Mycobacterium avium paratuberculosis Infection Suppresses Vitamin D Activation and Cathelicidin Production in Macrophages through Modulation of the TLR2-Dependent p38/MAPK-CYP27B1-VDR-CAMP Axis
Abstract
1. Introduction
2. Materials and Methods
2.1. THP-1 Macrophages and Caco-2 Monolayers Cell Culture
2.2. Infection and Treatment of THP-1 Macrophages and Caco-2 Monolayers
2.3. RNA Extraction, Reverse Transcription, and q-RT PCR to Measure CYP27B1, VDR, CAMP, NOX-1, IL-1β, and IL-10 in THP-1 Macrophages and Caco-2 Monolayers
2.4. Knockdown of TLR2 by siRNA Transfection
2.5. Measurement of Total and Phosphorylated p38/MAPK in THP-1 Macrophages
2.6. DHE Fluorescence Staining Assay for Caco-2 Monolayers
3. Statistical Analysis
4. Results
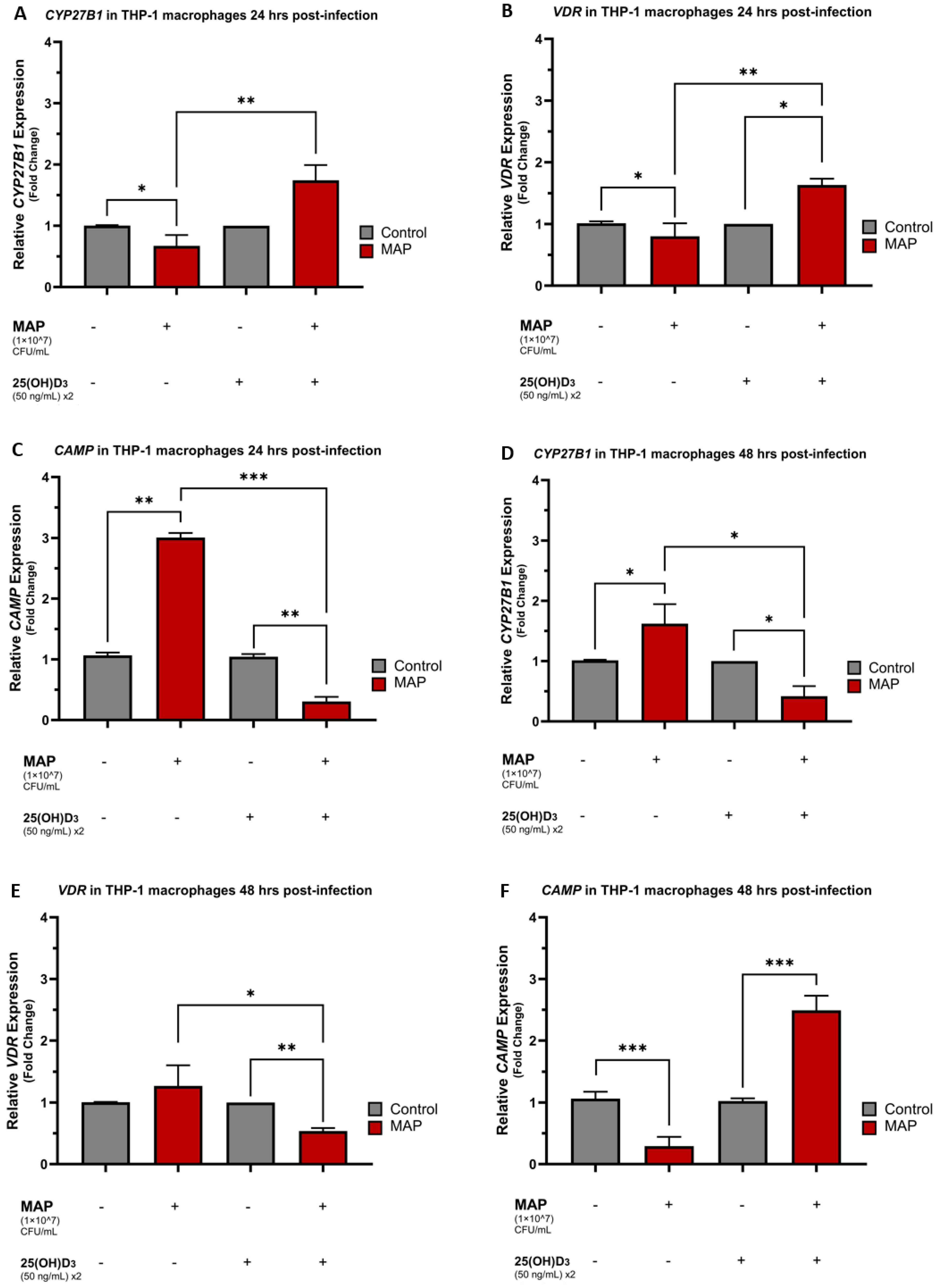
4.1. MAP Infection Influences CYP27B1, VDR, and CAMP Expression in a Time-Dependent Manner and Calcifediol Reverses MAP-Induced Expression Trends
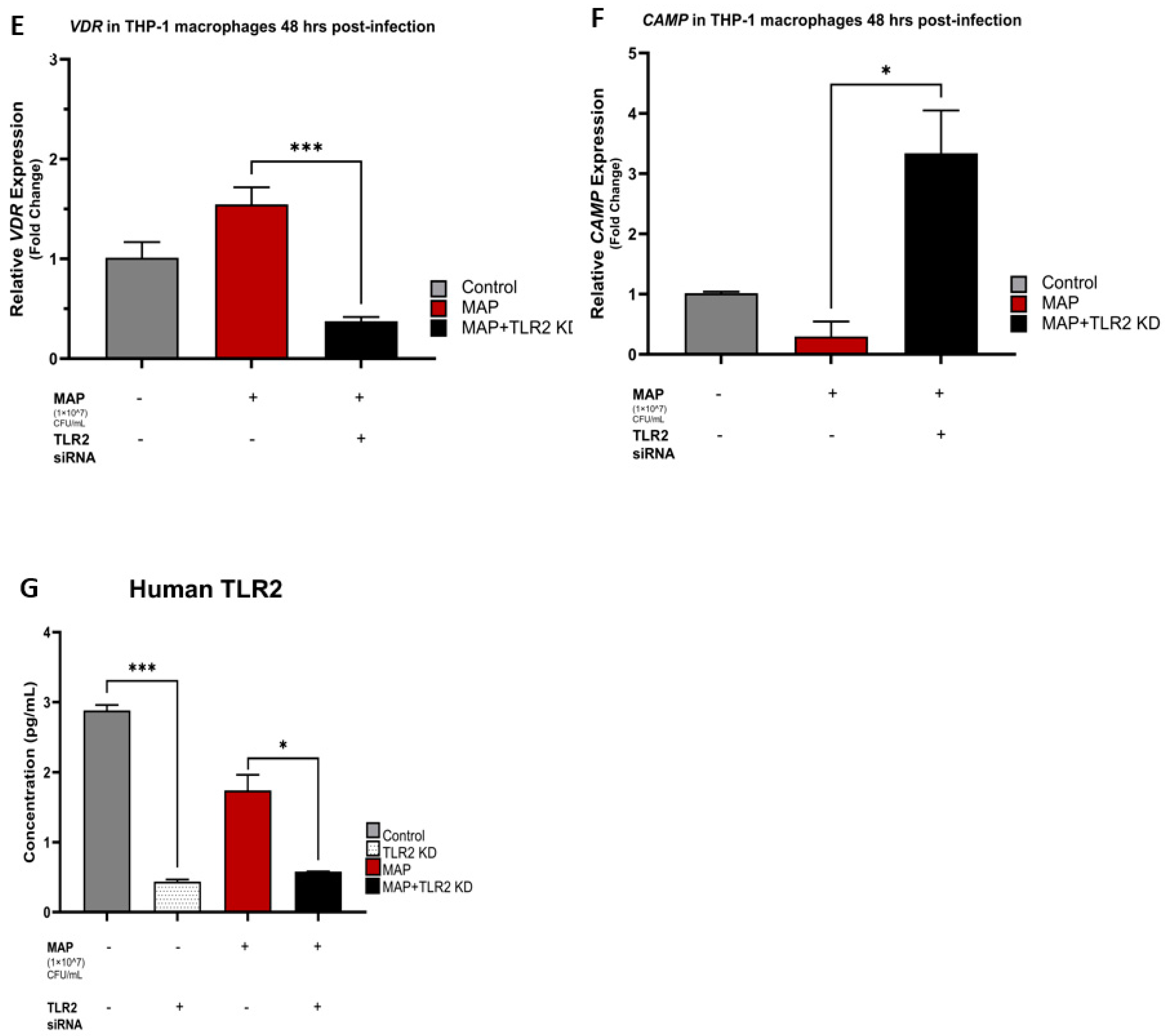
4.2. TLR2 Is Necessary for MAP Infection to Hinder Vitamin D Activation and Cathelicidin Production
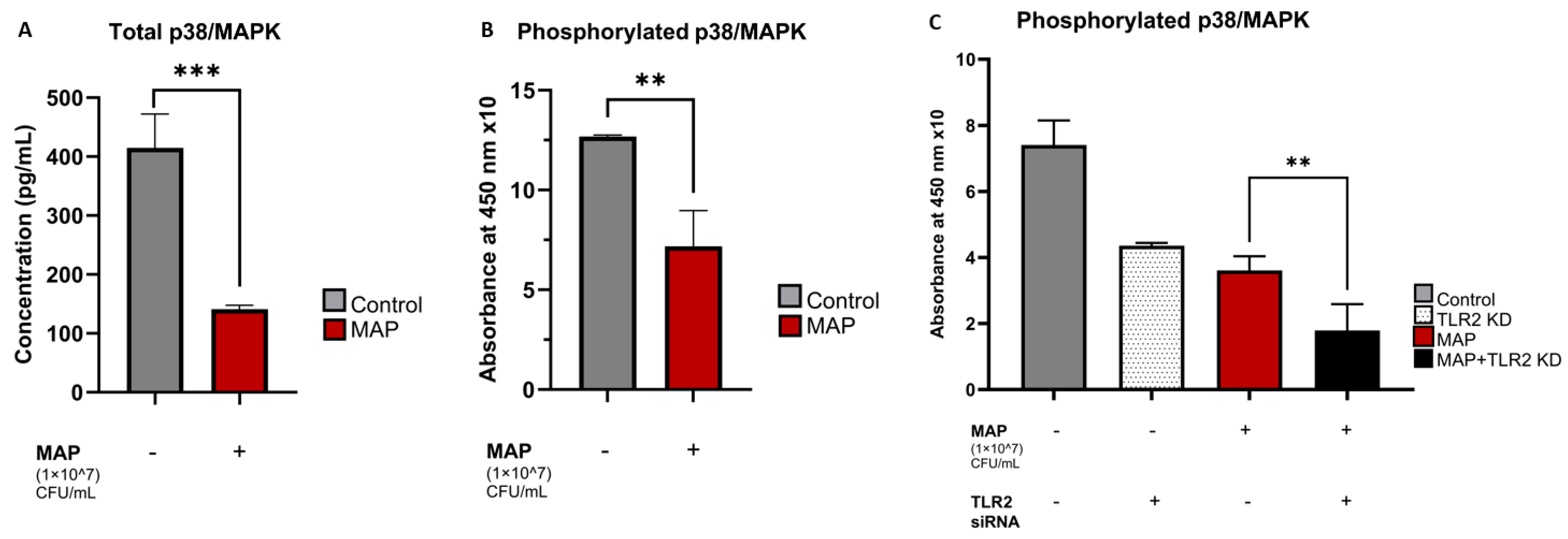
4.3. MAP Infection Decreases Total and Phosphorylated p38/MAPK Levels and the Pathway Inhibition Is Independent of TLR2 Presence
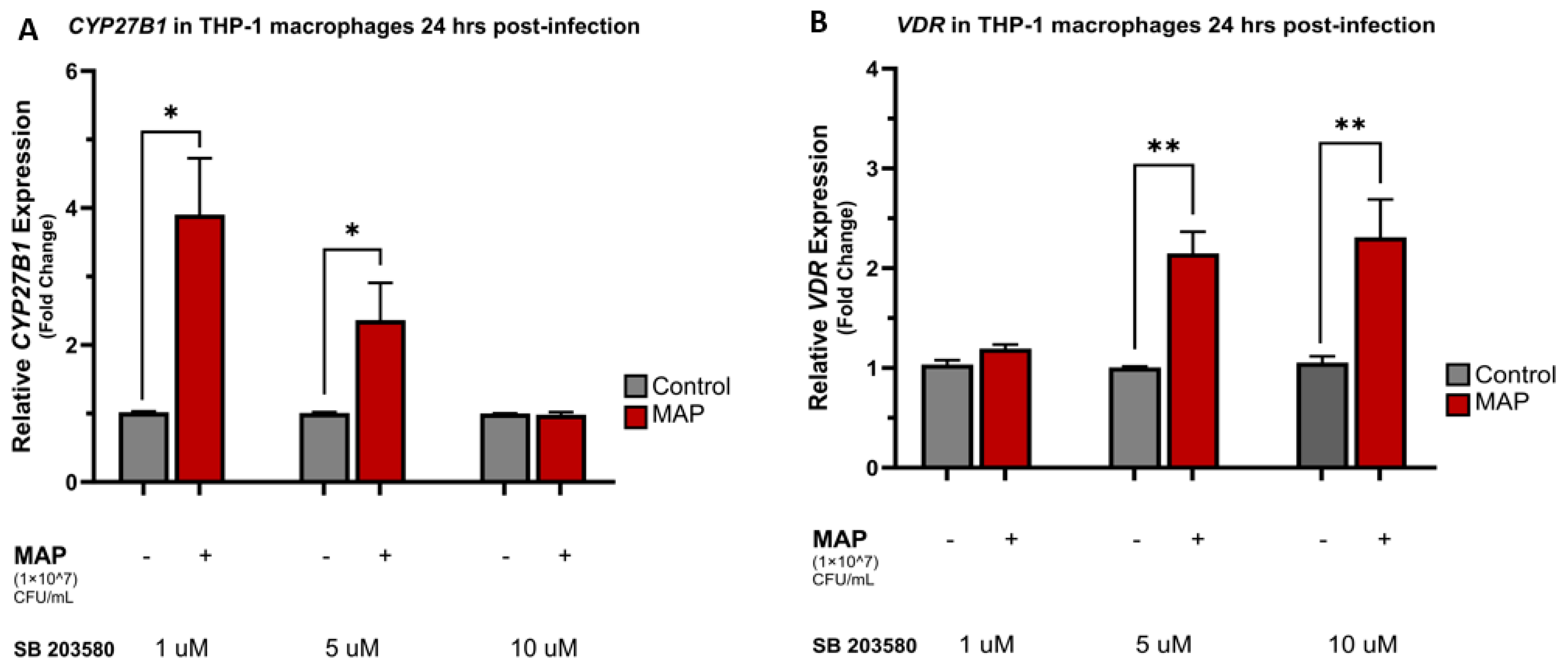
4.4. MAP Infection Alters CYP27B1, VDR, and CAMP Expression through Interaction with the p38/MAPK Pathway
4.5. Calcitriol and p38 Antagonism Reduce Oxidative Stress in Caco-2 Monolayers following Macrophage-Mediated MAP Infection
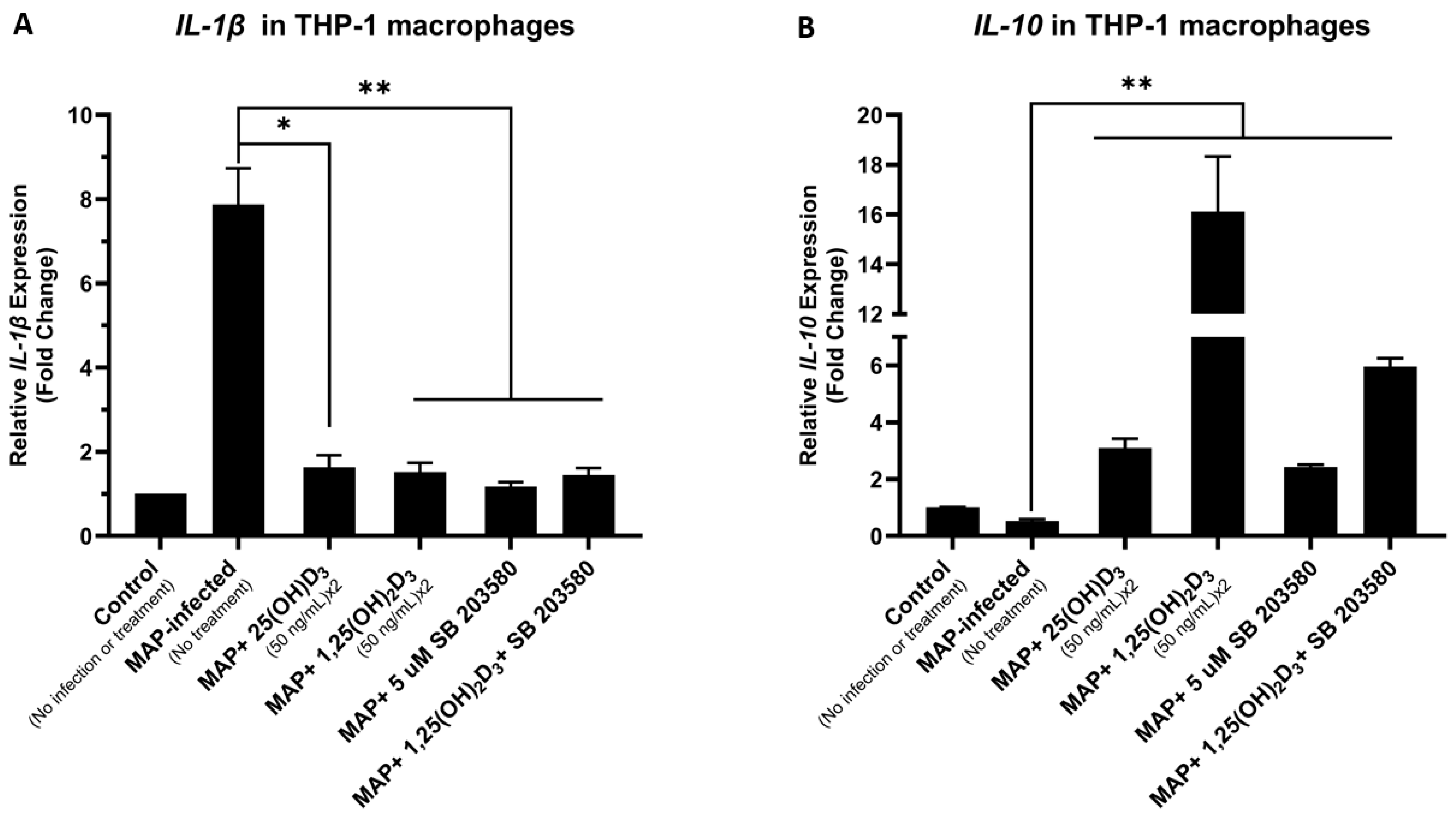
4.6. Calcitriol and p38 Antagonism Reduce Inflammation Secondary to MAP Infection in THP-1 Macrophages
5. Discussion
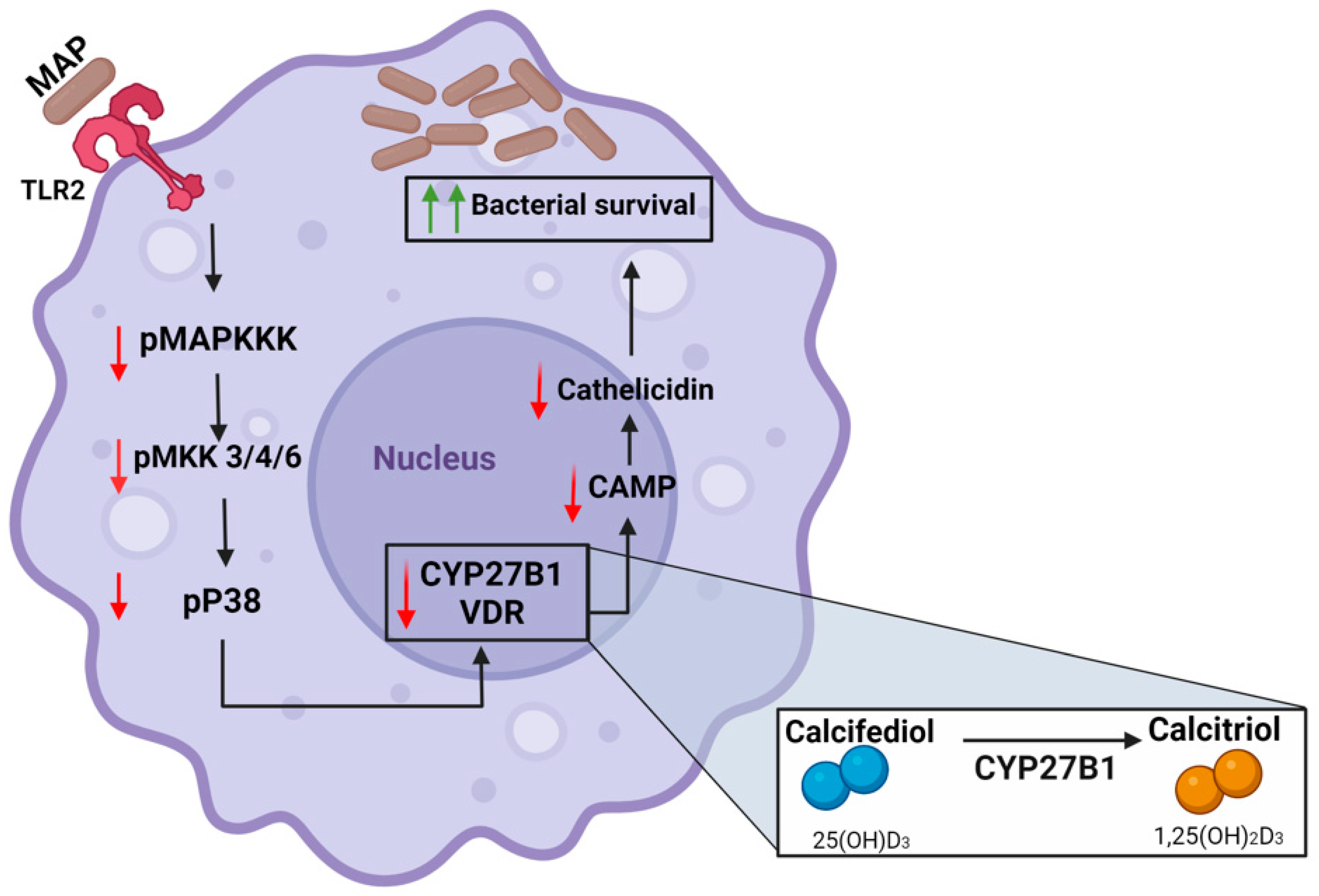
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goltzman, D.; Mannstadt, M.; Marcocci, C. Physiology of the Calcium-Parathyroid Hormone-Vitamin D Axis. In Vitamin D in Clinical Medicine; Giustina, A., Bilezikian, J.P., Eds.; S.Karger AG: Berlin, Germany, 2018; Volume 50. [Google Scholar]
- Meyer, M.B.; Pike, J.W. Mechanistic homeostasis of vitamin D metabolism in the kidney through reciprocal modulation of Cyp27b1 and Cyp24a1 expression. J. Steroid Biochem. Mol. Biol. 2020, 196, 105500. [Google Scholar] [CrossRef] [PubMed]
- Donati, S.; Palmini, G.; Aurilia, C.; Falsetti, I.; Marini, F.; Giusti, F.; Iantomasi, T.; Brandi, M.L. Calcifediol: Mechanisms of Action. Nutrients 2023, 15, 4409. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, J.A.; Qasem, A.; Naser, S.A. Cathelicidin Mediates an Anti-Inflammatory Role of Active Vitamin D (Calcitriol) During M. paratuberculosis Infection. Front. Cell Infect. Microbiol. 2022, 12, 875772. [Google Scholar] [CrossRef]
- Amagai, R.; Takahashi, T.; Terui, H.; Fujimura, T.; Yamasaki, K.; Aiba, S.; Asano, Y. The Antimicrobial Peptide Cathelicidin Exerts Immunomodulatory Effects via Scavenger Receptors. Int. J. Mol. Sci. 2023, 24, 875. [Google Scholar] [CrossRef] [PubMed]
- Freeman, H.J. Natural history and long-term clinical course of Crohn’s disease. World J. Gastroenterol. 2014, 20, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Saade, C.; Nasr, L.; Sharara, A.; Barada, K.; Soweid, A.; Murad, F.; Tawil, A.; Ghieh, D.; Asmar, K.; Tamim, H.; et al. Crohn’s disease: A retrospective analysis between computed tomography enterography, colonoscopy, and histopathology. Radiography 2019, 25, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, J.; El Ouali, S.; Qazi, T.; Cohen, B.; Steele, S.R.; Baker, M.E.; Rieder, F. Prevention and Treatment of Stricturing Crohn’s Disease—Perspectives and Challenges. Expert. Rev. Gastroenterol. Hepatol. 2021, 15, 401–411. [Google Scholar] [CrossRef]
- Pogacnik, J.S.; Salgado, G. Perianal Crohn’s Disease. Clin. Colon. Rectal Surg. 2019, 32, 377–385. [Google Scholar] [CrossRef]
- Aksan, A.; Farrag, K.; Blumenstein, I.; Schröder, O.; Dignass, A.U.; Stein, J. Chronic intestinal failure and short bowel syndrome in Crohn’s disease. World J. Gastroenterol. 2021, 27, 3440–3465. [Google Scholar] [CrossRef] [PubMed]
- Glassner, K.L.; Abraham, B.P.; Quigley, E.M.M. The microbiome and inflammatory bowel disease. J. Allergy Clin. Immunol. 2020, 145, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Fabisiak, N.; Fabisiak, A.; Watala, C.; Fichna, J. Fat-soluble Vitamin Deficiencies and Inflammatory Bowel Disease: Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2017, 51, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Mena Bares, L.; Benítez Cantero, J.M.; Iglesias Flores, E.; Gros Alcalde, B.; Moreno Ortega, E.; Maza Muret, F.R.; Carmona Asenjo, E.; García Sánchez Mª, V.; Vallejo Casas, J.A. Bile acid malabsorption in patients with chronic diarrhea and Crohn’s disease. Rev. Esp. Enferm. Dig. 2019, 111, 40–45. [Google Scholar] [CrossRef] [PubMed]
- McNees, A.L.; Markesich, D.; Zayyani, N.R.; Graham, D.Y. Mycobacterium paratuberculosis as a cause of Crohn’s disease. Expert. Rev. Gastroenterol. Hepatol. 2015, 9, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- Bharathy, S.; Gunaseelan, L.; Porteen, K. Exploring the potential hazard of Mycobacterium avium subspecies paratuberculosis as a cause for Crohn’s disease. Vet. World 2017, 10, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, G.; Aitken, J.; Hamblin, H.; Collins, M.; Borody, T.J. Putting Crohn’s on the MAP: Five Common Questions on the Contribution of Mycobacterium avium subspecies paratuberculosis to the Pathophysiology of Crohn’s Disease. Dig. Dis. Sci. 2021, 66, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Ssekitoleko, J.; Ojok, L.; Abd El Wahed, A.; Erume, J.; Amanzada, A.; Eltayeb, E.; Eltom, K.H.; Okuni, J.B. Mycobacterium avium subsp. paratuberculosis Virulence: A Review. Microorganisms 2021, 9, 2623. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, J.L.; Oak, E.; Garcia, J.; Liu, H.; Lorenzini, P.A.; Batra, D.; Chhabra, A.; Salazar, J.C.; Roca, X. Vitamin D modulates human macrophage response to Mycobacterium tuberculosis DNA. Tuberculosis 2019, 116, S131–S137. [Google Scholar] [CrossRef]
- Padhi, A.; Pattnaik, K.; Biswas, M.; Jagadeb, M.; Behera, A.; Sonawane, A. Mycobacterium tuberculosis LprE Suppresses TLR2-Dependent Cathelicidin and Autophagy Expression to Enhance Bacterial Survival in Macrophages. J. Immunol. 2019, 203, 2665–2678. [Google Scholar] [CrossRef]
- Louis, T.J.; Qasem, A.; Naser, S.A. Attenuation of Excess TNF-α Release in Crohn’s Disease by Silencing of iRHOMs 1/2 and the Restoration of TGF-β Mediated Immunosuppression Through Modulation of TACE Trafficking. Front. Immunol. 2022, 13, 887830. [Google Scholar] [CrossRef]
- Gajendran, M.; Loganathan, P.; Catinella, A.P.; Hashash, J.G. A comprehensive review and update on Crohn’s disease. Dis. Mon. 2018, 64, 20–57. [Google Scholar] [CrossRef]
- Mallikarjunappa, S.; Brito, L.F.; Pant, S.D.; Schenkel, F.S.; Meade, K.G.; Karrow, N.A. Johne’s Disease in Dairy Cattle: An Immunogenetic Perspective. Front. Vet. Sci. 2021, 8, 718987. [Google Scholar] [CrossRef] [PubMed]
- Qasem, A.; Naser, A.E.; Naser, S.A. Enteropathogenic infections modulate intestinal serotonin transporter (SERT) function by activating Toll-like receptor 2 (TLR-2) in Crohn’s disease. Sci. Rep. 2021, 11, 22624. [Google Scholar] [CrossRef]
- Naser, A.; Odeh, A.K.; Sharp, R.C.; Qasem, A.; Beg, S.; Naser, S.A. Polymorphisms in TNF Receptor Superfamily 1B (TNFRSF1B:rs3397) are Linked to Mycobacterium avium paratuberculosis Infection and Osteoporosis in Rheumatoid Arthritis. Microorganisms 2019, 7, 646. [Google Scholar] [CrossRef] [PubMed]
- Baban, Y.N.; Edicheria, C.M.; Joseph, J.; Kaur, P.; Mostafa, J.A. Osteoporosis Complications in Crohn’s Disease Patients: Factors, Pathogenesis, and Treatment Outlines. Cureus 2021, 13, e20564. [Google Scholar] [CrossRef]
- Harrison, S.R.; Li, D.; Jeffery, L.E.; Raza, K.; Hewison, M. Vitamin D, Autoimmune Disease and Rheumatoid Arthritis. Calcif. Tissue Int. 2020, 106, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.D.; Shane, E. Hypercalcemia: A Review. Jama 2022, 328, 1624–1636. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Li, H.; Li, H. Effects of vitamin D on thyroid autoimmunity markers in Hashimoto’s thyroiditis: Systematic review and meta-analysis. J. Int. Med. Res. 2021, 49, 3000605211060675. [Google Scholar] [CrossRef]
- Yu, J.; Sharma, P.; Girgis, C.M.; Gunton, J.E. Vitamin D and Beta Cells in Type 1 Diabetes: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 14434. [Google Scholar] [CrossRef]
- White, J.H. Vitamin D deficiency and the pathogenesis of Crohn’s disease. J. Steroid Biochem. Mol. Biol. 2018, 175, 23–28. [Google Scholar] [CrossRef]
- Wang, M.H.; Picco, M.F. Crohn’s Disease: Genetics Update. Gastroenterol. Clin. North. Am. 2017, 46, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Nabatov, A.A. The vesicle-associated function of NOD2 as a link between Crohn’s disease and mycobacterial infection. Gut Pathog. 2015, 7, 1. [Google Scholar] [CrossRef][Green Version]
- Salem, M.; Seidelin, J.B.; Eickhardt, S.; Alhede, M.; Rogler, G.; Nielsen, O.H. Species-specific engagement of human nucleotide oligomerization domain 2 (NOD)2 and Toll-like receptor (TLR) signalling upon intracellular bacterial infection: Role of Crohn’s associated NOD2 gene variants. Clin. Exp. Immunol. 2015, 179, 426–434. [Google Scholar] [CrossRef]
- Iizuka, M.; Konno, S. Wound healing of intestinal epithelial cells. World J. Gastroenterol. 2011, 17, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L. Nitric oxide-releasing mesalamine: Potential utility for treatment of inflammatory bowel disease. Dig. Liver Dis. 2003, 35 (Suppl. S2), S35–S40. [Google Scholar] [CrossRef] [PubMed]
- Kankuri, E.; Asmawi, M.Z.; Korpela, R.; Vapaatalo, H.; Moilanen, E. Induction of iNOS in a rat model of acute colitis. Inflammation 1999, 23, 141–152. [Google Scholar] [CrossRef]
- Akgun, E.; Caliskan, C.; Celik, H.A.; Ozutemiz, A.O.; Tuncyurek, M.; Aydin, H.H. Effects of N-acetylcysteine treatment on oxidative stress in acetic acid-induced experimental colitis in rats. J. Int. Med. Res. 2005, 33, 196–206. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talafha, M.M.; Qasem, A.; Naser, S.A. Mycobacterium avium paratuberculosis Infection Suppresses Vitamin D Activation and Cathelicidin Production in Macrophages through Modulation of the TLR2-Dependent p38/MAPK-CYP27B1-VDR-CAMP Axis. Nutrients 2024, 16, 1358. https://doi.org/10.3390/nu16091358
Talafha MM, Qasem A, Naser SA. Mycobacterium avium paratuberculosis Infection Suppresses Vitamin D Activation and Cathelicidin Production in Macrophages through Modulation of the TLR2-Dependent p38/MAPK-CYP27B1-VDR-CAMP Axis. Nutrients. 2024; 16(9):1358. https://doi.org/10.3390/nu16091358
Chicago/Turabian StyleTalafha, Muna M., Ahmad Qasem, and Saleh A. Naser. 2024. "Mycobacterium avium paratuberculosis Infection Suppresses Vitamin D Activation and Cathelicidin Production in Macrophages through Modulation of the TLR2-Dependent p38/MAPK-CYP27B1-VDR-CAMP Axis" Nutrients 16, no. 9: 1358. https://doi.org/10.3390/nu16091358
APA StyleTalafha, M. M., Qasem, A., & Naser, S. A. (2024). Mycobacterium avium paratuberculosis Infection Suppresses Vitamin D Activation and Cathelicidin Production in Macrophages through Modulation of the TLR2-Dependent p38/MAPK-CYP27B1-VDR-CAMP Axis. Nutrients, 16(9), 1358. https://doi.org/10.3390/nu16091358





