Anti-Inflammatory Diet and Protein-Enriched Diet Can Reduce the Risk of Cognitive Impairment among Older Adults: A Nationwide Cross-Sectional Research
Abstract
1. Introduction
2. Materials and Methods
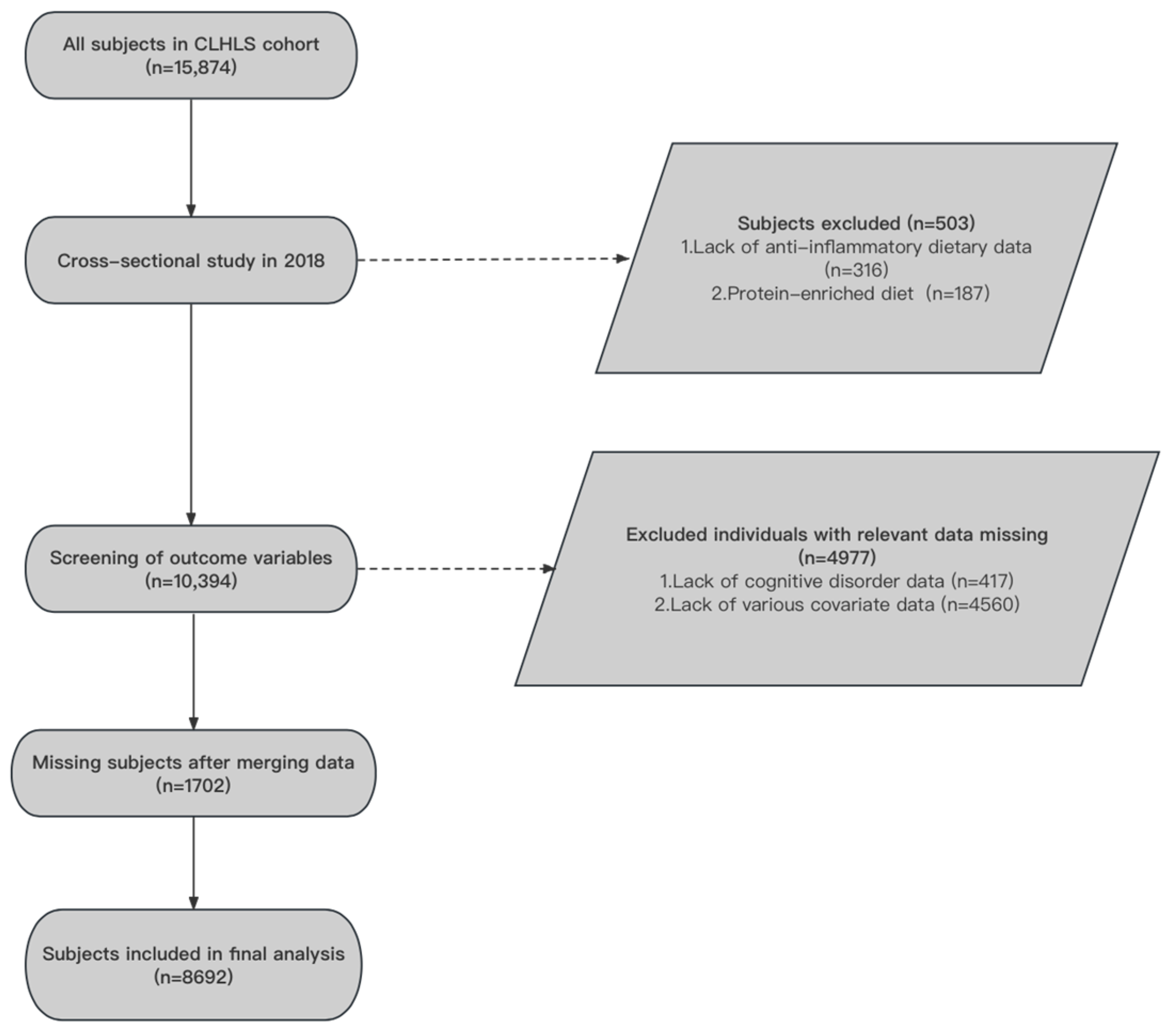
2.1. Participants and Process
2.2. Assessment of Dietary Index
2.3. Assessment of Cognitive Impairment
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. The Characteristics of Study Participants
3.2. Association between AID, PED, and CI
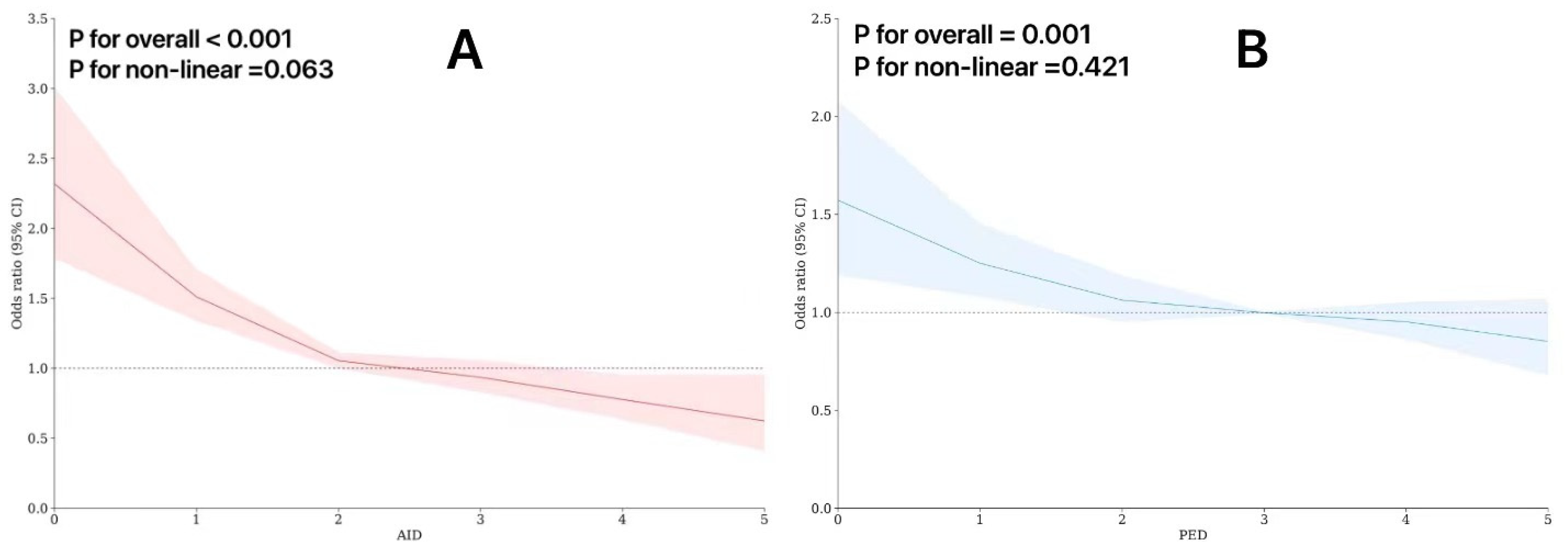
3.3. Restricted Cubic Splines in the Regression Model
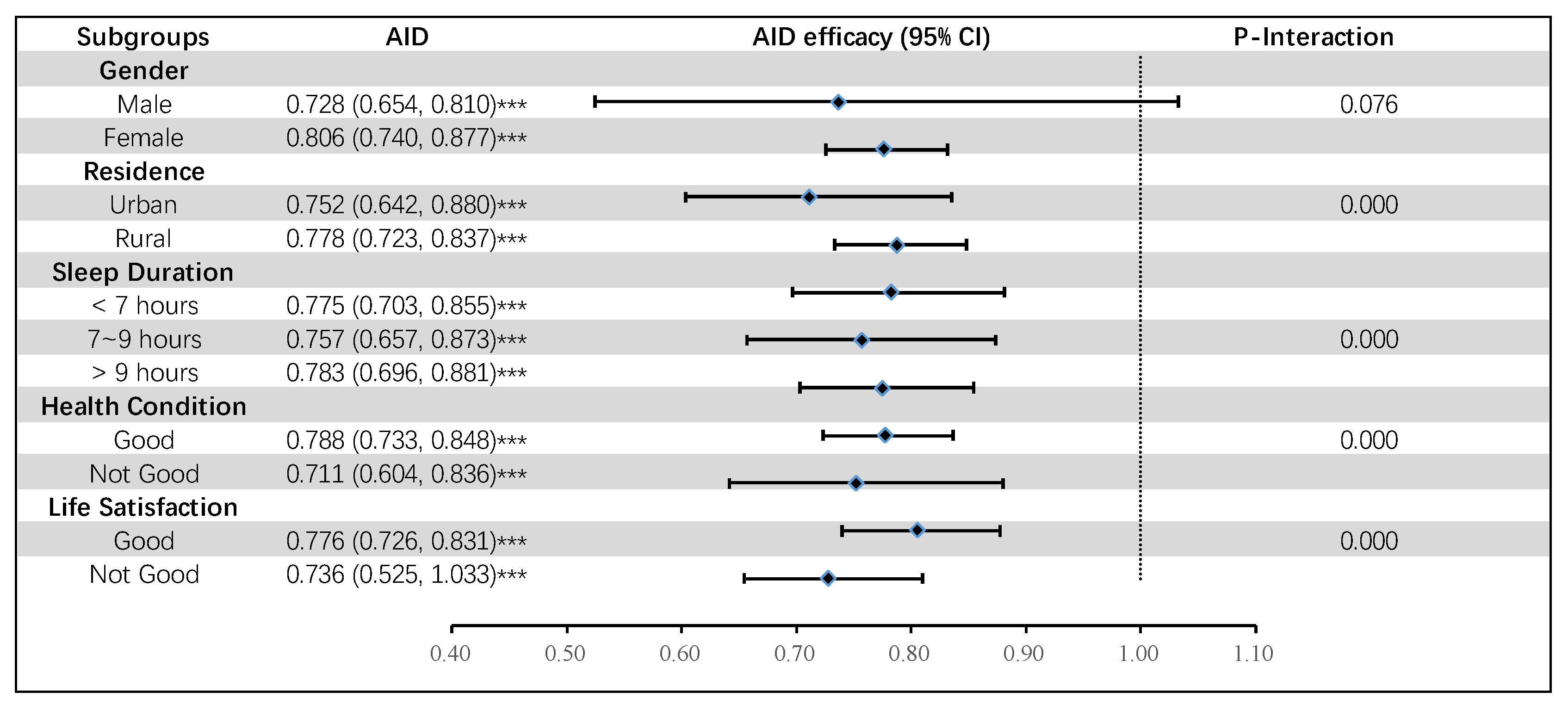
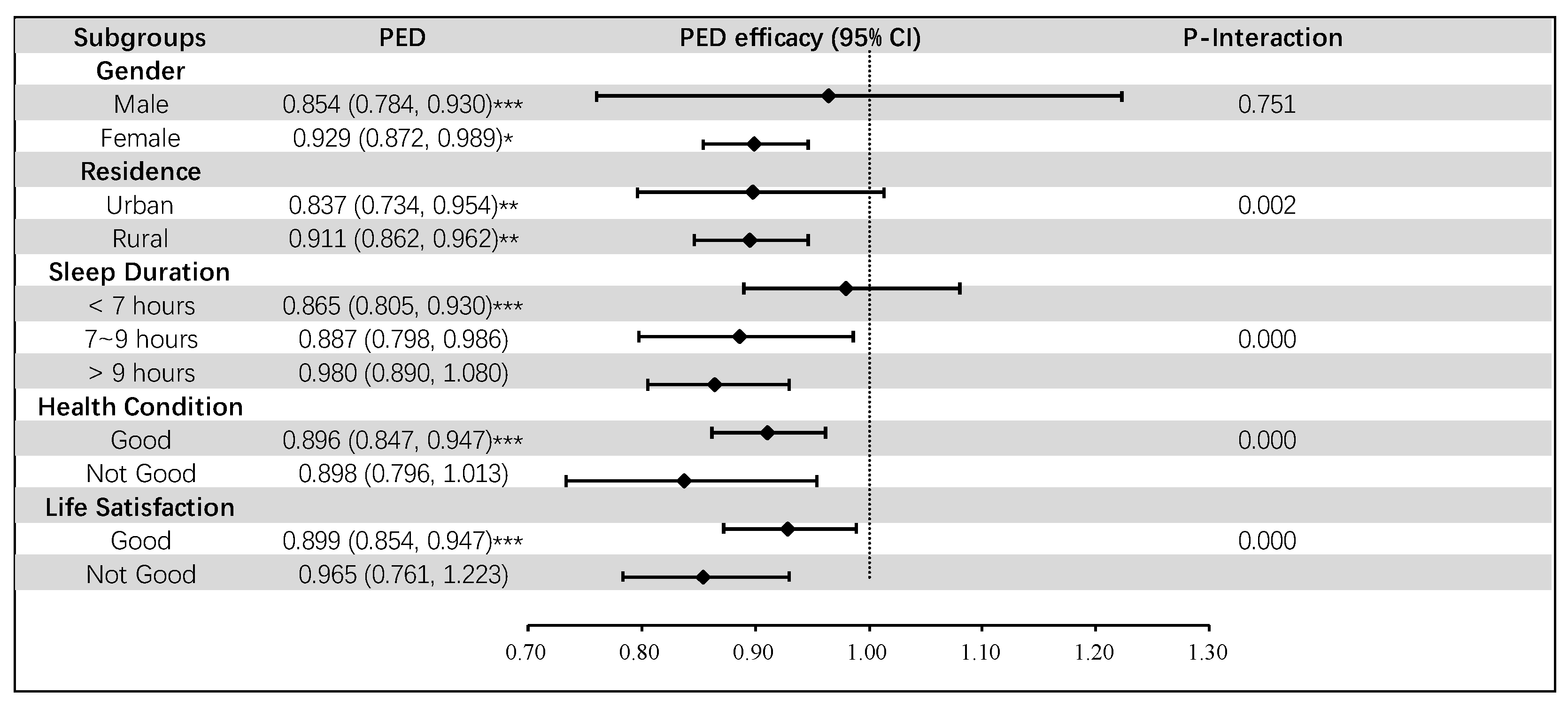
3.4. Subgroup Analysis
3.5. Sensitivity Analysis
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, E.F.; Xie, C.; Schenkel, J.A.; Wu, C.; Long, Q.; Cui, H.; Aman, Y.; Frank, J.; Liao, J.; Zou, H.; et al. A research agenda for ageing in China in the 21st century (2nd edition): Focusing on basic and translational research, long-term care, policy and social networks. Ageing Res. Rev. 2020, 64, 101174. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Zhang, X.; Guo, N.; Li, Z.; Lv, D.; Wang, H.; Jin, J.; Wen, X.; Zhao, S.; Xu, T.; et al. Prevalence of cognitive impairment in Chinese older inpatients and its relationship with 1-year adverse health outcomes: A multi-center cohort study. BMC Geriatr. 2021, 21, 595. [Google Scholar] [CrossRef] [PubMed]
- Tochel, C.; Smith, M.; Baldwin, H.; Gustavsson, A.; Ly, A.; Bexelius, C.; Nelson, M.; Bintener, C.; Fantoni, E.; Garre-Olmo, J.; et al. What outcomes are important to patients with mild cognitive impairment or Alzheimer’s disease, their caregivers, and health-care professionals? A systematic review. Alzheimer’s Dement. 2019, 11, 231–247. [Google Scholar] [CrossRef]
- Lipnicki, D.M.; Crawford, J.D.; Dutta, R.; Thalamuthu, A.; Kochan, N.A.; Andrews, G.; Lima-Costa, M.F.; Castro-Costa, E.; Brayne, C.; Matthews, F.E.; et al. Age-related cognitive decline and associations with sex, education and apolipoprotein E genotype across ethnocultural groups and geographic regions: A collaborative cohort study. PLoS Med. 2017, 14, e1002261. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.; Bryce, R.; Albanese, E.; Wimo, A.; Ribeiro, W.; Ferri, C.P. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2013, 9, 63–75.e2. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia prevention, intervention, and care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef]
- Ma, W.; Wu, B.; Gao, X.; Zhong, R. Association between frailty and cognitive function in older Chinese people: A moderated mediation of social relationships and depressive symptoms. J. Affect. Disord. 2022, 316, 223–232. [Google Scholar] [CrossRef]
- Frankish, H.; Horton, R. Prevention and management of dementia: A priority for public health. Lancet 2017, 390, 2614–2615. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Shu, X.; Yu, G.; Wu, X.; Välimäki, M.; Feng, H. A Risk Prediction Model Based on Machine Learning for Cognitive Impairment Among Chinese Community-Dwelling Elderly People With Normal Cognition: Development and Validation Study. J. Med. Internet Res. 2021, 23, e20298. [Google Scholar] [CrossRef]
- Jia, L.; Du, Y.; Chu, L.; Zhang, Z.; Li, F.; Lyu, D.; Li, Y.; Li, Y.; Zhu, M.; Jiao, H.; et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: A cross-sectional study. Lancet Public Health 2020, 5, e661–e671. [Google Scholar] [CrossRef]
- Rowe, J.W.; Kahn, R.L. Successful aging. Gerontologist 1997, 37, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Gallardo-Gómez, D.; Del Pozo-Cruz, J.; Noetel, M.; Álvarez-Barbosa, F.; Alfonso-Rosa, R.M.; Del Pozo Cruz, B. Optimal dose and type of exercise to improve cognitive function in older adults: A systematic review and bayesian model-based network meta-analysis of RCTs. Ageing Res. Rev. 2022, 76, 101591. [Google Scholar] [CrossRef] [PubMed]
- Sewell, K.R.; Erickson, K.I.; Rainey-Smith, S.R.; Peiffer, J.J.; Sohrabi, H.R.; Brown, B.M. Relationships between physical activity, sleep and cognitive function: A narrative review. Neurosci. Biobehav. Rev. 2021, 130, 369–378. [Google Scholar] [CrossRef]
- Bossers, W.J.; van der Woude, L.H.; Boersma, F.; Hortobágyi, T.; Scherder, E.J.; van Heuvelen, M.J. A 9-Week Aerobic and Strength Training Program Improves Cognitive and Motor Function in Patients with Dementia: A Randomized, Controlled Trial. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2015, 23, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Abrego-Guandique, D.M.; Bonet, M.L.; Caroleo, M.C.; Cannataro, R.; Tucci, P.; Ribot, J.; Cione, E. The Effect of Beta-Carotene on Cognitive Function: A Systematic Review. Brain Sci. 2023, 13, 1468. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Torrisi, S.A.; Mogavero, M.P.; Currenti, W.; Castellano, S.; Godos, J.; Ferri, R.; Galvano, F.; Leggio, G.M.; Grosso, G.; et al. Polyphenols and neuroprotection: Therapeutic implications for cognitive decline. Pharmacol. Ther. 2022, 232, 108013. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Jones, J.; Lee, G.J. Plant-Based Dietary Patterns, Plant Foods, and Age-Related Cognitive Decline. Adv. Nutr. 2019, 10, S422–S436. [Google Scholar] [CrossRef] [PubMed]
- Tseng, P.T.; Zeng, B.S.; Suen, M.W.; Wu, Y.C.; Correll, C.U.; Zeng, B.Y.; Kuo, J.S.; Chen, Y.W.; Chen, T.Y.; Tu, Y.K.; et al. Efficacy and acceptability of anti-inflammatory eicosapentaenoic acid for cognitive function in Alzheimer’s dementia: A network meta-analysis of randomized, placebo-controlled trials with omega-3 fatty acids and FDA-approved pharmacotherapy. Brain Behav. Immun. 2023, 111, 352–364. [Google Scholar] [CrossRef]
- Stavrinou, P.S.; Andreou, E.; Aphamis, G.; Pantzaris, M.; Ioannou, M.; Patrikios, I.S.; Giannaki, C.D. The Effects of a 6-Month High Dose Omega-3 and Omega-6 Polyunsaturated Fatty Acids and Antioxidant Vitamins Supplementation on Cognitive Function and Functional Capacity in Older Adults with Mild Cognitive Impairment. Nutrients 2020, 12, 325. [Google Scholar] [CrossRef]
- von Schacky, C. Importance of EPA and DHA Blood Levels in Brain Structure and Function. Nutrients 2021, 13, 1074. [Google Scholar] [CrossRef] [PubMed]
- Gou, R.; Qin, J.; Pang, W.; Cai, J.; Luo, T.; He, K.; Xiao, S.; Tang, X.; Zhang, Z.; Li, Y. Correlation between dietary patterns and cognitive function in older Chinese adults: A representative cross-sectional study. Front. Nutr. 2023, 10, 1093456. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Bosco, N.; Bourdet-Sicard, R.; Capuron, L.; Delzenne, N.; Doré, J.; Franceschi, C.; Lehtinen, M.J.; Recker, T.; Salvioli, S.; et al. Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res. Rev. 2017, 40, 95–119. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhou, R.; Li, F.; Chen, L.; Wu, K.; Huang, J.; Liu, H.; Huang, Z.; Xu, L.; Yuan, Z.; et al. Association between dietary diversity and cognitive impairment among the oldest-old: Findings from a nationwide cohort study. Clin. Nutr. 2021, 40, 1452–1462. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Smargiassi, A.; Hudson, M.; Fritzler, M.J.; Bernatsky, S. Investigating associations between anti-nuclear antibody positivity and combined long-term exposures to NO2, O3, and PM2.5 using a Bayesian kernel machine regression approach. Environ. Int. 2020, 136, 105472. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. 1), S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Capri, M.; Monti, D.; Giunta, S.; Olivieri, F.; Sevini, F.; Panourgia, M.P.; Invidia, L.; Celani, L.; Scurti, M.; et al. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 2007, 128, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.A.; Ficek, B.N.; Westbrook, R. Understanding the Role of Systemic Inflammation in Alzheimer's Disease. ACS Chem. Neurosci. 2019, 10, 3340–3342. [Google Scholar] [CrossRef]
- Charisis, S.; Ntanasi, E.; Yannakoulia, M.; Anastasiou, C.A.; Kosmidis, M.H.; Dardiotis, E.; Gargalionis, A.N.; Patas, K.; Chatzipanagiotou, S.; Mourtzinos, I.; et al. Diet Inflammatory Index and Dementia Incidence: A Population-Based Study. Neurology 2021, 97, e2381–e2391. [Google Scholar] [CrossRef]
- Tolkien, K.; Bradburn, S.; Murgatroyd, C. An anti-inflammatory diet as a potential intervention for depressive disorders: A systematic review and meta-analysis. Clin. Nutr. 2019, 38, 2045–2052. [Google Scholar] [CrossRef]
- Lv, X.; Sun, S.; Wang, J.; Chen, H.; Li, S.; Hu, Y.; Yu, M.; Zeng, Y.; Gao, X.; Xu, Y.; et al. Anti-Inflammatory Dietary Diversity and Depressive Symptoms among Older Adults: A Nationwide Cross-Sectional Analysis. Nutrients 2022, 14, 5062. [Google Scholar] [CrossRef] [PubMed]
- Valls-Pedret, C.; Sala-Vila, A.; Serra-Mir, M.; Corella, D.; de la Torre, R.; Martínez-González, M.; Martínez-Lapiscina, E.H.; Fitó, M.; Pérez-Heras, A.; Salas-Salvadó, J.; et al. Mediterranean Diet and Age-Related Cognitive Decline: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Petersson, S.D.; Philippou, E. Mediterranean Diet, Cognitive Function, and Dementia: A Systematic Review of the Evidence. Adv. Nutr. 2016, 7, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Siervo, M.; Shannon, O.M.; Llewellyn, D.J.; Stephan, B.C.; Fontana, L. Mediterranean diet and cognitive function: From methodology to mechanisms of action. Free Radic. Biol. Med. 2021, 176, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Júnior, H.J.; Trichopoulou, A.; Panza, F. Cross-sectional and longitudinal associations between adherence to Mediterranean diet with physical performance and cognitive function in older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2021, 70, 101395. [Google Scholar] [CrossRef]
- Barnes, L.L.; Dhana, K.; Liu, X.; Carey, V.J.; Ventrelle, J.; Johnson, K.; Hollings, C.S.; Bishop, L.; Laranjo, N.; Stubbs, B.J.; et al. Trial of the MIND Diet for Prevention of Cognitive Decline in Older Persons. N. Engl. J. Med. 2023, 389, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Barnes, L.L.; Bennett, D.A.; Aggarwal, N.T. MIND diet slows cognitive decline with aging. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2015, 11, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Kheirouri, S.; Alizadeh, M. MIND diet and cognitive performance in older adults: A systematic review. Crit. Rev. Food Sci. Nutr. 2022, 62, 8059–8077. [Google Scholar] [CrossRef]
- Berendsen, A.A.M.; Kang, J.H.; van de Rest, O.; Feskens, E.J.M.; de Groot, L.; Grodstein, F. The Dietary Approaches to Stop Hypertension Diet, Cognitive Function, and Cognitive Decline in American Older Women. J. Am. Med. Dir. Assoc. 2017, 18, 427–432. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, H.; Meng, G.; Zhang, Q.; Liu, L.; Wu, H.; Gu, Y.; Zhang, S.; Wang, X.; Zhang, J.; et al. Anti-inflammatory dietary pattern is associated with handgrip strength decline: A prospective cohort study. Eur. J. Nutr. 2023, 62, 3207–3216. [Google Scholar] [CrossRef]
- Duchowny, K.A.; Ackley, S.F.; Brenowitz, W.D.; Wang, J.; Zimmerman, S.C.; Caunca, M.R.; Glymour, M.M. Associations Between Handgrip Strength and Dementia Risk, Cognition, and Neuroimaging Outcomes in the UK Biobank Cohort Study. JAMA Netw. Open 2022, 5, e2218314. [Google Scholar] [CrossRef] [PubMed]
- Kuo, K.; Zhang, Y.R.; Chen, S.D.; He, X.Y.; Huang, S.Y.; Wu, B.S.; Deng, Y.T.; Yang, L.; Ou, Y.N.; Guo, Y.; et al. Associations of grip strength, walking pace, and the risk of incident dementia: A prospective cohort study of 340212 participants. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2023, 19, 1415–1427. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhan, W.; Huang, X.; Zhang, L.; Zhang, Z.; Zhou, M.; Wang, Z.; Ma, Y. The Relationship Between Mild Cognitive Impairment and Anti-Inflammatory/Pro-Inflammatory Nutrients in the Elderly in Northern China: A Bayesian Kernel Machine Regression Approach. J. Inflamm. Res. 2022, 15, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Dietary protein intake and human health. Food Funct. 2016, 7, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- La Rue, A.; Koehler, K.M.; Wayne, S.J.; Chiulli, S.J.; Haaland, K.Y.; Garry, P.J. Nutritional status and cognitive functioning in a normally aging sample: A 6-y reassessment. Am. J. Clin. Nutr. 1997, 65, 20–29. [Google Scholar] [CrossRef] [PubMed]
- van der Zwaluw, N.L.; van de Rest, O.; Tieland, M.; Adam, J.J.; Hiddink, G.J.; van Loon, L.J.; de Groot, L.C. The impact of protein supplementation on cognitive performance in frail elderly. Eur. J. Nutr. 2014, 53, 803–812. [Google Scholar] [CrossRef]
- Roschel, H.; Gualano, B.; Ostojic, S.M.; Rawson, E.S. Creatine Supplementation and Brain Health. Nutrients 2021, 13, 586. [Google Scholar] [CrossRef]
- Elechi, J.O.G.; Guandique, D.M.A.; Cannataro, R. Creatine in Cognitive Performance: A Commentary. Curr. Mol. Pharmacol. 2024, 17, e18761429272915. [Google Scholar] [CrossRef]
- Sakurai, K.; Okada, E.; Anzai, S.; Tamura, R.; Shiraishi, I.; Inamura, N.; Kobayashi, S.; Sato, M.; Matsumoto, T.; Kudo, K.; et al. Protein-Balanced Dietary Habits Benefit Cognitive Function in Japanese Older Adults. Nutrients 2023, 15, 770. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Wang, W.; Zhang, D. Association between Dietary Protein Intake and Cognitive Function in Adults Aged 60 Years and Older. J. Nutr. Health Aging 2020, 24, 223–229. [Google Scholar] [CrossRef]
- O’Neill, R.F.; Brennan, L.; Prinelli, F.; Sergi, G.; Trevisan, C.; De Groot, L.; Volkert, D.; Maggi, S.; Noale, M.; Conti, S.; et al. PROtein enriched MEDiterranean diet to combat undernutrition and promote healthy neuroCOGnitive ageing in older adults: The PROMED-COG consortium project. Nutr. Bull. 2022, 47, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, J.; Qiu, J.; Li, Y.; Wang, J.; Jiao, J. Intakes of fish and polyunsaturated fatty acids and mild-to-severe cognitive impairment risks: A dose-response meta-analysis of 21 cohort studies. Am. J. Clin. Nutr. 2016, 103, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Talaei, M.; Feng, L.; Yuan, J.M.; Pan, A.; Koh, W.P. Dairy, soy, and calcium consumption and risk of cognitive impairment: The Singapore Chinese Health Study. Eur. J. Nutr. 2020, 59, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R.M.; Requejo, A.M.; Andrés, P.; López-Sobaler, A.M.; Quintas, M.E.; Redondo, M.R.; Navia, B.; Rivas, T. Dietary intake and cognitive function in a group of elderly people. Am. J. Clin. Nutr. 1997, 66, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Velho, S.; Marques-Vidal, P.; Baptista, F.; Camilo, M.E. Dietary intake adequacy and cognitive function in free-living active elderly: A cross-sectional and short-term prospective study. Clin. Nutr. 2008, 27, 77–86. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Liu, G.G. Cognitive impairment and mortality among the oldest-old Chinese. Int. J. Geriatr. Psychiatry 2016, 31, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z. Gender differentials in cognitive impairment and decline of the oldest old in China. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2006, 61, S107–S115. [Google Scholar] [CrossRef] [PubMed]
- Plassman, B.L.; Williams, J.W., Jr.; Burke, J.R.; Holsinger, T.; Benjamin, S. Systematic review: Factors associated with risk for and possible prevention of cognitive decline in later life. Ann. Intern. Med. 2010, 153, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, C.E.; Liu, M. Gender differences in cognitive impairment among the old and the oldest-old in China. Geriatr. Gerontol. Int. 2019, 19, 586–592. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, X.; Feng, Q.; Zeng, Y. The Effects of “Diet-Smoking-Gender” Three-Way Interactions on Cognitive Impairment among Chinese Older Adults. Nutrients 2022, 14, 2144. [Google Scholar] [CrossRef]
- Baker, A.H.; Wardle, J. Sex differences in fruit and vegetable intake in older adults. Appetite 2003, 40, 269–275. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, D.; Parrott, M.D.; Greenwood, C.E.; Ferland, G.; Gaudreau, P.; Belleville, S.; Laurin, D.; Anderson, N.D.; Kergoat, M.J.; Morais, J.A.; et al. Sex differences in the relationship between dietary pattern adherence and cognitive function among older adults: Findings from the NuAge study. Nutr. J. 2020, 19, 58. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shang, S.; Li, P.; Deng, M.; Chen, C.; Jiang, Y.; Dang, L.; Qu, Q. Association between current smoking and cognitive impairment depends on age: A cross-sectional study in Xi’an, China. Med. Clin. 2017, 149, 203–208. [Google Scholar] [CrossRef]
- Chen, J.C.; Espeland, M.A.; Brunner, R.L.; Lovato, L.C.; Wallace, R.B.; Leng, X.; Phillips, L.S.; Robinson, J.G.; Kotchen, J.M.; Johnson, K.C.; et al. Sleep duration, cognitive decline, and dementia risk in older women. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2016, 12, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liang, L.; Zheng, F.; Shi, L.; Zhong, B.; Xie, W. Association Between Sleep Duration and Cognitive Decline. JAMA Netw. Open 2020, 3, e2013573. [Google Scholar] [CrossRef]
- Keil, S.A.; Schindler, A.G.; Wang, M.X.; Piantino, J.; Silbert, L.C.; Elliott, J.E.; Werhane, M.L.; Thomas, R.G.; Willis, S.; Lim, M.M.; et al. Longitudinal Sleep Patterns and Cognitive Impairment in Older Adults. JAMA Netw. Open 2023, 6, e2346006. [Google Scholar] [CrossRef]
- Muhammad, T. Life course rural/urban place of residence, depressive symptoms and cognitive impairment among older adults: Findings from the Longitudinal Aging Study in India. BMC Psychiatry 2023, 23, 391. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Huang, X.; Hilal, S. Association between Marital Status and Cognitive Impairment in a Multi-Ethnic Asian Population. Neuroepidemiology 2024, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Y.; Burgard, S.A.; Needham, B.L. Marital status and cognitive impairment in the United States: Evidence from the National Health and Aging Trends Study. Ann. Epidemiol. 2019, 38, 28–34.e2. [Google Scholar] [CrossRef]
- Zhu, X.; Luchetti, M.; Aschwanden, D.; Sesker, A.A.; Stephan, Y.; Sutin, A.R.; Terracciano, A. Satisfaction With Life and Risk of Dementia: Findings From the Korean Longitudinal Study of Aging. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2022, 77, 1831–1840. [Google Scholar] [CrossRef]
- Ding, G.; Zhao, X.; Wang, Y.; Song, D.; Chen, D.; Deng, Y.; Xing, W.; Dong, H.; Zhou, Y.; Li, D.; et al. Evaluation of the relationship between cognitive impairment and suboptimal health status in a northern Chinese population: A cross-sectional study. J. Glob. Health 2020, 10, 010804. [Google Scholar] [CrossRef]
- Scarmeas, N.; Anastasiou, C.A.; Yannakoulia, M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018, 17, 1006–1015. [Google Scholar] [CrossRef]
- Cherbuin, N.; Anstey, K.J. The Mediterranean Diet is Not Related to Cognitive Change in a Large Prospective Investigation: The PATH Through Life Study. Am. J. Geriatr. Psychiatry 2012, 20, 635–639. [Google Scholar] [CrossRef]
- Laitinen, M.H.; Ngandu, T.; Rovio, S.; Helkala, E.-L.; Uusitalo, U.; Viitanen, M.; Nissinen, A.; Tuomilehto, J.; Soininen, H.; Kivipelto, M. Fat Intake at Midlife and Risk of Dementia and Alzheimer’s Disease: A Population-Based Study. Dement. Geriatr. Cogn. Disord. 2006, 22, 99–107. [Google Scholar] [CrossRef]
- Jayedi, A.; Shab-Bidar, S.; Eimeri, S.; Djafarian, K. Fish consumption and risk of all-cause and cardiovascular mortality: A dose–response meta-analysis of prospective observational studies. Public Health Nutr. 2018, 21, 1297–1306. [Google Scholar] [CrossRef]
- Van De Rest, O.; Wang, Y.; Barnes, L.L.; Tangney, C.; Bennett, D.A.; Morris, M.C. APOE e4 and the associations of seafood and long-chain omega-3 fatty acids with cognitive decline. Neurology 2016, 86, 2063–2070. [Google Scholar] [CrossRef]
- Ozawa, M.; Ohara, T.; Ninomiya, T.; Hata, J.; Yoshida, D.; Mukai, N.; Nagata, M.; Uchida, K.; Shirota, T.; Kitazono, T.; et al. Milk and Dairy Consumption and Risk of Dementia in an Elderly Japanese Population: The Hisayama Study. J. Am. Geriatr. Soc. 2014, 62, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yin, Y.; Niu, L.; Yang, X.; Du, X.; Tian, Q. Association between Changes in Protein Intake and Risk of Cognitive Impairment: A Prospective Cohort Study. Nutrients 2022, 15, 2. [Google Scholar] [CrossRef]
- Milte, C.M.; McNaughton, S.A. Dietary patterns and successful ageing: A systematic review. Eur. J. Nutr. 2016, 55, 423–450. [Google Scholar] [CrossRef]
- Matthews, F.E.; Arthur, A.; Barnes, L.E.; Bond, J.; Jagger, C.; Robinson, L.; Brayne, C. A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: Results of the Cognitive Function and Ageing Study I and II. Lancet 2013, 382, 1405–1412. [Google Scholar] [CrossRef]
- Christensen, K.; Thinggaard, M.; Oksuzyan, A.; Steenstrup, T.; Andersen-Ranberg, K.; Jeune, B.; McGue, M.; Vaupel, J.W. Physical and cognitive functioning of people older than 90 years: A comparison of two Danish cohorts born 10 years apart. Lancet 2013, 382, 1507–1513. [Google Scholar] [CrossRef]
- Qiu, C.; von Strauss, E.; Bäckman, L.; Winblad, B.; Fratiglioni, L. Twenty-year changes in dementia occurrence suggest decreasing incidence in central Stockholm, Sweden. Neurology 2013, 80, 1888–1894. [Google Scholar] [CrossRef]
- Allès, B.; Samieri, C.; Féart, C.; Jutand, M.A.; Laurin, D.; Barberger-Gateau, P. Dietary patterns: A novel approach to examine the link between nutrition and cognitive function in older individuals. Nutr. Res. Rev. 2012, 25, 207–222. [Google Scholar] [CrossRef]
- Kesse-Guyot, E.; Assmann, K.E.; Andreeva, V.A.; Touvier, M.; Neufcourt, L.; Shivappa, N.; Hébert, J.R.; Wirth, M.D.; Hercberg, S.; Galan, P.; et al. Long-term association between the dietary inflammatory index and cognitive functioning: Findings from the SU.VI.MAX study. Eur. J. Nutr. 2017, 56, 1647–1655. [Google Scholar] [CrossRef]
- Shin, D.; Kwon, S.C.; Kim, M.H.; Lee, K.W.; Choi, S.Y.; Shivappa, N.; Hébert, J.R.; Chung, H.K. Inflammatory potential of diet is associated with cognitive function in an older adult Korean population. Nutrients 2018, 55–56, 56–62. [Google Scholar] [CrossRef]
- Hayden, K.M.; Beavers, D.P.; Steck, S.E.; Hebert, J.R.; Tabung, F.K.; Shivappa, N.; Casanova, R.; Manson, J.E.; Padula, C.B.; Salmoirago-Blotcher, E.; et al. The association between an inflammatory diet and global cognitive function and incident dementia in older women: The Women’s Health Initiative Memory Study. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2017, 13, 1187–1196. [Google Scholar] [CrossRef]
- Samson, M.E.; Yeung, L.F.; Rose, C.E.; Qi, Y.P.; Taylor, C.A.; Crider, K.S. Vitamin B-12 malabsorption and renal function are critical considerations in studies of folate and vitamin B-12 interactions in cognitive performance: NHANES 2011–2014. Am. J. Clin. Nutr. 2022, 116, 74–85. [Google Scholar] [CrossRef]
- Makhlouf, S.; Messelmani, M.; Zaouali, J.; Mrissa, R. Cognitive impairment in celiac disease and non-celiac gluten sensitivity: Review of literature on the main cognitive impairments, the imaging and the effect of gluten free diet. Acta Neurol. Belg. 2018, 118, 21–27. [Google Scholar] [CrossRef]
- Croall, I.D.; Tooth, C.; Venneri, A.; Poyser, C.; Sanders, D.S.; Hoggard, N.; Hadjivassiliou, M. Cognitive Impairment in Coeliac Disease with Respect to Disease Duration and Gluten-Free Diet Adherence: A Pilot Study. Nutrients 2020, 12, 2028. [Google Scholar] [CrossRef]
- Araújo, J.R.; Martel, F.; Borges, N.; Araújo, J.M.; Keating, E. Folates and aging: Role in mild cognitive impairment, dementia and depression. Ageing Res. Rev. 2015, 22, 9–19. [Google Scholar] [CrossRef]
| Variable | Frequency (%) | AID (M ± SD) | p Value | PED (M ± SD) | p Value | CI (M ± SD) | p Value |
|---|---|---|---|---|---|---|---|
| Age, years | 8692 (100%) | 2.32 ± 1.18 | p < 0.001 | 2.95 ± 1.44 | 0.093 | 25.28 ± 6.01 | p < 0.001 |
| BMI, kg/m2 | |||||||
| <18.5 | 1304 (15%) | 2.05 ± 1.12 | p < 0.001 | 2.82 ± 1.38 | p < 0.001 | 22.65 ± 7.42 | p < 0.001 |
| 18.5–23.9 | 4740 (54.5%) | 2.27 ± 1.18 | 2.91 ± 1.45 | 25.25 ± 5.97 | |||
| 23.9–27.9 | 2145 (24.7%) | 2.54 ± 1.18 | 3.11 ± 1.43 | 26.68 ± 4.80 | |||
| >27.9 | 503 (5.8%) | 2.49 ± 1.15 | 3.01 ± 1.45 | 26.46 ± 4.57 | |||
| Gender | |||||||
| Male | 3896 (44.8%) | 2.47 ± 1.22 | p < 0.001 | 3.08 ± 1.39 | p < 0.001 | 26.54 ± 4.97 | p < 0.001 |
| Female | 4796 (55.2%) | 2.19 ± 1.14 | 2.84 ± 1.46 | 24.27 ± 6.57 | |||
| Nationality | |||||||
| Han | 8262 (95.1%) | 2.33 ± 1.19 | p < 0.001 | 2.98 ± 1.44 | 0.024 | 25.30 ± 5.99 | 0.066 |
| Others | 430 (4.9%) | 2.03 ± 1.08 | 2.45 ± 1.27 | 24.92 ± 6.52 | |||
| Education (years) | |||||||
| 0 | 3940 (45.3%) | 1.98 ± 1.06 | p < 0.001 | 2.65 ± 1.43 | p < 0.001 | 22.78 ± 6.87 | p < 0.001 |
| 1–6 | 2965 (34.1%) | 2.34 ± 1.14 | 2.94 ± 1.38 | 26.94 ± 4.37 | |||
| >6 | 1787 (20.6%) | 3.03 ± 1.18 | 3.63 ± 1.31 | 28.07 ± 3.73 | |||
| Marital status | |||||||
| Unmarried | 68 (0.8%) | 1.93 ± 1.14 | p < 0.001 | 2.46 ± 1.49 | p < 0.001 | 25.32 ± 5.18 | p < 0.001 |
| Married | 3854 (44.3%) | 2.51 ± 1.21 | 3.05 ± 1.42 | 27.53 ± 3.74 | |||
| Divorce or Separation | 184 (2.1%) | 2.47 ± 1.21 | 3.17 ± 1.41 | 26.88 ± 4.50 | |||
| Widowed | 4586 (52.8%) | 2.16 ± 1.13 | 2.87 ± 1.45 | 23.33 ± 6.89 | |||
| Smoking | |||||||
| Yes | 1379 (15.9%) | 2.40 ± 1.19 | 0.275 | 2.94 ± 1.38 | 0.017 | 26.60 ± 4.83 | p < 0.001 |
| No | 7313 (84.1%) | 2.30 ± 1.18 | 2.95 ± 1.45 | 25.04 ± 6.18 | |||
| Drinking | |||||||
| Yes | 1292 (14.9%) | 2.56 ± 1.23 | p < 0.001 | 3.11 ± 1.37 | 0.007 | 26.58 ± 4.81 | p < 0.001 |
| No | 7400 (85.1%) | 2.28 ± 1.17 | 2.92 ± 1.45 | 25.06 ± 6.17 | |||
| Sleep Duration (h) | |||||||
| <7 h | 4628 (53.2%) | 2.31 ± 1.20 | p < 0.001 | 2.91 ± 1.48 | 0.014 | 25.71 ± 5.58 | p < 0.001 |
| 7~9 h | 2451 (28.2%) | 2.40 ± 1.16 | 3.02 ± 1.40 | 26.04 ± 5.40 | |||
| >9 h | 1613 (18.6%) | 2.22 ± 1.14 | 2.97 ± 1.34 | 22.91 ± 7.36 | |||
| Life Satisfaction | |||||||
| Good | 8431 (97%) | 2.34 ± 1.18 | 0.096 | 2.98 ± 1.43 | 0.202 | 25.39 ± 5.93 | p < 0.001 |
| Not Good | 261 (3%) | 1.68 ± 1.10 | 2.19 ± 1.50 | 22.01 ± 7.60 | |||
| Residence | |||||||
| Urban | 1512 (17.4%) | 2.95 ± 1.19 | 0.485 | 3.71 ± 1.31 | p < 0.001 | 26.58 ± 5.37 | p < 0.001 |
| Rural | 7180 (82.6%) | 2.19 ± 1.14 | 2.79 ± 1.41 | 25.01 ± 6.11 | |||
| Health Condition | |||||||
| Good | 7516 (86.5%) | 2.37 ± 1.18 | p < 0.001 | 3.00 ± 1.42 | p < 0.001 | 25.52 ± 5.83 | p < 0.001 |
| Not Good | 1176 (13.5%) | 2.00 ± 1.16 | 2.67 ± 1.50 | 23.78 ± 6.90 |
| Model | AID | PED |
|---|---|---|
| Model 1 | 0.694 (0.657, 0.734) *** | 0.902 (0.864, 0.941) *** |
| Model 2 | 0.795 (0.746, 0.846) *** | 0.914 (0.871, 0.960) *** |
| Model 3 | 0.798 (0.749, 0.850) *** | 0.917 (0.874, 0.962) *** |
| Model 4 | 0.789 (0.740, 0.842) *** | 0.910 (0.866, 0.956) *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Xian, X.; Zhou, M.; Xu, K.; Cao, S.; Cheng, J.; Dai, W.; Zhang, W.; Ye, M. Anti-Inflammatory Diet and Protein-Enriched Diet Can Reduce the Risk of Cognitive Impairment among Older Adults: A Nationwide Cross-Sectional Research. Nutrients 2024, 16, 1333. https://doi.org/10.3390/nu16091333
Wang L, Xian X, Zhou M, Xu K, Cao S, Cheng J, Dai W, Zhang W, Ye M. Anti-Inflammatory Diet and Protein-Enriched Diet Can Reduce the Risk of Cognitive Impairment among Older Adults: A Nationwide Cross-Sectional Research. Nutrients. 2024; 16(9):1333. https://doi.org/10.3390/nu16091333
Chicago/Turabian StyleWang, Liang, Xiaobing Xian, Mengting Zhou, Ke Xu, Shiwei Cao, Jingyu Cheng, Weizhi Dai, Wenjia Zhang, and Mengliang Ye. 2024. "Anti-Inflammatory Diet and Protein-Enriched Diet Can Reduce the Risk of Cognitive Impairment among Older Adults: A Nationwide Cross-Sectional Research" Nutrients 16, no. 9: 1333. https://doi.org/10.3390/nu16091333
APA StyleWang, L., Xian, X., Zhou, M., Xu, K., Cao, S., Cheng, J., Dai, W., Zhang, W., & Ye, M. (2024). Anti-Inflammatory Diet and Protein-Enriched Diet Can Reduce the Risk of Cognitive Impairment among Older Adults: A Nationwide Cross-Sectional Research. Nutrients, 16(9), 1333. https://doi.org/10.3390/nu16091333






