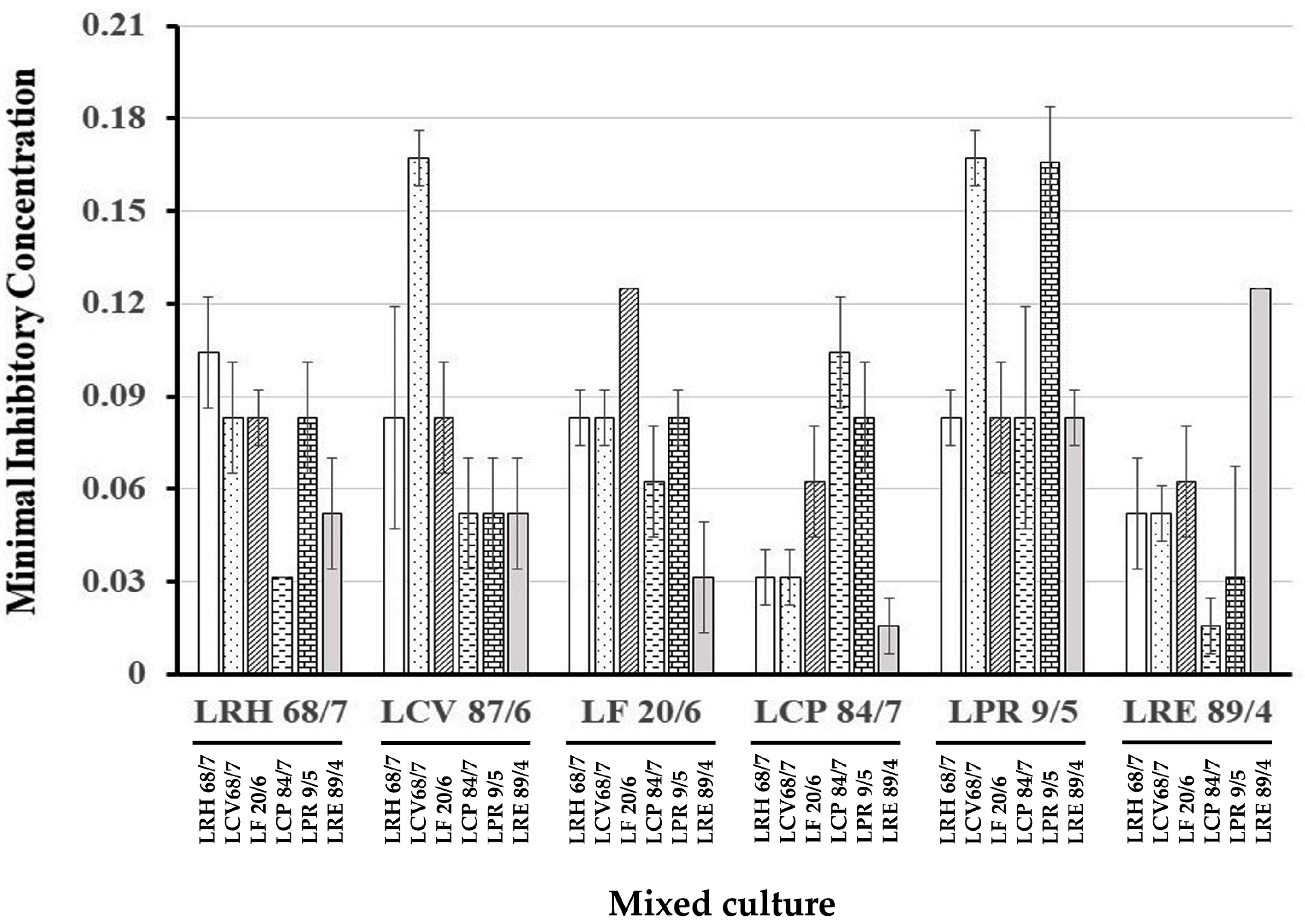
3.2. Determination of Anticandidal Activity
3.3. Anticandidal Activity of the Cell-Free Supernatant of Synbiotic Cultures
3.4. Fractional Inhibitory Concentration Index of Anticandidal Activity of LAB Culture Combination
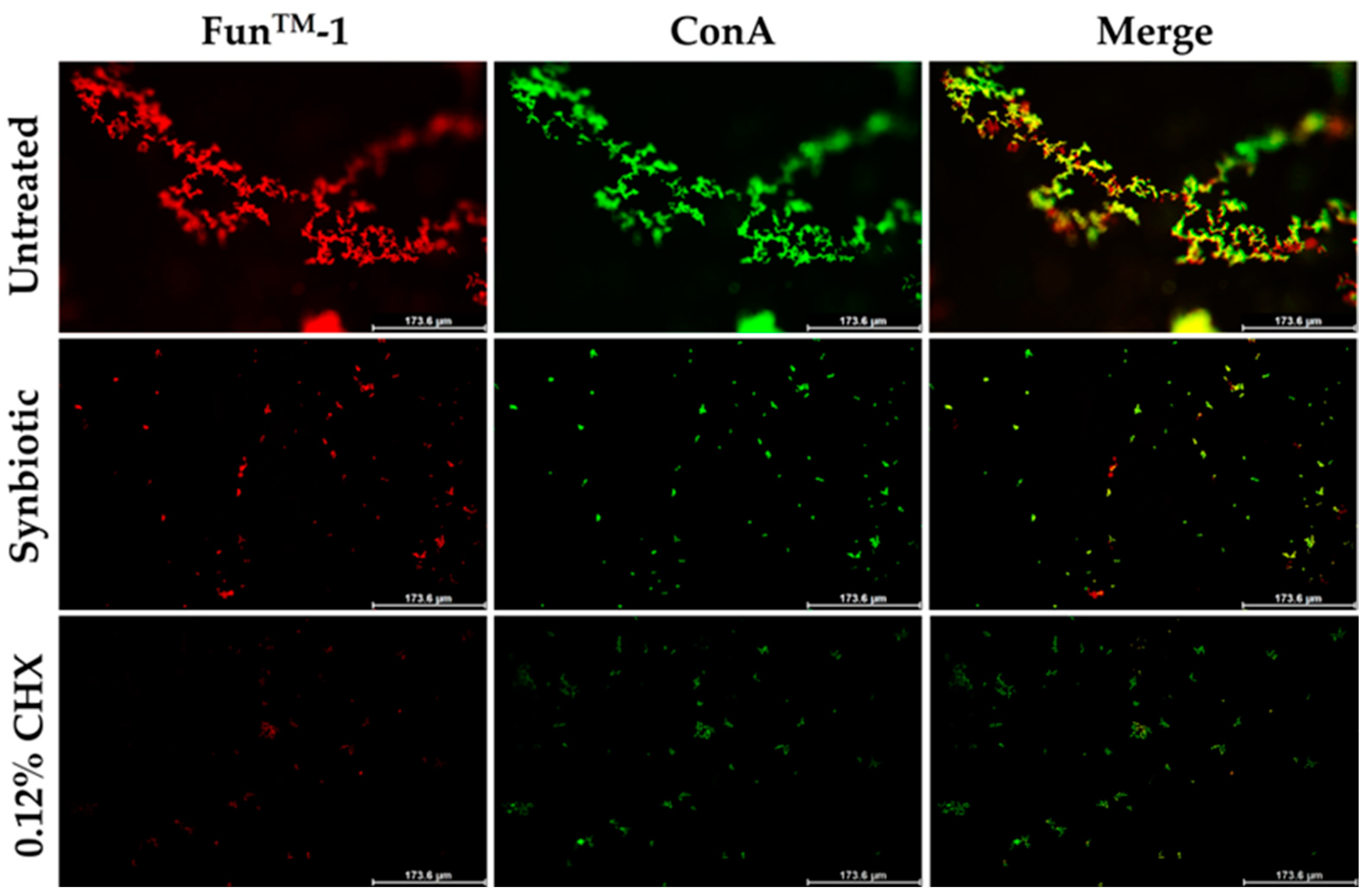
3.5. Inhibition of Candidal Viability and Biofilm Formation Detected by Fluorescence Microscopy
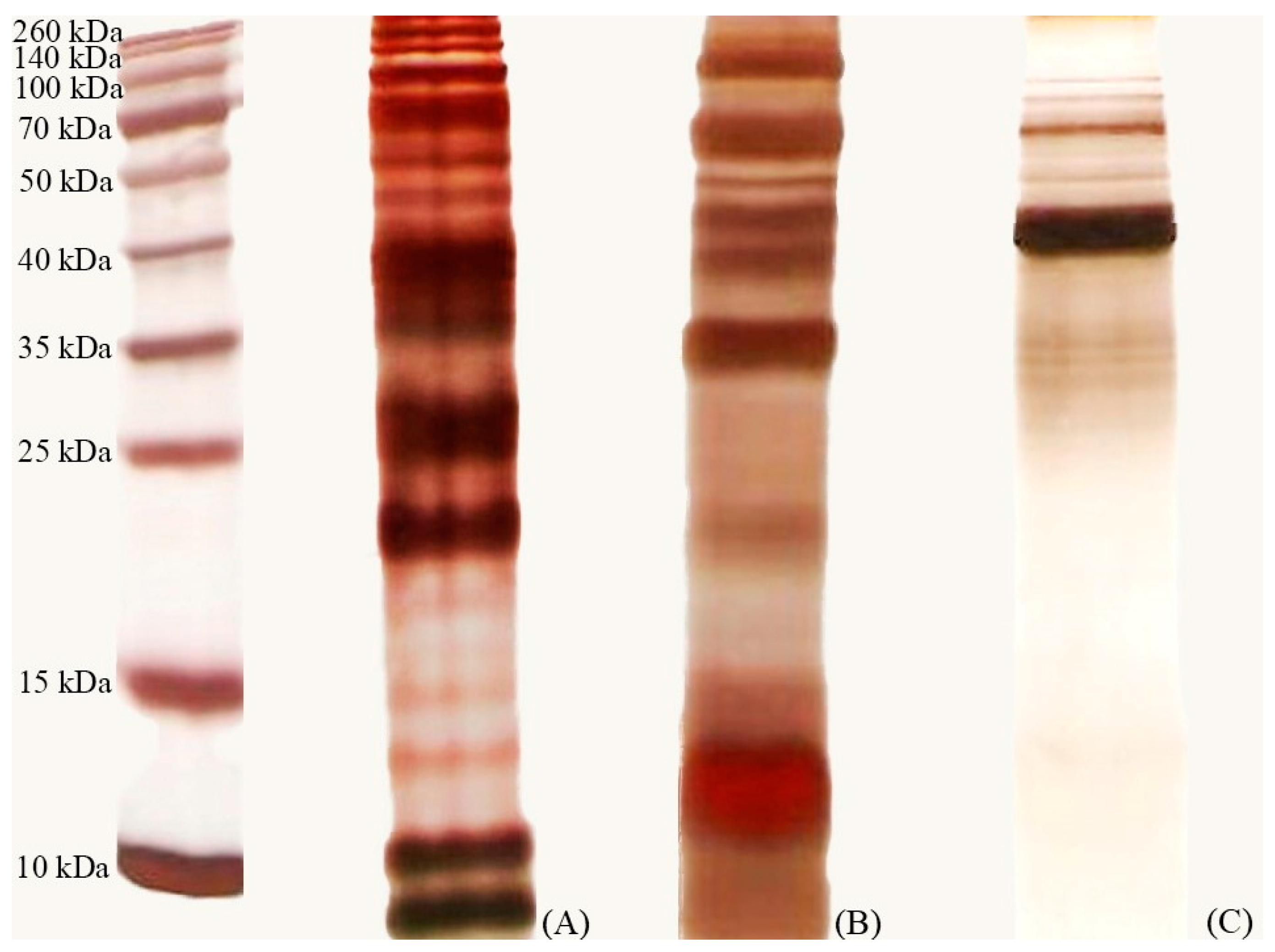
3.6. Characterization of Inhibitory Substances
3.7. One-Dimensional Polyacrylamide Electrophoresis
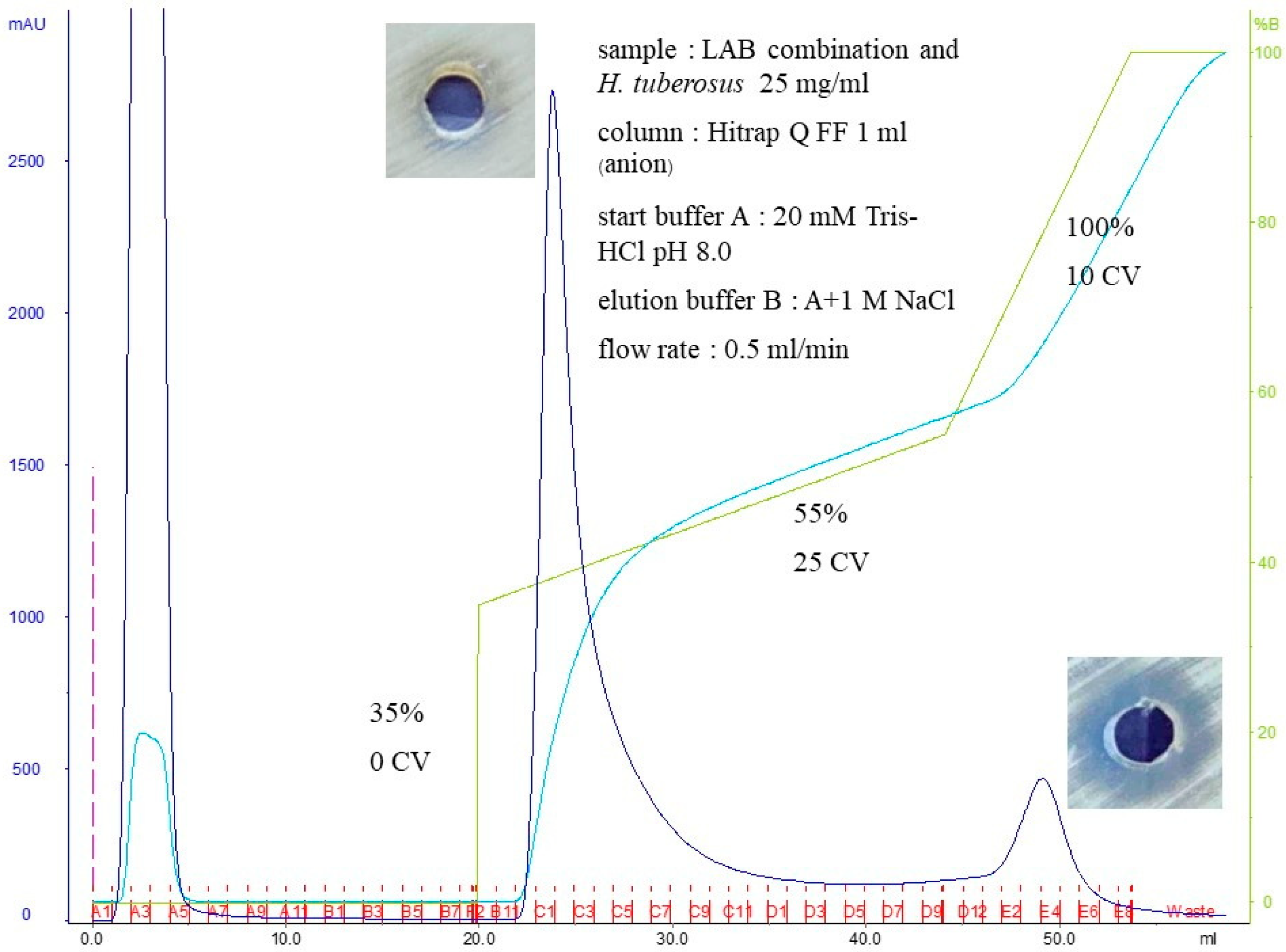
3.8. Purification of Potent Bacteriocins
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Oliveira Santos, G.C.; Vasconcelos, C.C.; Lopes, A.J.O.; de Sousa Cartágenes, M.d.S.; Filho, A.K.D.B.; do Nascimento, F.R.F.; Ramos, R.M.; Pires, E.R.R.B.; de Andrade, M.S.; Rocha, F.M.G.; et al. Candida infections and therapeutic strategies: Mechanisms of action for traditional and alternative agents. Front. Microbiol. 2018, 9, 1351. [Google Scholar] [CrossRef] [PubMed]
- Mårdh, P.A.; Rodrigues, A.G.; Genç, M.; Novikova, N.; Martinez-de-Oliveira, J.; Guaschino, S. Facts and myths on recurrent vulvovaginal candidosis—A review on epidemiology, clinical manifestations, diagnosis, pathogenesis and therapy. Int. J. STD AIDS 2002, 13, 522–539. [Google Scholar] [CrossRef]
- Pai, V.; Ganavalli, A.; Kikkeri, N.N. Antifungal resistance in dermatology. Indian J. Dermatol. 2018, 63, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Panizo, M.M.; Reviákina, V.; Dolande, M.; Selgrad, S. Candida spp. in vitro susceptibility profile to four antifungal agents. Resistance surveillance study in Venezuelan strains. Med. Mycol. 2009, 47, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Seddighi, N.S.; Salari, S.; Izadi, A.R. Evaluation of antifungal effect of iron-oxide nanoparticles against different Candida species. IET Nanobiotechnol. 2017, 11, 883–888. [Google Scholar] [CrossRef]
- Strus, M.; Kucharska, A.; Kukla, G.; Brzychczy-Włoch, M.; Maresz, K.; Heczko, P.B. The in vitro activity of vaginal Lactobacillus with probiotic properties against Candida. Infect. Dis. Obstet. Gynecol. 2005, 13, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Larsen, B.; White, S. Antifungal effect of hydrogen peroxide on catalase-producing strains of Candida spp. Infect. Dis. Obstet. Gynecol. 1995, 3, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Munoz, R.; Dongari-Bagtzoglou, A. Anticandidal activities by Lactobacillus species: An update on mechanisms of action. Front. Oral Health 2021, 2, 689382. [Google Scholar] [CrossRef]
- Yang, S.C.; Lin, C.H.; Sung, C.T.; Fang, J.Y. Antibacterial activities of bacteriocins: Application in foods and pharmaceuticals. Front. Microbiol. 2014, 5, 241. [Google Scholar] [CrossRef]
- Cleveland, J.; Montville, T.J.; Nes, I.F.; Chikindas, M.L. Bacteriocins: Safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 2001, 71, 1–20. [Google Scholar] [CrossRef]
- Hefzy, E.M.; Khalil, M.A.F.; Amin, A.A.I.; Ashour, H.M.; Abdelaliem, Y.F. Bacteriocin-like inhibitory substances from probiotics as therapeutic agents for Candida vulvovaginitis. Antibiotics 2021, 10, 306. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, A.; Abriouel, H.; López, R.L.; Ben Omar, N. Bacteriocin-based strategies for food biopreservation. Int. J. Food Microbiol. 2007, 120, 51–70. [Google Scholar] [CrossRef]
- Prudêncio, C.V.; Dos Santos, M.T.; Vanetti, M.C. Strategies for the use of bacteriocins in Gram-negative bacteria: Relevance in food microbiology. J. Food Sci. Technol. 2015, 52, 5408–5417. [Google Scholar] [CrossRef]
- Barbosa, A.A.T.; de Melo, M.R.; da Silva, C.M.R.; Jain, S.; Dolabella, S.S. Nisin resistance in Gram-positive bacteria and approaches to circumvent resistance for successful therapeutic use. Crit. Rev. Microbiol. 2021, 47, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Sookkhee, S.; Kumsang, Y.; Sathian, B. Bacteriocin harvested from the synbiotic culture of selected lactic acid bacteria with various vegetables, cereals, fruits, medicinal and tuber plants: Inhibition of Vibrio parahaemolyticus. CMU J. Nat. Sci. 2022, 21, e2022050. [Google Scholar] [CrossRef]
- Sharma, A.; Srivastava, S. Anti-Candida activity of two-peptide bacteriocins, plantaricins (Pln E/F and J/K) and their mode of action. Fungal Biol. 2014, 118, 264–275. [Google Scholar] [CrossRef]
- Santos, C.M.A.; Pires, M.C.V.; Leão, T.L.; Silva, A.K.S.; Miranda, L.S.; Martins, F.S.; Silva, A.M.; Nicoli, J.R. Anti-inflammatory effect of two Lactobacillus strains during infection with Gardnerella vaginalis and Candida albicans in a HeLa cell culture model. Microbiology 2018, 164, 349–358. [Google Scholar] [CrossRef]
- Graham, C.E.; Cruz, M.R.; Garsin, D.A.; Lorenz, M.C. Enterococcus faecalis bacteriocin EntV inhibits hyphal morphogenesis, biofilm formation, and virulence of Candida albicans. Proc. Natl. Acad. Sci. USA 2017, 114, 4507–4512. [Google Scholar] [CrossRef] [PubMed]
- Sookkhee, S.; Chulasiri, M.; Prachyabrued, W. Lactic acid bacteria from healthy oral cavity of Thai volunteers: Inhibition of oral pathogens. J. Appl. Microbiol. 2001, 90, 172–179. [Google Scholar] [CrossRef]
- Chew, S.Y.; Cheah, Y.K.; Seow, H.F.; Sandai, D.; Than, L.T. Probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 exhibit strong antifungal effects against vulvovaginal candidiasis-causing Candida glabrata isolates. J. Appl. Microbiol. 2015, 118, 1180–1190. [Google Scholar] [CrossRef]
- Jørgensen, M.R.; Kragelund, C.; Jensen, P.; Keller, M.K.; Twetman, S. Probiotic Lactobacillus reuteri has antifungal effects on oral Candida species in vitro. J. Oral Microbiol. 2017, 9, 1274582. [Google Scholar] [CrossRef] [PubMed]
- Vilela, S.F.; Barbosa, J.O.; Rossoni, R.D.; Santos, J.D.; Prata, M.C.; Anbinder, A.L.; Jorge, A.O.; Junqueira, J.C. Lactobacillus acidophilus ATCC 4356 inhibits biofilm formation by C. albicans and attenuates the experimental candidiasis in Galleria mellonella. Virulence 2015, 6, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Pyar, H.; Kok, P. Confirmation of the identity of Lactobacillus species using carbohydrate fermentation test (API 50 CHL) identification system. J. Appl. Sci. 2019, 19, 797–802. [Google Scholar] [CrossRef]
- Singhavejsakul, P.; Niamsup, P.; Malairungsakul, N.; Chaisaen, Y.; Pooriwarangkakul, P.; Nimlamool, W.; Khamnoi, P.; Sastraruji, T.; Sookkhee, S. Clavulanic acid—Susceptible peptide spectra tested with agar disk diffusion and E-test of urinary tract infected Escherichia coli isolated from the community-acquired infection. CMU J. Nat. Sci. 2022, 21, e2022035. [Google Scholar] [CrossRef]
- Lananta, S.; Siriratanagool, P.; Sommanawan, N.; Lerttrakarnnon, P.; Boonchuay, S.; Jirawattanapong, S.; Manochomphu, S.; Sastraruji, T.; Siriwoot, S. Different responses of ESBL indicative peptide spectra to various β- lactam exposures among community acquired urinary tract infected Escherichia coli by using the MALDI-TOF technique. CMU J. Nat. Sci. 2021, 20, e2021095. [Google Scholar] [CrossRef]
- Sookkhee, S.; Manonuek, S.; Apichartpiyakul, C.; Sakonwasun, C. Antibacterial, sperm immobilization and spermicidal activities of bacteriocins produced by vaginal lactobacilli. CMU J. Nat. Sci. 2022, 21, e2022015. [Google Scholar] [CrossRef]
- Bonev, B.; Hooper, J.; Parisot, J. Principles of assessing bacterial susceptibility to antibiotics using the agar diffusion method. J. Antimicrob. Chemother. 2008, 61, 1295–1301. [Google Scholar] [CrossRef]
- Clinical Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; CLSI: Wayne, PA, USA, 2020; Available online: https://www.nih.org.pk/wp-content/uploads/2021/02/CLSI-2020.pdf (accessed on 30 March 2022).
- Sookkhee, S.; Sakonwasun, C.; Mungkornasawakul, P.; Khamnoi, P.; Wikan, N.; Nimlamool, W. Synergistic effects of some methoxyflavones extracted from rhizome of Kaempferia parviflora combined with gentamicin against carbapenem-resistant strains of Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii. Plants 2022, 11, 3128. [Google Scholar] [CrossRef]
- Santos, R.C.; Kushima, H.; Rodrigues, C.M.; Sannomiya, M.; Rocha, L.R.; Bauab, T.M.; Tamashiro, J.; Vilegas, W.; Hiruma-Lima, C.A. Byrsonima intermedia A. Juss.: Gastric and duodenal anti-ulcer, antimicrobial and antidiarrheal effects in experimental rodent models. J. Ethnopharmacol. 2012, 140, 203–212. [Google Scholar] [CrossRef]
- Chanpa, P.; Owittayakul, D.; Wanachantararak, P.; Chaiyana, W.; Sookkhee, S. Formulation of coconut oil mouthwash with mixed emulsifier and its growth inhibition of Candida albicans biofilms. Nat. Life Sci. Comm. 2023, 22, 1–16. [Google Scholar] [CrossRef]
- Montelongo-Jauregui, D.; Saville Stephen, P.; Lopez-Ribot Jose, L. Contributions of Candida albicans dimorphism, adhesive interactions, and extracellular matrix to the formation of dual-species biofilms with Streptococcus gordonii. mBio 2019, 10, e01179-01119. [Google Scholar] [CrossRef] [PubMed]
- Achkar, J.M.; Fries, B.C. Candida infections of the genitourinary tract. Clin. Microbiol. Rev. 2010, 23, 253–273. [Google Scholar] [CrossRef] [PubMed]
- Petrova, M.I.; Lievens, E.; Malik, S.; Imholz, N.; Lebeer, S. Lactobacillus species as biomarkers and agents that can promote various aspects of vaginal health. Front. Physiol. 2015, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Madhivanan, P.; Alleyn, H.N.; Raphael, E.; Krupp, K.; Ravi, K.; Nebhrajani, R.; Arun, A.; Reingold, A.L.; Riley, L.W.; Klausner, J.D. Identification of culturable vaginal Lactobacillus species among reproductive age women in Mysore, India. J. Med. Microbiol. 2015, 64, 636–641. [Google Scholar] [CrossRef] [PubMed]
- De Seta, F.; Parazzini, F.; De Leo, R.; Banco, R.; Maso, G.P.; De Santo, D.; Sartore, A.; Stabile, G.; Inglese, S.; Tonon, M.; et al. Lactobacillus plantarum P17630 for preventing Candida vaginitis recurrence: A retrospective comparative study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 182, 136–139. [Google Scholar] [CrossRef]
- Tulasidas, S.; Rao, P.; Bhat, S.; Manipura, R. A study on biofilm production and antifungal drug resistance among Candida species from vulvovaginal and bloodstream infections. Infect. Drug Resist. 2018, 11, 2443–2448. [Google Scholar] [CrossRef]
- Parolin, C.; Marangoni, A.; Laghi, L.; Foschi, C.; Ñahui Palomino, R.A.; Calonghi, N.; Cevenini, R.; Vitali, B. Isolation of vaginal lactobacilli and characterization of anti-Candida activity. PLoS ONE 2015, 10, e0131220. [Google Scholar] [CrossRef] [PubMed]
- Antonio, M.A.; Hawes, S.E.; Hillier, S.L. The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. J. Infect. Dis. 1999, 180, 1950–1956. [Google Scholar] [CrossRef]
- Borges, S.; Silva, J.; Teixeira, P. The role of lactobacilli and probiotics in maintaining vaginal health. Arch. Gynecol. Obstet. 2014, 289, 479–489. [Google Scholar] [CrossRef]
- O’Hanlon, D.E.; Moench, T.R.; Cone, R.A. Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PLoS ONE 2013, 8, e80074. [Google Scholar] [CrossRef]
- Aroutcheva, A.; Gariti, D.; Simon, M.; Shott, S.; Faro, J.; Simoes, J.A.; Gurguis, A.; Faro, S. Defense factors of vaginal lactobacilli. Am. J. Obstet. Gynecol. 2001, 185, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Dover, S.E.; Aroutcheva, A.A.; Faro, S.; Chikindas, M.L. Natural antimicrobials and their role in vaginal health: A short review. Int. J. Probiotics Prebiotics 2008, 3, 219–230. [Google Scholar] [PubMed]
- Köhler, G.A.; Assefa, S.; Reid, G. Probiotic interference of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 with the opportunistic fungal pathogen Candida albicans. Infect. Dis. Obstet. Gynecol. 2012, 2012, 636474. [Google Scholar] [CrossRef] [PubMed]
- Parolin, C.; Croatti, V.; Laghi, L.; Giordani, B.; Tondi, M.R.; De Gregorio, P.R.; Foschi, C.; Vitali, B. Lactobacillus biofilms influence anti-Candida activity. Front. Microbiol. 2021, 12, 750368. [Google Scholar] [CrossRef] [PubMed]
- Madhu, A.N.; Prapulla, S.G. Evaluation and functional characterization of a biosurfactant produced by Lactobacillus plantarum CFR 2194. Appl. Biochem. Biotechnol. 2014, 172, 1777–1789. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Barragán, A.; Caballero-Guerrero, B.; Martín, V.; Ruiz-Barba, J.L.; Rodríguez, J.M. Purification and genetic characterization of gassericin E, a novel co-culture inducible bacteriocin from Lactobacillus gasseri EV1461 isolated from the vagina of a healthy woman. BMC Microbiol. 2016, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Todorov, S.D. Bacteriocins from Lactobacillus plantarum—Production, genetic organization and mode of action. Braz. J. Microbiol. 2009, 40, 209–221. [Google Scholar] [CrossRef]
- Man, L.L.; Xiang, D.J. Characterization of a broad spectrum bacteriocin produced by Lactobacillus plantarum MXG-68 from Inner Mongolia traditional fermented koumiss. Folia Microbiol. (Praha) 2019, 64, 821–834. [Google Scholar] [CrossRef]
- Banerjee, S.P.; Dora, K.C.; Chowdhury, S. Detection, partial purification and characterization of bacteriocin produced by Lactobacillus brevis FPTLB3 isolated from freshwater fish: Bacteriocin from Lb. brevis FPTLB3. J. Food Sci. Technol. 2013, 50, 17–25. [Google Scholar] [CrossRef]
| LAB Species | Numbers of Isolates |
|---|---|
| Bifidobacteria, Lactobacilli, Lactococci, and Pediococci: | |
| Bifidobacterium lactis | 6 |
| Bifidobacterium longum | 12 |
| Lactobacillus acidophilus | 27 |
| Lactobacillus crispatus | 27 |
| Lactobacillus johnsonii | 15 |
| Lactobacillus delbrueckii subsp. delbrueckii | 33 |
| Lactobacillus acetotolerans | 4 |
| Lactobacillus helveticus | 21 |
| Lactobacillus jensenii | 9 |
| Lactococcus lactis | 33 |
| Pediococcus acidilactici | 36 |
| Homofermentative LABs: | |
| Companilactobacillus kimchi * | 1 |
| Fructilactobacillus fructivorans * | 1 |
| Lacticaseibacillus casei * | 24 |
| Lacticaseibacillus paracasei subsp. paracasei * | 90 |
| Lacticaseibacillus rhamnosus * | 27 |
| Latilactobacillus sakei subsp. sakei * | 15 |
| Latilactobacillus curvatus * | 21 |
| Lactiplantibacillus plantarum subsp. plantarum * | 90 |
| Lentilactobacillus buchneri * | 5 |
| Lentilactobacillus kefiri * | 1 |
| Levilactobacillus brevis * | 3 |
| Levilactobacillus cerevisiae * | 1 |
| Liquorilactobacillus capillatus * | 3 |
| Heterofermentative LABs: | |
| Limosilactobacillus fermentum * | 54 |
| Limosilactobacillus reuteri * | 39 |
| Secundilactobacillus kimchicus * | 1 |
| Secundilactobacillus oryzae * | 1 |
| No. | Scientific Names | Thai Names | General Names |
|---|---|---|---|
| Cereals | |||
| 1 | Bruguiera cylindrica | Ma-led-tua-kao | Navy bean |
| 2 | Cajanus cajan | Tua-rae | Pigeon pea |
| 3 | Glycine max | Tua-lueng | Soybean |
| 4 | Phaseolus vulgaris | Tua-daeng | Red bean |
| 5 | Sesamum indicum | Nga-dum | Black sesame |
| 6 | Zea mays | Kao-pod | Sweet corn |
| Vegetables | |||
| 7 | Brassica oleracea | Broc-co-li | Broccoli |
| 8 | Cucurbita moschata | Fag-thong | Pumpkin |
| 9 | Cynara scolymus | Ar-ti-choke | Globe artichoke |
| 10 | Sechium edule | Cha-yo-te | Chayote |
| 11 | Solanum melongena var. serpentinum | Ma-kue-maung | Eggplant |
| Fruits | |||
| 12 | Ananas comosus | Sup-pa-rod | Pineapple |
| 13 | Averrhoa carambola | Ma-fueng | Star fruit |
| 14 | Citrus maxima | Som-o-thong-dee | Pummelo |
| 15 | Hylocereus undatus | Kaew-mung-korn | Dragon fruit |
| 16 | Malus domestica | Ap-ple | Gala apple |
| 17 | Passiflora edulis | Saw-wa-rod | Passion fruit |
| 18 | Prunus salicina | Luk-nai-daeng | Japanese plum |
| 19 | Pyrus pyrifolia | Sa-lee-hi-mah | Nashi pear |
| 20 | Vitis labrusca | A-ngoon-moung | Purple table grapes |
| Medicinal plants | |||
| 21 | Angelica sinensis | Tung-kui | Dong quai |
| 22 | Kaempferia parviflora | Kra-chai-dum | Black ginger |
| Tuber plants | |||
| 23 | Arctium lappa | Som-jak-ka-pat | Greater burdock |
| 24 | Daucus carota | Car-rot | Carrot |
| 25 | Helianthus tuberosus | Kaen-ta-wan | Jerusalem artichoke |
| 26 | Raphanus sativus var. longipinnatus | Hua-chi-tao | Chinese radish |
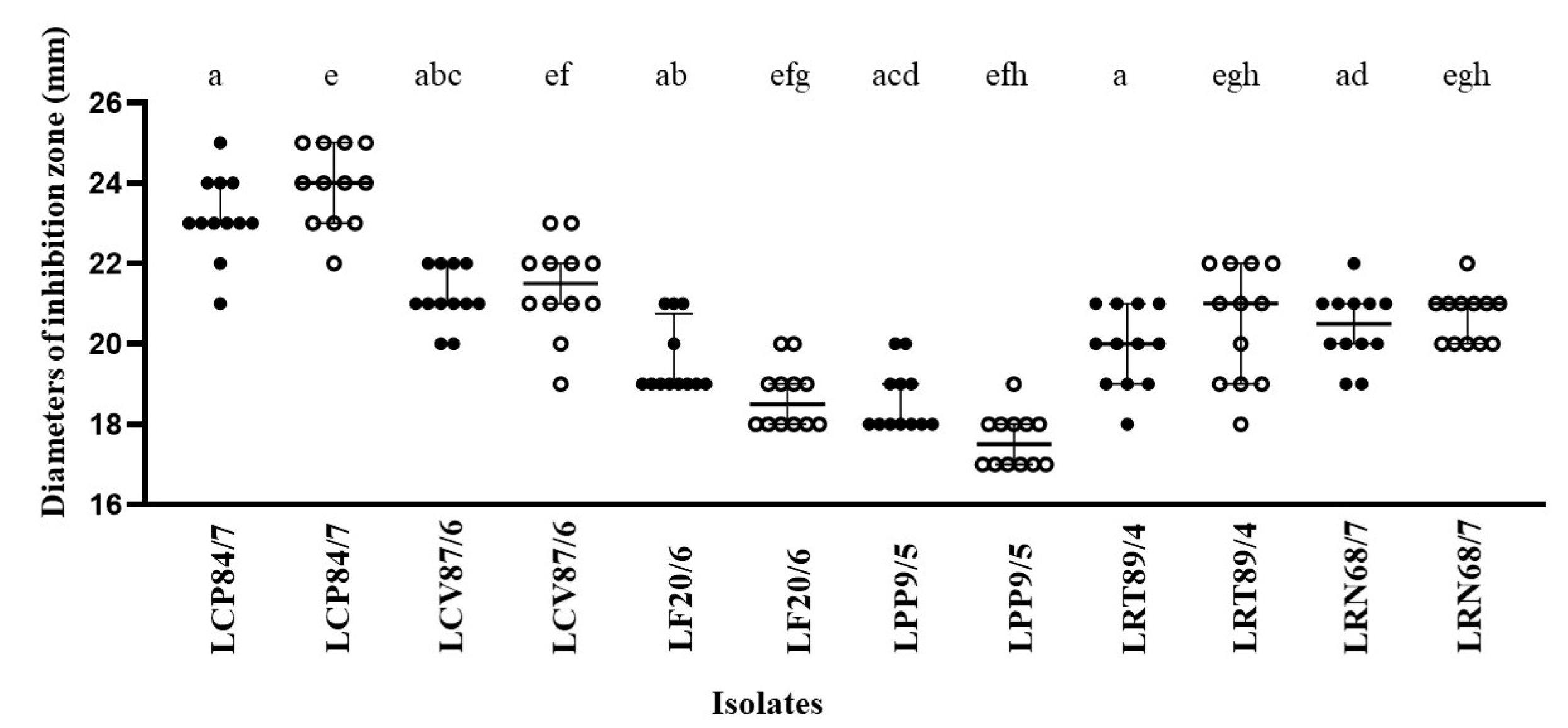
| LAB Species | S. aureus ATCC 25923 | S. lutea ATCC 9341 | E. coli ATCC 29213 | B. subtilis ATCC 6633 |
|---|---|---|---|---|
| L. acetotolerans 601 | 24.00 ± 0.50 * | 20.00 ± 1.00 | 20.00 ± 0.87 | 22.50 ± 1.50 |
| L. acidophilus 18/1 | 22.50 ± 0.67 * | 22.00 ± 1.67 | 18.50 ± 0.33 | 21.50 ± 1.25 |
| L. buchneri D09 | 22.00 ± 0.50 | 21.50 ± 0.50 | 24.00 ± 0.50 * | 28.50 ± 0.67 * |
| L. buchneri D33 | 22.00 ± 1.50 | 25.50 ± 0.87 * | 22.50 ± 0.67 | 25.50 ± 1.50 * |
| L. crispatus 23/1 | 20.00 ± 0.87 | 18.50 ± 0.67 | 22.00 ± 0.50 | 20.00 ± 1.50 |
| L. crispatus 27/9 | 22.00 ± 0.50 | 25.50 ± 0.87 * | 20.50 ± 0.76 | 28.50 ± 0.67 * |
| L. crispatus 33/9 | 22.00+1.67 | 21.50 ± 1.25 | 20.00 ± 1.00 | 24.00 ± 0.29 * |
| L. crispatus 84/7 # | 25.00 ± 0.87 * | 31.50 ± 0.29 * | 24.50 ± 0.29 * | 29.50 ± 0.76 * |
| L. crispatus 55/9 | 18.50 ± 0.67 | 21.00 ± 0.87 | 23.50 ± 1.25 * | 28.50 ± 0.67 * |
| L. crispatus 22/2 | 19.50 ± 0.29 | 17.50 ± 0.29 | 25.50 ± 0.87 * | 20.50 ± 0.76 |
| L. curvatus 74/6 | 22.50 ± 0.67 * | 19.00 ± 0.29 | 18.50 ± 0.67 | 21.00 ± 0.29 |
| L. curvatus 87/6 # | 25.00 ± 0.29 * | 30.50 ± 0.87 * | 20.50 ± 0.76 | 28.00 ± 0.87 * |
| L. curvatus 92/6 | 25.50 ± 0.67 * | 21.50 ± 0.50 | 25.50 ± 0.87 * | 19.50 ± 0.29 |
| L. delbrueckii 77/2 | 22.50 ± 0.67 * | 22.00 ± 0.29 | 18.50 ± 0.67 | 22.00 ± 1.67 |
| L. fermentum 20/6 # | 26.00 ± 1.00 * | 29.50 ± 0.76 * | 22.00 ± 1.00 | 29.50 ± 1.00 * |
| L. fermentum 32/6 | 23.50 ± 1.25 * | 19.00 ± 0.29 | 18.50 ± 0.67 | 18.50 ± 0.33 |
| L. fermentum 44/6 | 22.50 ± 0.67 * | 23.50 ± 0.67 | 24.00 ± 0.50 * | 22.00 ± 1.67 |
| L. fermentum 48/6 | 19.50 ± 0.29 | 22.50 ± 0.67 | 21.50 ± 0.50 | 22.50 ± 0.67 |
| L. jensenii 881 | 23.00 ± 0.50 * | 19.00 ± 0.29 | 21.00 ± 0.87 | 20.00 ± 1.50 |
| L. lactis H57 | 22.50 ± 0.67 * | 22.00 ± 0.50 | 24.00 ± 0.29 * | 22.00 ± 0.29 |
| L. paracasei 6/5 | 22.00 ± 0.29 | 20.00 ± 1.00 | 21.50 ± 0.50 | 22.50 ± 1.50 |
| L. paracasei 9/5 # | 25.00 ± 0.76 * | 32.00 ± 0.76 * | 19.50 ± 0.29 | 29.00 ± 0.87 * |
| L. paracasei 18/5 | 18.50 ± 0.67 | 22.00 ± 0.50 | 22.00 ± 1.67 | 19.00 ± 0.29 |
| L. paracasei 41/5 | 21.50 ± 0.50 | 22.50 ± 0.67 | 21.00 ± 0.29 | 22.50 ± 0.67 |
| L. plantarum 8/8 | 22.00 ± 0.67 | 22.50 ± 1.50 | 24.00 ± 0.29 * | 28.50 ± 0.67 * |
| L. plantarum 56/8 | 21.00 ± 0.29 | 22.50 ± 0.67 | 23.00 ± 0.50 * | 22.50 ± 1.50 |
| L. plantarum 86/8 | 18.50 ± 0.67 | 22.50 ± 1.50 | 21.00 ± 2.00 | 20.00 ± 1.50 |
| L. plantarum 90/8 | 25.50 ± 1.50 * | 23.50 ± 1.25 | 19.00 ± 0.29 | 24.00 ± 0.29 * |
| L. reuteri 22/4 | 19.00 ± 0.29 | 20.00 ± 1.50 | 23.00 ± 0.50 * | 23.50 ± 1.25 * |
| L. reuteri 66/4 | 21.00 ± 2.00 | 22.50 ± 1.50 | 25.50 ± 0.87 * | 29.00 ± 0.29 * |
| L. reuteri 71/4 | 18.50 ± 0.67 | 22.50 ± 0.67 | 22.00 ± 0.50 | 22.00 ± 0.29 |
| L. reuteri 89/4 # | 26.00 ± 0.76 * | 31.50 ± 0.87 * | 27.00 ± 0.29 * | 29.50 ± 0.76 * |
| L. reuteri B16 | 21.50 ± 1.25 | 21.50 ± 0.50 | 21.00 ± 2.00 | 20.00 ± 1.50 |
| L. rhamnosus 22/5 | 19.00 ± 0.29 | 22.00 ± 0.29 | 25.50 ± 0.87 * | 23.50 ± 1.25 * |
| L. rhamnosus 61/7 | 23.50 ± 1.25 * | 21.00 ± 0.29 | 19.50 ± 0.29 | 20.00 ± 1.50 |
| L. rhamnosus 68/7 # | 24.50 ± 0.76 * | 31.00 ± 0.29 * | 21.00 ± 0.76 | 23.50 ± 0.76 * |
| L. rhamnosus 83/7 | 20.00 ± 1.00 | 18.50 ± 0.33 | 22.50 ± 0.67 | 24.00 ± 0.29 * |
| L. sakei 26/7 | 22.50 ± 1.50 * | 21.50 ± 0.50 | 22.00 ± 0.29 | 25.50 ± 0.87 * |
| L. sakei 29/7 | 23.00 ± 0.50 * | 20.00 ± 1.50 | 23.50 ± 1.25 * | 22.00 ± 0.50 |
| P. acidilactici G02 | 18.50 ± 0.67 | 25.50 ± 0.87 * | 18.50 ± 0.33 | 20.00 ± 0.87 |
| P. acidilactici G53 | 21.50 ± 1.25 | 19.50 ± 0.29 | 22.00 ± 1.67 | 20.00 ± 1.50 |
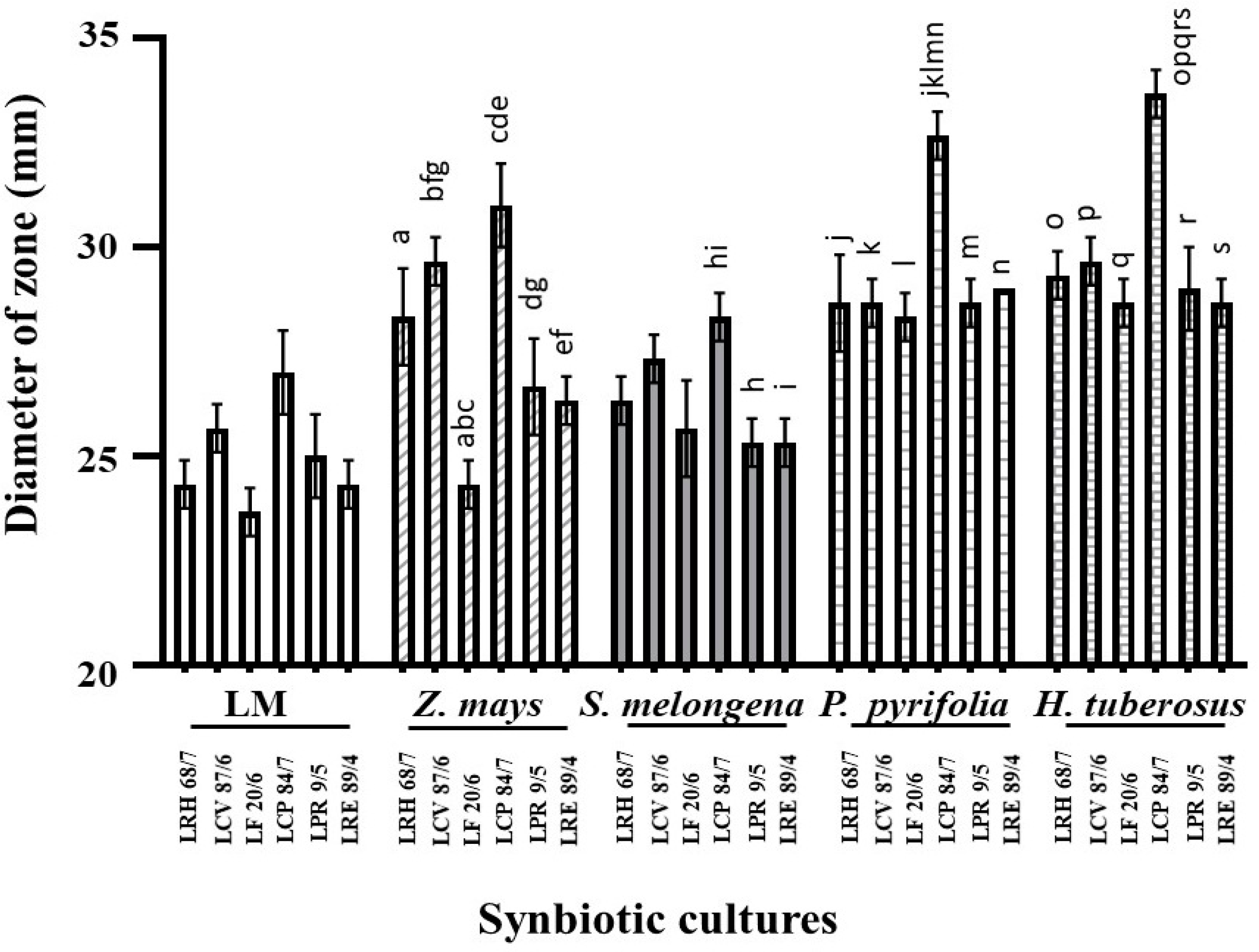
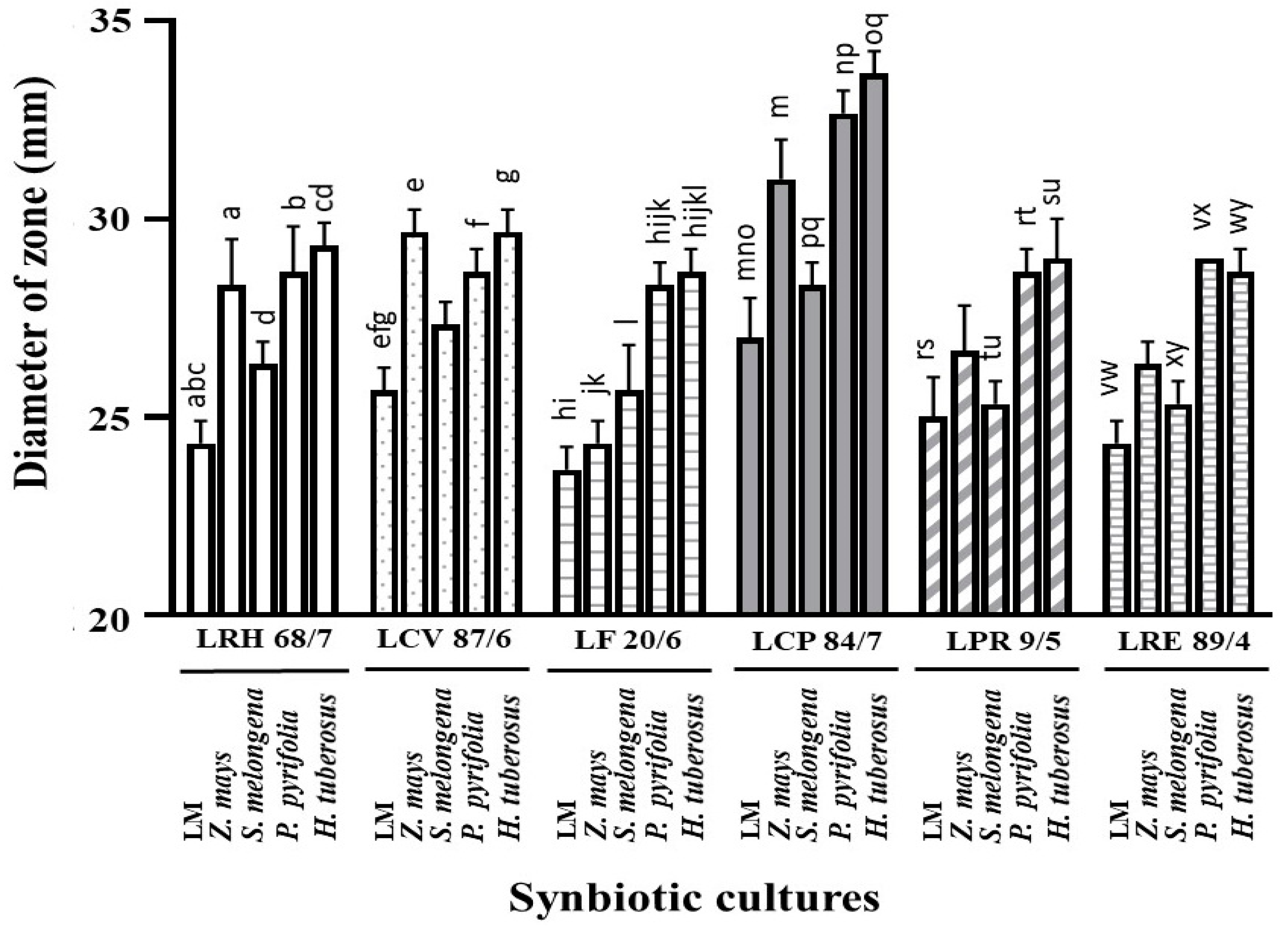
| No. | Plants | Average Fold Change in LPM Growth after Compared with Each LM | |||||
|---|---|---|---|---|---|---|---|
| LRH68/7 | LCV87/6 | LF20/6 | LCP84/7 | LPR9/5 | LRE89/4 | ||
| LM | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |
| 1 | B. cylindrica | 1.12 ± 0.04 | 1.13 ± 0.13 | 1.10 ± 0.12 | 1.10 ± 0.04 | 1.08 ± 0.01 | 1.13 ± 0.03 |
| 2 | C.cajan | 1.08 ± 0.04 | 1.12 ± 0.01 | 1.09 ± 0.04 | 1.17 ± 0.06 | 1.07 ± 0.04 | 1.14 ± 0.02 |
| 3 | G.max | 1.12 ± 0.04 | 1.09 ± 0.01 | 1.19 ± 0.04 | 1.10 ± 0.01 | 1.17 ± 0.13 | 1.17 ± 0.07 |
| 4 | P. vulgaris | 1.10 ± 0.03 | 1.09 ± 0.07 | 1.09 ± 0.11 | 1.17 ± 0.06 | 1.19 ± 0.08 | 1.10 ± 0.03 |
| 5 | S. indicum | 1.11 ± 0.04 | 1.20 ± 0.16 | 1.19 ± 0.04 | 1.11 ± 0.04 | 1.18 ± 0.09 | 1.11 ± 0.02 |
| 6 | Z. mays | 1.31 ± 0.11 * | 1.39 ± 0.19 * | 1.32 ± 0.06 * | 1.41 ± 0.06 * | 1.45 ± 0.04 * | 1.39 ± 0.03 * |
| 7 | B. oleracea | 1.10 ± 0.04 | 1.10 ± 0.04 | 1.12 ± 0.01 | 1.19 ± 0.11 | 1.14 ± 0.11 | 1.12 ± 0.01 |
| 8 | C. moschata | 1.10 ± 0.02 | 1.16 ± 0.02 | 1.13 ± 0.06 | 1.15 ± 0.09 | 1.19 ± 0.11 | 1.16 ± 0.09 |
| 9 | C. scolymus | 1.12 ± 0.05 | 1.24 ± 0.11 | 1.21 ± 0.01 | 1.16 ± 0.07 | 1.16 ± 0.14 | 1.17 ± 0.07 |
| 10 | S. edule | 1.16 ± 0.08 | 1.08 ± 0.01 | 1.12 ± 0.01 | 1.12 ± 0.01 | 1.07 ± 0.01 | 1.14 ± 0.02 |
| 11 | S. melongena | 1.27 ± 0.06 * | 1.25 ± 0.02 * | 1.23 ± 0.06 * | 1.24 ± 0.05 * | 1.18 ± 0.08 * | 1.22 ± 0.01 * |
| 12 | A. comosus | 1.10 ± 0.01 | 1.20 ± 0.04 | 1.14 ± 0.08 | 1.13 ± 0.04 | 1.12 ± 0.04 | 1.16 ± 0.01 |
| 13 | A. carambola | 1.19 ± 0.04 | 1.20 ± 0.05 | 1.13 ± 0.01 | 1.13 ± 0.07 | 1.12 ± 0.06 | 1.16 ± 0.02 |
| 14 | C. maxima | 1.19 ± 0.11 | 1.17 ± 0.01 | 1.09 ± 0.01 | 1.16 ± 0.01 | 1.13 ± 0.06 | 1.10 ± 0.08 |
| 15 | H. undatus | 1.23 ± 0.17 | 1.16 ± 0.10 | 1.11 ± 0.01 | 1.10 ± 0.01 | 1.17 ± 0.05 | 1.14 ± 0.06 |
| 16 | M. domestica | 1.20 ± 0.11 | 1.11 ± 0.03 | 1.12 ± 0.03 | 1.16 ± 0.18 | 1.13 ± 0.02 | 1.16 ± 0.01 |
| 17 | P. edulis | 1.14 ± 0.01 | 1.11 ± 0.01 | 1.09 ± 0.01 | 1.18 ± 0.06 | 1.14 ± 0.02 | 1.22 ± 0.08 |
| 18 | P. salicina | 1.09 ± 0.03 | 1.09 ± 0.04 | 1.13 ± 0.01 | 1.22 ± 0.09 | 1.10 ± 0.03 | 1.11 ± 0.16 |
| 19 | P. pyrifolia | 1.42 ± 0.05 * | 1.44 ± 0.06 * | 1.44 ± 0.09 * | 1.46 ± 0.07 * | 1.42 ± 0.04 * | 1.38 ± 0.01 * |
| 20 | V. labrusca | 1.25 ± 0.06 | 1.14 ± 0.04 | 1.11 ± 0.01 | 1.14 ± 0.01 | 1.16 ± 0.01 | 1.22 ± 0.01 |
| 21 | A. sinensis | 1.07 ± 0.08 | 1.13 ± 0.02 | 1.08 ± 0.10 | 1.18 ± 0.17 | 1.17 ± 0.10 | 1.23 ± 0.01 |
| 22 | K. parviflora | 1.06 ± 0.11 | 1.07 ± 0.06 | 1.13 ± 0.04 | 1.16 ± 0.11 | 1.16 ± 0.03 | 1.17 ± 0.08 |
| 23 | A. lappa | 1.17 ± 0.05 | 1.15 ± 0.06 | 1.15 ± 0.09 | 1.10 ± 0.07 | 1.15 ± 0.04 | 1.20 ± 0.01 |
| 24 | D. carota | 1.11 ± 0.03 | 1.16 ± 0.09 | 1.16 ± 0.05 | 1.14 ± 0.11 | 1.11 ± 0.04 | 1.17 ± 0.01 |
| 25 | H. tuberosus | 1.42 ± 0.08 * | 1.41 ± 0.04 * | 1.46 ± 0.05 * | 1.44 ± 0.11 * | 1.41 ± 0.11 * | 1.43 ± 0.10 * |
| 26 | R. sativus | 1.07 ± 0.02 | 1.11 ± 0.02 | 1.11 ± 0.03 | 1.18 ± 0.04 | 1.13 ± 0.06 | 1.18 ± 0.01 |
| Probiotics | Average Diameter of Inhibition Zone (mm) against C. albicans ATCC10231 of | LPM/LM | |
|---|---|---|---|
| LPM | LM | ||
| LCP84/7 plus LRE 89/4 | 33.33 ± 0.58 | 24.17 ± 0.29 | 1.38 |
| LCP84/7 alone | 28.33 ± 0.58 | 23.33 ± 0.58 | 1.21 |
| LRE 89/4 alone | 27.67 ± 0.58 | 22.67 ± 0.58 | 1.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sookkhee, S.; Khamnoi, P.; Sastraruji, T.; Boonkum, S.; Wikan, N.; Nimlamool, W. Synergistic Inhibition of Synbiotic Cultures among Lactobacilli and Plant Extracts against Vaginal Discharge Causing Candida albicans. Nutrients 2024, 16, 1372. https://doi.org/10.3390/nu16091372
Sookkhee S, Khamnoi P, Sastraruji T, Boonkum S, Wikan N, Nimlamool W. Synergistic Inhibition of Synbiotic Cultures among Lactobacilli and Plant Extracts against Vaginal Discharge Causing Candida albicans. Nutrients. 2024; 16(9):1372. https://doi.org/10.3390/nu16091372
Chicago/Turabian StyleSookkhee, Siriwoot, Phadungkiat Khamnoi, Thanapat Sastraruji, Sathian Boonkum, Nitwara Wikan, and Wutigri Nimlamool. 2024. "Synergistic Inhibition of Synbiotic Cultures among Lactobacilli and Plant Extracts against Vaginal Discharge Causing Candida albicans" Nutrients 16, no. 9: 1372. https://doi.org/10.3390/nu16091372
APA StyleSookkhee, S., Khamnoi, P., Sastraruji, T., Boonkum, S., Wikan, N., & Nimlamool, W. (2024). Synergistic Inhibition of Synbiotic Cultures among Lactobacilli and Plant Extracts against Vaginal Discharge Causing Candida albicans. Nutrients, 16(9), 1372. https://doi.org/10.3390/nu16091372






