Blood Lead Level as Marker of Increased Risk of Ovarian Cancer in BRCA1 Carriers
Abstract
1. Introduction
2. Materials and Methods
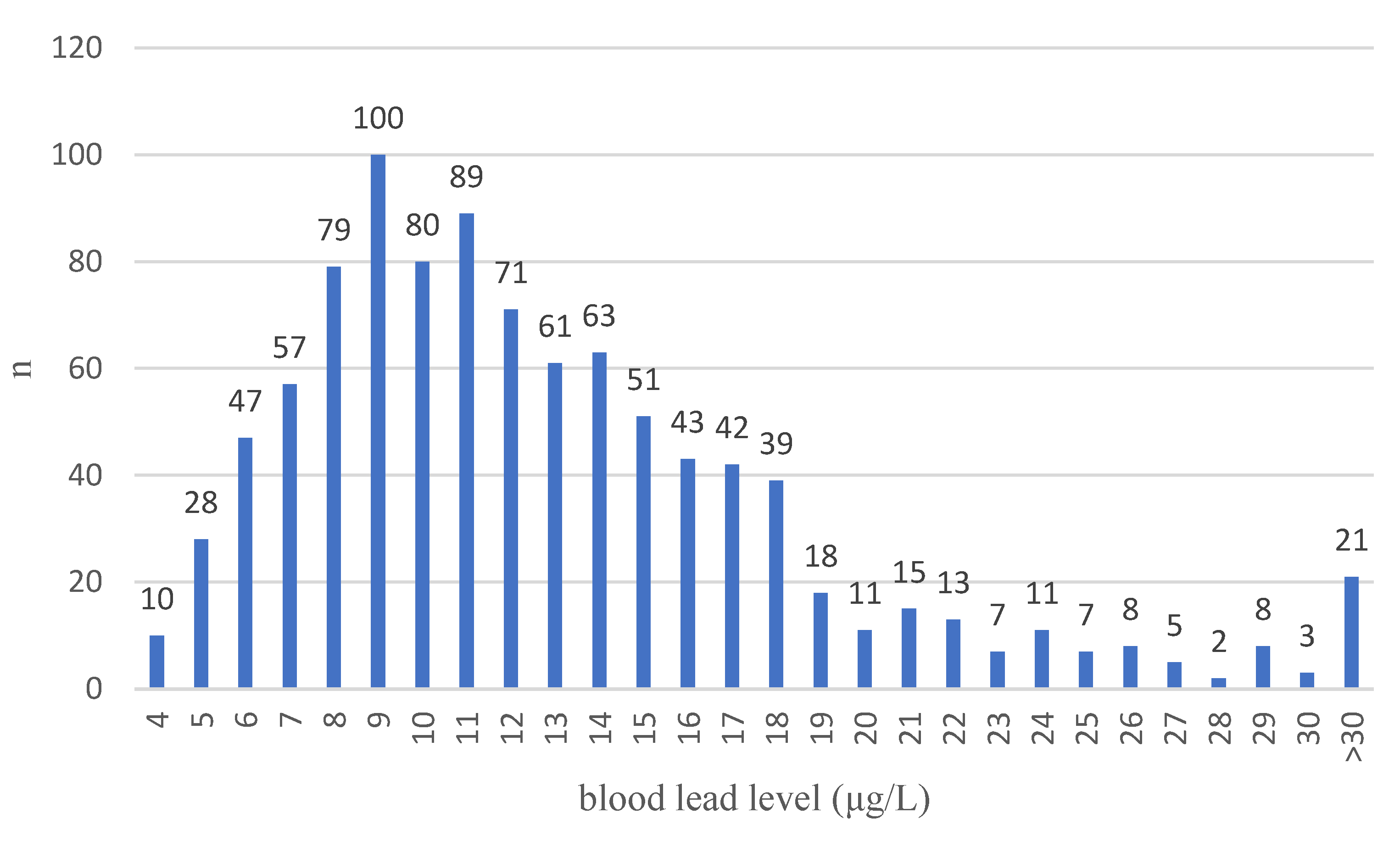
2.1. Measurement of Blood Lead Levels
2.2. Statistical Analysis
3. Results
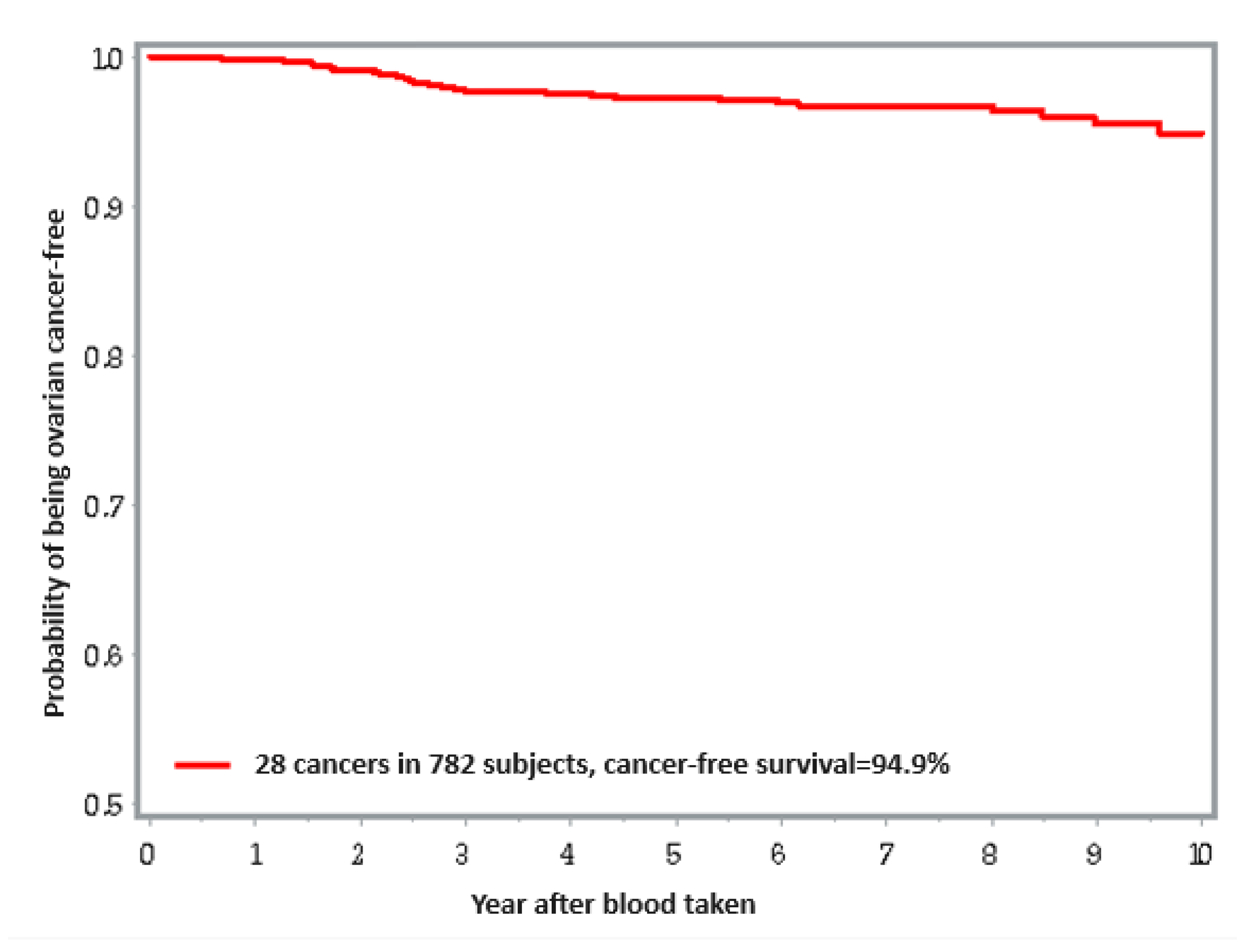
Ovarian Cancer
4. Discussion
- -
- Lysophosphatidic acid (LPA) is involved in the carcinogenesis of ovarian cancer, but not breast cancer. LPA induces the proliferation, survival, drug resistance, invasion, opening of tight intercellular junctions and closing of gap junctions, cell migration, or metastasis of ovarian cancer cells. No direct effect of lead on this signaling pathway has been demonstrated [16].
- -
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.-A.; Mooij, T.M.; Roos-Blom, M.-J.; Jervis, S.; van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 2017, 317, 2402. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Silvestri, V.; Leslie, G.; Rebbeck, T.R.; Neuhausen, S.L.; Hopper, J.L.; Nielsen, H.R.; Lee, A.; Yang, X.; McGuffog, L.; et al. Cancer Risks Associated With BRCA1 and BRCA2 Pathogenic Variants. J. Clin. Oncol. 2022, 40, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Friebel, T.M.; Domchek, S.M.; Rebbeck, T.R. Modifiers of Cancer Risk in BRCA1 and BRCA2 Mutation Carriers: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2014, 106, dju091. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, W.; Matoušek, T.; Domchek, S.; Paradiso, A.; Patruno, M.; Irmejs, A.; Roderte, I.; Derkacz, R.; Baszuk, P.; Kuświk, M.; et al. Blood Arsenic Levels as a Marker of Breast Cancer Risk among BRCA1 Carriers. Cancers 2021, 13, 3345. [Google Scholar] [CrossRef] [PubMed]
- Levy, L.; Silsoe, B. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Inorganic and Organic Lead Compounds. IARC Monogr. Eval. Carcinog. Risks Hum. 2006, 87, 1–471. [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lead and Lead Compounds. IARC Monogr. Eval. Carcinog. Risk Chem. Hum. 1980, 23, 325–415. [Google Scholar]
- Steenland, K.; Barry, V.; Anttila, A.; Sallmen, M.; Mueller, W.; Ritchie, P.; McElvenny, D.M.; Straif, K. Cancer Incidence among Workers with Blood Lead Measurements in Two Countries. Occup. Environ. Med. 2019, 76, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Anttila, A.; Uuksulainen, S.; Rantanen, M.; Sallmén, M. Lung Cancer Incidence among Workers Biologically Monitored for Occupational Exposure to Lead: A Cohort Study. Scand. J. Work Environ. Health 2022, 48, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Steenland, K.; Boffetta, P. Lead and Cancer in Humans: Where Are We Now? Am. J. Ind. Med. 2000, 38, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Boffetta, P. Cancer and Occupational Exposure to Inorganic Lead Compounds: A Meta-Analysis of Published Data. Occup. Environ. Med. 1995, 52, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, M.M.; Deubler, E.L.; Kelly, R.S.; Ryan Diver, W.; Teras, L.R.; Hodge, J.M.; Levine, K.E.; Haines, L.G.; Lundh, T.; Lenner, P.; et al. Blood Levels of Cadmium and Lead in Relation to Breast Cancer Risk in Three Prospective Cohorts. Int. J. Cancer 2019, 144, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, J.; Liu, C.; Luo, Y.; Chen, J.; Fu, Y.; Xu, Y.; Wu, H.; Li, X.; Wang, H. Relationships Between Biological Heavy Metals and Breast Cancer: A Systematic Review and Meta-Analysis. Front. Nutr. 2022, 9, 838762. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, K.; Kupnicka, P.; Witczak, G.; Tousty, P.; Bosiacki, M.; Kurzawski, M.; Chlubek, D.; Cymbaluk-Płoska, A. Assessment of Cadmium (Cd) and Lead (Pb) Blood Concentration on the Risk of Endometrial Cancer. Biology 2023, 12, 717. [Google Scholar] [CrossRef]
- Benderli Cihan, Y.; Sözen, S.; Öztürk Yıldırım, S. Trace Elements and Heavy Metals in Hair of Stage III Breast Cancer Patients. Biol. Trace Elem. Res. 2011, 144, 360–379. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.J.; Singh, S.; Mehrotra, P.; Singh, K.; Sarangi, R. Comparison of Some Trace Elements Concentration in Blood, Tumor Free Breast and Tumor Tissues of Women with Benign and Malignant Breast Lesions: An Indian Study. Environ. Int. 2006, 32, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Yung, Y.C.; Stoddard, N.C.; Chun, J. LPA Receptor Signaling: Pharmacology, Physiology, and Pathophysiology. J. Lipid Res. 2014, 55, 1192–1214. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, M.; Garambois, V.; Colombo, P.-E.; Chentouf, M.; Gros, L.; Brouillet, J.-P.; Robert, B.; Jarlier, M.; Dumas, K.; Martineau, P.; et al. Anti-Müllerian Hormone (AMH) Autocrine Signaling Promotes Survival and Proliferation of Ovarian Cancer Cells. Sci. Rep. 2021, 11, 2231. [Google Scholar] [CrossRef] [PubMed]
- Kastora, S.L.; Triantafyllidou, O.; Kolovos, G.; Kastoras, A.; Sigalos, G.; Vlahos, N. Combinational Approach of Retrospective Clinical Evidence and Transcriptomics Highlight AMH Superiority to FSH, as Successful ICSI Outcome Predictor. J. Assist. Reprod. Genet. 2020, 37, 1623–1635. [Google Scholar] [CrossRef] [PubMed]
- Gentile, D.; Losurdo, A.; Sagona, A.; Zuradelli, M.; Gatzemeier, W.; Barbieri, E.; Testori, A.; Errico, V.; Bianchi, P.; Biondi, E.; et al. Surgical Management of BRCA-Mutation Carriers: A Single Institution Experience. Eur. J. Surg. Oncol. 2022, 48, 1706–1712. [Google Scholar] [CrossRef] [PubMed]
| n = 989 | |
|---|---|
| Age at enrollment | |
| <50 | 775 (78.36%) |
| ≥50 | 214 (21.64%) |
| Smoking | |
| -never | 720 (72.80%) |
| -ever | 264 (26.70%) |
| -missing data | 5 (0.50%) |
| Hormonal replacement therapy | |
| -never | 720 (72.80%) |
| -ever | 263 (26.59%) |
| -missing data | 6 (0.61%) |
| Oophorectomy | |
| -no | 413 (41.76%) |
| -yes | 576 (58.24%) |
| -missing data | 0 (0.00%) |
| Oral Contraceptive use | |
| -never | 501 (50.66%) |
| -ever | 481 (48.64%) |
| -missing data | 7 (0.70%) |
| Diabetes | |
| -no | 880 (88.98%) |
| -yes | 62 (6.27%) |
| -missing data | 47 (4.75%) |
| Body Mass Index | |
| <18.5 | 56 (5.66%) |
| 18.5–24.9 | 553 (55.92%) |
| 25.0–29.9 | 237 (23.96%) |
| ≥30.0 | 95 (9.61%) |
| -missing data | 48 (4.85%) |
| Dietary supplements usage | |
| -never | 500 (50.56%) |
| -ever | 489 (49.44%) |
| -missing data | 0 (0.00%) |
| New cancer site (n = 174) (by the first cancer) | |
| breast | 122 (70.11%) |
| ovarian | 29 (16.67%) |
| bladder | 2 (1.15%) |
| cervix | 3 (1.72%) |
| colon | 2 (1.15%) |
| kidney | 1 (0.57%) |
| leukemia | 2 (1.15%) |
| lung | 3 (1.72%) |
| pancreas | 1 (0.57%) |
| salivary gland | 1 (0.57%) |
| sarcoma | 1 (0.57%) |
| site unknown | 1 (0.57%) |
| skin | 1 (0.57%) |
| thyroid | 3 (1.72%) |
| urothelial | 1 (0.57%) |
| abdomen—CSU | 1 (0.57%) |
| Variables | Ovarian Cases/ Total | Univariate HR (95% CI) P | Multivariate * HR (95% CI) P |
|---|---|---|---|
| Lead level | |||
| ≤9.6 μg/L | 5/260 | 1 | 1 |
| 9.6–13.6 μg/L | 6/261 | 1.12 (0.34–3.69) 0.85 | 0.98 (0.29–3.25) 0.97 |
| >13.6 μg/L | 18/261 | 3.33 (1.23–9.00) 0.02 | 2.10 (0.73–6.01) 0.17 |
| Total | 29/782 | ||
| Date of birth | |||
| ≤1965 | 10/101 | 1 | 1 |
| 1965–1975 | 9/164 | 0.49 (0.20–1.22) 0.13 | 1.43 (0.07–28.1) 0.82 |
| 1975–1985 | 9/328 | 0.25 (0.10–0.64) 0.003 | 0.44 (0.02–11.0) 0.62 |
| >1985 | 1/189 | 0.06 (0.01–0.50) 0.006 | 0.09 (0.00–3.73) 0.22 |
| Age at blood draw (years) | |||
| ≤40 | 14/556 | 1 | 1 |
| 40–50 | 5/129 | 1.53 (0.55–4.23) 0.42 | 0.44 (0.12–1.66) 0.22 |
| >50 | 10/97 | 4.49 (1.99–10.1) 0.0003 | 1.27 (0.05–30.5) 0.88 |
| Oral contraceptive use | |||
| No | 18/374 | 1 | 1 |
| Yes | 11/402 | 0.54 (0.25–1.14) 0.10 | 0.82 (0.35–1.91) 0.65 |
| Missing | 0/6 | ||
| Hormone replacement therapy | |||
| No | 26/662 | 1 | 1 |
| Yes | 3/154 | 0.40 (0.12–1.32) 0.13 | 0.34 (0.10–1.17) 0.09 |
| Missing | 0/6 | ||
| Smoking | |||
| No | 12/447 | 1 | 1 |
| Current | 7/176 | 1.46 (0.58–3.71) 0.42 | 1.27 (0.49–3.30) 0.63 |
| Former | 10/154 | 2.53 (1.09–5.85) 0.03 | 2.35 (0.99–5.59) 0.05 |
| BMI at blood draw | |||
| ≤median (23.0) | 11/396 | 1 | 1 |
| >median (23.0) | 16/339 | 1.70 (0.79–3.65) 0.18 | 0.98 (0.42–2.29) 0.96 |
| Missing | 2/47 |
| Mechanism | BRCA1 Interactions | Breast Cancer | Ovarian Cancer | Lead Interactions |
|---|---|---|---|---|
| NF-kB (signaling path) | No | Yes | Yes | Yes |
| MAPK (signaling path) | Yes | Yes | Yes | Yes |
| ErbB (signaling path) | No | Yes | Yes | |
| AMH (signaling path) | No | No | Yes | |
| LPA (signaling path) | Yes | No | Yes | |
| PI3K (signaling path) | Yes | Yes | Yes | No |
| Estrogen Receptors (signaling path) | Yes | ERα+ | ERβ+ | |
| D1-CDK4/6-RB (signaling path) | Yes | Yes | No | Yes |
| FGF (signaling path) | Yes | Yes | No | |
| EGF (signaling path) | No | Yes | Yes | Yes |
| VEGF (signaling path) | Yes | No | Yes | Yes |
| SRC | No | Yes | Yes | |
| JAK | Yes | Yes | Yes | |
| HER2 | No | Yes | Yes | |
| IGF-1 (signaling path) | Yes | Yes | Yes | No |
| NOTCH (signaling path) | Yes | Yes | Yes | Yes |
| E-cadherin-integrin | No | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiljańczyk, A.; Matuszczak, M.; Marciniak, W.; Derkacz, R.; Stempa, K.; Baszuk, P.; Bryśkiewicz, M.; Lubiński, K.; Cybulski, C.; Dębniak, T.; et al. Blood Lead Level as Marker of Increased Risk of Ovarian Cancer in BRCA1 Carriers. Nutrients 2024, 16, 1370. https://doi.org/10.3390/nu16091370
Kiljańczyk A, Matuszczak M, Marciniak W, Derkacz R, Stempa K, Baszuk P, Bryśkiewicz M, Lubiński K, Cybulski C, Dębniak T, et al. Blood Lead Level as Marker of Increased Risk of Ovarian Cancer in BRCA1 Carriers. Nutrients. 2024; 16(9):1370. https://doi.org/10.3390/nu16091370
Chicago/Turabian StyleKiljańczyk, Adam, Milena Matuszczak, Wojciech Marciniak, Róża Derkacz, Klaudia Stempa, Piotr Baszuk, Marta Bryśkiewicz, Krzysztof Lubiński, Cezary Cybulski, Tadeusz Dębniak, and et al. 2024. "Blood Lead Level as Marker of Increased Risk of Ovarian Cancer in BRCA1 Carriers" Nutrients 16, no. 9: 1370. https://doi.org/10.3390/nu16091370
APA StyleKiljańczyk, A., Matuszczak, M., Marciniak, W., Derkacz, R., Stempa, K., Baszuk, P., Bryśkiewicz, M., Lubiński, K., Cybulski, C., Dębniak, T., Gronwald, J., Huzarski, T., Lener, M. R., Jakubowska, A., Szwiec, M., Stawicka-Niełacna, M., Godlewski, D., Prusaczyk, A., Jasiewicz, A., ... Lubiński, J. (2024). Blood Lead Level as Marker of Increased Risk of Ovarian Cancer in BRCA1 Carriers. Nutrients, 16(9), 1370. https://doi.org/10.3390/nu16091370









