Ser9p-GSK3β Modulation Contributes to the Protective Effects of Vitamin C in Neuroinflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatments
2.2. Animals and Treatment Protocols
2.3. Immunohistochemistry
2.4. MTT Assay
2.5. Protein Extraction and Immunoblotting
2.6. RNA Isolation and RT-PCR
2.7. Immunofluorescence
2.8. Cell Image Processing and Morphometric Analysis
2.9. Statistical Analysis
3. Results
3.1. Vit C Effects on BV-2 Cell Viability
3.2. Vit C Regulation of Pro-Inflammatory Mediators
3.3. Vit C Regulation of GSK3β
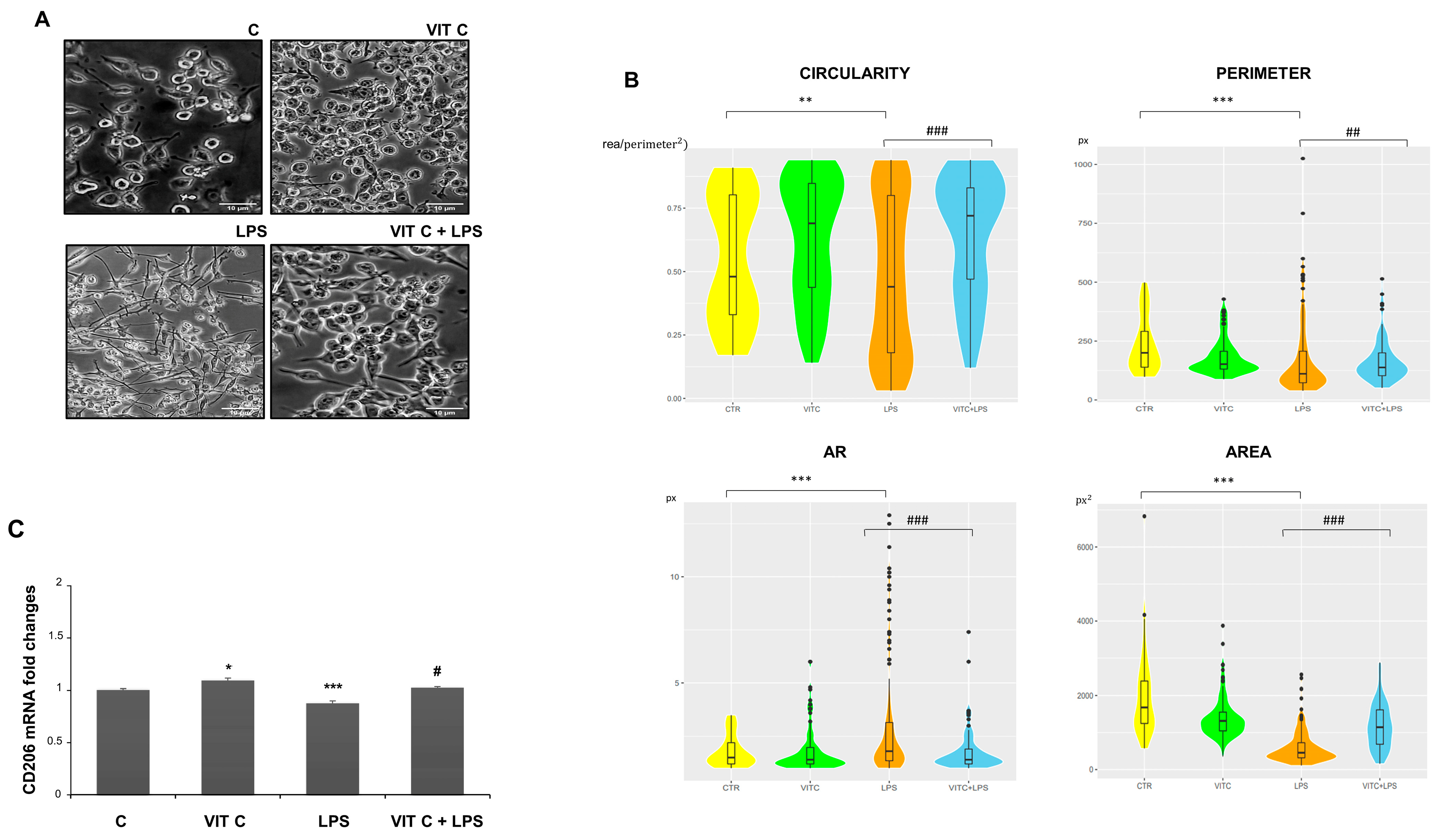
3.4. Vit C Effects on Microglial Phenotype
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kölliker-Frers, R.; Udovin, L.; Otero-Losada, M.; Kobiec, T.; Herrera, M.I.; Palacios, J.; Razzitte, G.; Capani, F. Neuroinflammation: An Integrating Overview of Reactive-Neuroimmune Cell Interactions in Health and Disease. Mediat. Inflamm. 2021, 2021, 9999146. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflamm. 2014, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Panaro, M.A.; Cianciulli, A. Current opinions and perspectives on the role of immune system in the pathogenesis of Parkinson’sdisease. Curr. Pharm. Des. 2012, 18, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Morissette, M.; Samadi, P.; Tahar, A.H.; Bélanger, N.; Di Paolo, T. Striatal Akt/GSK3 signaling pathway in the development of L-Dopa-induced dyskinesias in MPTP monkeys. Progr. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Mínguez-Mínguez, S.; Solís-García Del Pozo, J.; Jordán, J. Rasagiline in Parkinson’s disease: A review based on meta-analysis of clinical data. Pharmacol. Res. 2013, 74, 78–86. [Google Scholar] [CrossRef]
- Hur, E.M.; Zhou, F.Q. (GSK3 signalling in neural development. Nat. Rev. Neurosci. 2010, 11, 539–551. [Google Scholar] [CrossRef]
- Patel, S.; Werstuck, G.H. Macrophage Function and the Role of GSK3. Int. J. Mol. Sci. 2021, 22, 2206. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Vieyra, R.; Silva-García, O.; Gómez-García, A.; Gutiérrez-Castellanos, S.; Álvarez-Aguilar, C.; Baizabal-Aguirre, V.M. Glycogen Synthase Kinase 3β Modulates the Inflammatory Response Activated by Bacteria, Viruses, and Parasites. Front. Immunol. 2021, 12, 75751. [Google Scholar] [CrossRef]
- Li, J.; Ma, S.; Chen, J.; Hu, K.; Li, Y.; Zhang, Z.; Su, Z.; Woodgett, J.R.; Li, M.; Huang, Q. GSK-3β Contributes to Parkinsonian Dopaminergic Neuron Death: Evidence from Conditional Knockout Mice and Tideglusib. Front. Mol. Neurosci. 2020, 13, 81. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Activity of Ascorbic Acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef]
- De Nuccio, F.; Cianciulli, A.; Porro, C.; Kashyrina, M.; Ruggiero, M.; Calvello, R.; Miraglia, A.; Nicolardi, G.; Lofrumento, D.D.; Panaro, M.A. Inflammatory Response Modulation by Vitamin C in an MPTP Mouse Model of Parkinson’s Disease. Biology 2021, 10, 1155. [Google Scholar] [CrossRef] [PubMed]
- Jackson-Lewis, V.; Przedborski, S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat. Protoc. 2007, 2, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Ko, R.; Lee, S.Y. Glycogen synthase kinase 3β in Toll-like receptor signaling. BMB Rep. 2016, 49, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wu, S.; Xing, D. High fluence low-power laser irradiation induces apoptosis via inactivation of Akt/GSK3β signaling pathway. J. Cell. Physiol. 2011, 226, 588–601. [Google Scholar] [CrossRef] [PubMed]
- Lobo, J.; See, E.Y.S.; Biggs, M.; Pandit, A. An insight into morphometric descriptors of cell shape that pertain to regenerative medicine: Cell shape analysis descriptors that pertain to regenerative medicine. J. Tissue Eng. Regen. Med. 2016, 10, 539–553. [Google Scholar] [CrossRef] [PubMed]
- La Torre, M.E.; Cianciulli, A.; Monda, V.; Monda, M.; Filannino, F.M.; Antonucci, L.; Valenzano, A.; Cibelli, G.; Porro, C.; Messina, G.; et al. α-Tocopherol Protects Lipopolysaccharide-Activated BV2 Microglia. Molecules 2023, 28, 3340. [Google Scholar] [CrossRef] [PubMed]
- Calvello, R.; Cianciulli, A.; Nicolardi, G.; De Nuccio, F.; Giannotti, L.; Salvatore, R.; Porro, C.; Trotta, T.; Panaro, M.A.; Lofrumento, D.D. Vitamin D Treatment Attenuates Neuroinflammation and Dopaminergic Neurodegeneration in an Animal Model of Parkinson’s Disease, Shifting M1 to M2 Microglia Responses. J. Neuroimmune Pharmacol. 2017, 12, 327–339. [Google Scholar] [CrossRef] [PubMed]
- May, J.M. Vitamin C transport and its role in the central nervous system. Subcell. Biochem. 2012, 56, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Moretti, M.; Fraga, D.B.; Rodrigues, A.L.S. Preventive and therapeutic potential of ascorbic acid in neurodegenerative diseases. CNS Neurosci. Ther. 2017, 23, 921–929. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Xu, Z.P.; Wang, W.; Cao, J.B.; Fu, Q.; Zhao, W.X.; Li, Y.; Huo, X.L.; Zhang, L.M.; Li, Y.F.; et al. Vitamin C alleviates LPS-induced cognitive impairment in mice by suppressing neuroinflammation and oxidative stress. Int. Immunopharmacol. 2018, 65, 438–447. [Google Scholar] [CrossRef]
- Sil, S.; Ghosh, T.; Gupta, P.; Ghosh, R.; Kabir, S.N.; Roy, A. Dual Role of Vitamin C on the Neuroinflammation Mediated Neurodegeneration and Memory Impairments in Colchicine Induced Rat Model of Alzheimer Disease. J. Mol. Neurosci. 2016, 60, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Shah, S.A.; Badshah, H.; Kim, M.J.; Ali, T.; Yoon, G.H.; Kim, T.H.; Abid, N.B.; Rehman, S.U.; Khan, S.; et al. Neuroprotection by vitamin C against ethanol -induced neuroinflammation associated neurodegeneration in developing rat brain. CNS Neurol. Disord. Drug. Targets 2016, 15, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Skrzypczak-Wiercioch, A.; Sałat, K. Lipopolysaccharide-Induced Model of Neuroinflammation: Mechanisms of Action, Research Application and Future Directions for Its Use. Molecules 2022, 27, 5481. [Google Scholar] [CrossRef] [PubMed]
- Cianciulli, A.; Porro, C.; Calvello, R.; Trotta, T.; Lofrumento, D.D.; Panaro, M.A. Microglia Mediated Neuroinflammation: Focus on PI3K Modulation. Biomolecules 2020, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Krishnankutty, A.; Kimura, T.; Saito, T.; Aoyagi, K.; Asada, A.; Takahashi, S.I.; Ando, K.; Ohara-Imaizumi, M.; Ishiguro, K.; Hisanaga, S.I. In vivo regulation of glycogen synthase kinase 3β activity in neurons and brains. Sci. Rep. 2017, 7, 8602. [Google Scholar] [CrossRef] [PubMed]
- Calvo, B.; Fernandez, M.; Rincon, M.; Tranque, P. GSK3β Inhibition by Phosphorylation at Ser389 Controls Neuroinflammation. Int. J. Mol. Sci. 2022, 24, 337. [Google Scholar] [CrossRef] [PubMed]
- Fichtner-Feigl, S.; Kesselring, R.; Martin, M.; Obermeier, F.; Ruemmele, P.; Kitani, A.; Brunner, S.M.; Haimerl, M.; Geissler, E.K.; Strober, W.; et al. IL-13 orchestrates resolution of chronic intestinal inflammation via phosphorylation of glycogen synthase kinase-3β. J. Immunol. 2014, 192, 3969–3980. [Google Scholar] [CrossRef] [PubMed]
- Nagao, M.; Hayashi, H. Glycogen synthase kinase-3beta is associated with Parkinson’s disease. Neurosci. Lett. 2009, 449, 103–107. [Google Scholar] [CrossRef]
- Choi, C.H.; Lee, B.H.; Ahn, S.G.; Oh, S.H. Proteasome inhibition-induced p38 MAPK/ERK signaling regulates autophagy and apoptosis through the dual phosphorylation of glycogen synthase kinase 3β. Biochem. Biophys. Res. Commun. 2012, 418, 759–764. [Google Scholar] [CrossRef]
- Yao, Y.Y.; Bian, L.G.; Yang, P.; Sui, Y.; Li, R.; Chen, Y.L.; Sun, L.; Ai, Q.L.; Zhong, L.M.; Lu, D. Gastrodin attenuates proliferation and inflammatory responses in activated microglia through Wnt/β-catenin signaling pathway. Brain Res. 2019, 1717, 190–203. [Google Scholar] [CrossRef]
- Cao, Q.; Karthikeyan, A.; Dheen, S.T.; Kaur, C.; Ling, E.A. Production of proinflammatory mediators in activated microglia is synergistically regulated by Notch-1, glycogen synthase kinase (GSK-3β) and NF-κB/p65 signalling. PLoS ONE 2017, 12, e0186764. [Google Scholar] [CrossRef]
- Armentero, M.T.; Sinforiani, E.; Ghezzi, C.; Bazzini, E.; Levandis, G.; Ambrosi, G.; Zangaglia, R.; Pacchetti, C.; Cereda, C.; Cova, E.; et al. Peripheral expression of key regulatory kinases in Alzheimer’s disease and Parkinson’s disease. Neurobiol. Aging 2011, 32, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Kozikowski, A.P.; Gaisina, I.N.; Petukhov, P.A.; Sridhar, J.; King, L.T.; Blond, S.Y.; Duka, T.; Rusnak, M.; Sidhu, A. Highly Potent and Specific GSK-3β Inhibitors That Block Tau Phosphorylation and Decrease α-Synuclein Protein Expression in a Cellular Model of Parkinson’s Disease. Chem. Med. Chem. 2006, 1, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Duka, T.; Duka, V.; Joyce, J.N.; Sidhu, A. α-Synuclein contributes to GSK-3β-catalyzed Tau phosphorylation in Parkinson’s disease models. FASEB J. 2009, 23, 2820–2830. [Google Scholar] [CrossRef] [PubMed]
- Ho, I.C.; Miaw, S.C. Regulation of IL-4 Expression in Immunity and Diseases. Adv. Exp. Med. Biol. 2016, 94, 31–77. [Google Scholar] [CrossRef]
- Savchenko, V.L. Regulation of NADPH oxidase gene expression with PKA and cytokine IL-4 in neurons and microglia. Neurotox. Res. 2013, 23, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, W.; Su, Z.Y.; Kong, A.N. The complexity of the Nrf2 pathway: Beyond the antioxidant response. J. Nutr. Biochem. 2015, 26, 1401–1413. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab. Rev. 2006, 38, 769–789. [Google Scholar] [CrossRef]
- Kim, J.; Cha, Y.N.; Surh, Y.J. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat. Res. 2010, 690, 12–23. [Google Scholar] [CrossRef]
- Van Steenwinckel, J.; Schang, A.L.; Krishnan, M.L.; Degos, V.; Delahaye-Duriez, A.; Bokobza, C.; Csaba, Z.; Verdonk, F.; Montané, A.; Sigaut, S.; et al. Decreased microglial Wnt/β-catenin signalling drives microglial pro-inflammatory activation in the developing brain. Brain 2019, 142, 3806–3833. [Google Scholar] [CrossRef]
- Jridi, I.; Canté-Barrett, K.; Pike-Overzet, K.; Staal, F.J.T. Inflammation and Wnt Signaling: Target for Immunomodulatory Therapy? Front. Cell. Dev. Biol. 2021, 8, 615131. [Google Scholar] [CrossRef] [PubMed]
- Klamer, G.; Song, E.; Ko, K.H.; O’Brien, T.A.; Dolnikov, A. Using small molecule GSK3β inhibitors to treat inflammation. Curr. Med. Chem. 2010, 17, 2873–2881. [Google Scholar] [CrossRef] [PubMed]
- Dun, Y.; Yang, Y.; Xiong, Z.; Feng, M.; Zhang, Y.; Wang, M.; Xiang, J.; Li, G.; Ma, R. Induction of Dickkopf-1 contributes to the neurotoxicity of MPP+ in PC12 cells via inhibition of the canonical Wnt signaling pathway. Neuropharmacology 2013, 67, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zu, G.; Zhang, X.; Wang, X.; Li, S.; Gong, X.; Liang, Z.; Zhao, J. Neuroprotective effects of ginsenoside Rg1 through the Wnt/β-catenin signaling pathway in both in vivo and in vitro models of Parkinson’s disease. Neuropharmacology 2016, 101, 480–489. [Google Scholar] [CrossRef]
- Tam, W.Y.; Ma, C.H.E. Bipolar/rod-shaped microglia are proliferating microglia with distinct M1/M2 phenotypes. Sci. Rep. 2014, 4, 7279. [Google Scholar] [CrossRef]






| cDNA Target | Sequence (5′–>3′) | Sequence References |
|---|---|---|
| IL4 | FW: CTCCTAGCAACCACGGCCCC RW: GCTAGGCATAACGCACTAGGTT | NM_021283.2 |
| GAPDH | FW: ACCACAGTCCCTGCCATCAG RW: TCCACCACCCTGTTGCTGTA | NM_001411840.1 |
| CD206 | FW: AACCAGTTCCTTGAGCTCGG RW: CTGATTAGGGCAGCCGGTAG | NM_008625.2 |
| NRF2 | FW: CAAGACTTGGGCCACTTAAAAGAC RW: AGTAAGGCTTTCCATCCTCATCAC | XM_021193142.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruggiero, M.; Cianciulli, A.; Calvello, R.; Porro, C.; De Nuccio, F.; Kashyrina, M.; Miraglia, A.; Lofrumento, D.D.; Panaro, M.A. Ser9p-GSK3β Modulation Contributes to the Protective Effects of Vitamin C in Neuroinflammation. Nutrients 2024, 16, 1121. https://doi.org/10.3390/nu16081121
Ruggiero M, Cianciulli A, Calvello R, Porro C, De Nuccio F, Kashyrina M, Miraglia A, Lofrumento DD, Panaro MA. Ser9p-GSK3β Modulation Contributes to the Protective Effects of Vitamin C in Neuroinflammation. Nutrients. 2024; 16(8):1121. https://doi.org/10.3390/nu16081121
Chicago/Turabian StyleRuggiero, Melania, Antonia Cianciulli, Rosa Calvello, Chiara Porro, Francesco De Nuccio, Marianna Kashyrina, Alessandro Miraglia, Dario Domenico Lofrumento, and Maria Antonietta Panaro. 2024. "Ser9p-GSK3β Modulation Contributes to the Protective Effects of Vitamin C in Neuroinflammation" Nutrients 16, no. 8: 1121. https://doi.org/10.3390/nu16081121
APA StyleRuggiero, M., Cianciulli, A., Calvello, R., Porro, C., De Nuccio, F., Kashyrina, M., Miraglia, A., Lofrumento, D. D., & Panaro, M. A. (2024). Ser9p-GSK3β Modulation Contributes to the Protective Effects of Vitamin C in Neuroinflammation. Nutrients, 16(8), 1121. https://doi.org/10.3390/nu16081121








