The Role of PNPLA3_rs738409 Gene Variant, Lifestyle Factors, and Bioactive Compounds in Nonalcoholic Fatty Liver Disease: A Population-Based and Molecular Approach towards Healthy Nutrition
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Basic Characteristics of the Participants and Biochemical Measurements
2.3. Food and Nutrient Intake and Dietary Pattern Using a Semi-Quantitative Food Frequency Questionnaire (SQFFQ)
2.4. Definition of NAFLD
2.5. Genotyping and Quality Control
2.6. Genetic Variants Affecting NAFLD in Koreans
2.7. Screening of Bioactive Compounds in Foods to Have Low Binding Energy with PNPLA3
2.8. Molecular Dynamics Simulation (MDS)
2.9. Cell Culture
2.10. Realtime PCR
2.11. Statistical Analysis
3. Results
3.1. Basic Characteristics of the Participants
3.2. Genetic Variations Associated with NAFLD Risk
3.3. Interaction of PNPLA3_rs738409 with Lifestyles
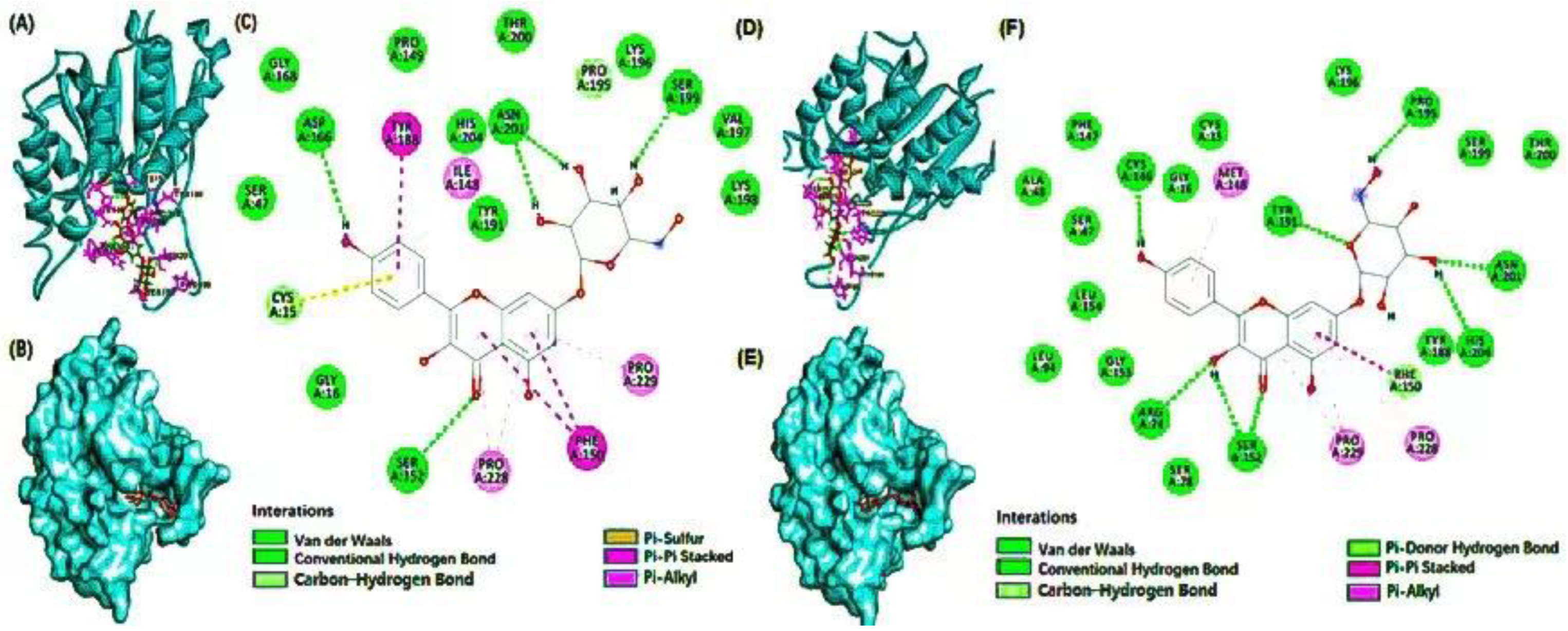
3.4. Molecular Docking
3.5. Molecular Dynamics (MD) Simulation
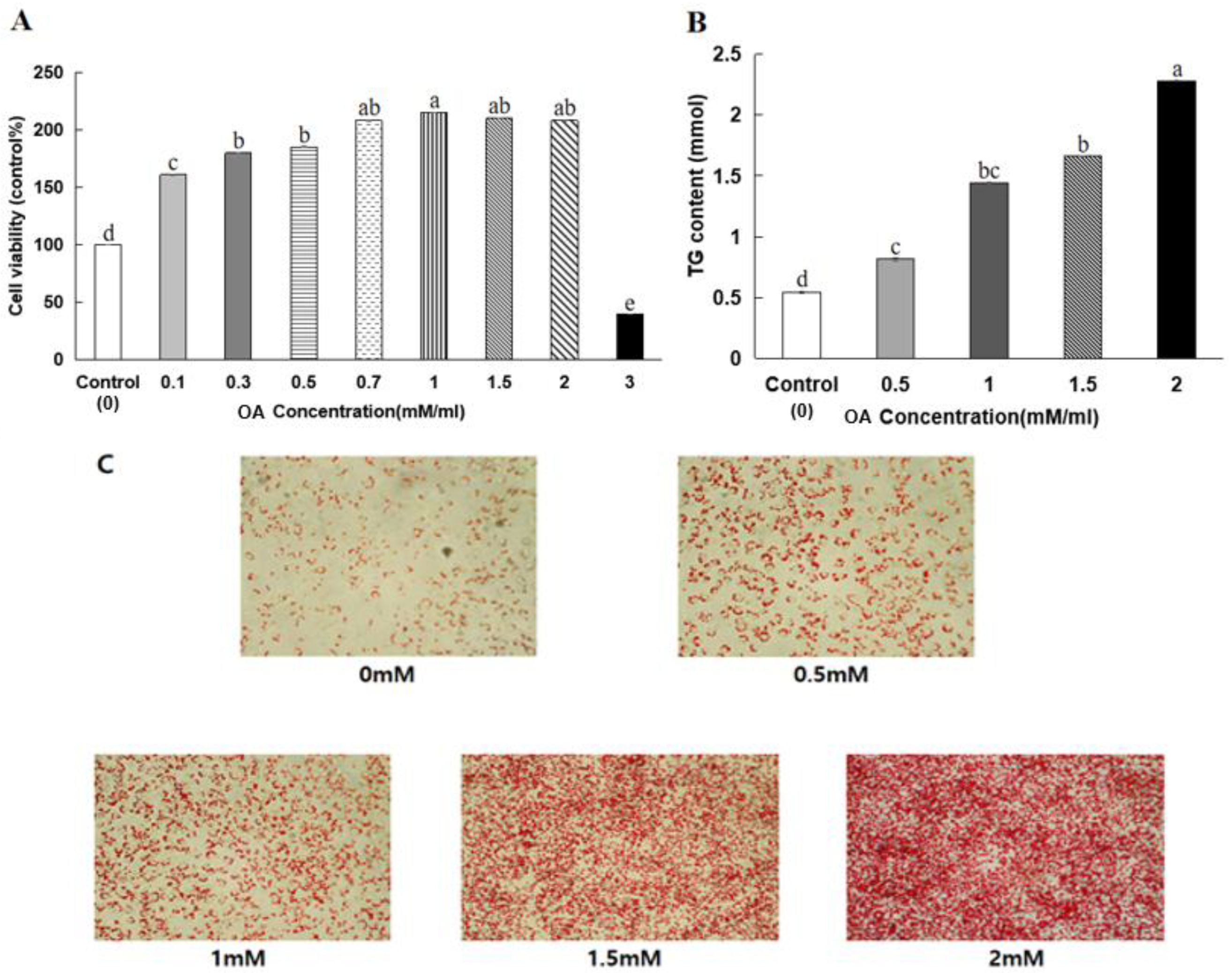
3.6. HepG2 Cell Viability
3.7. Lipid Peroxide Contents in HeG2 Cells
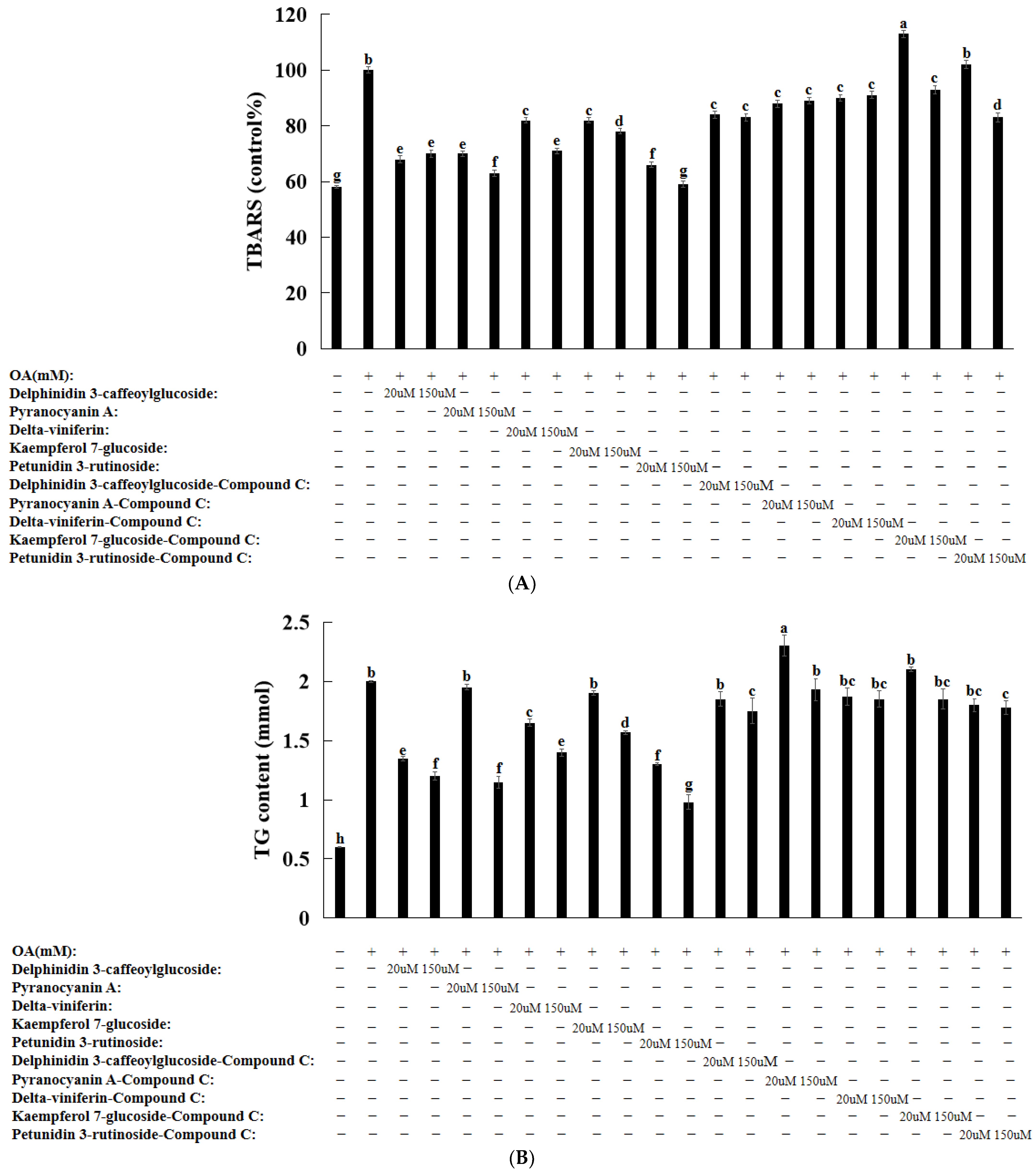
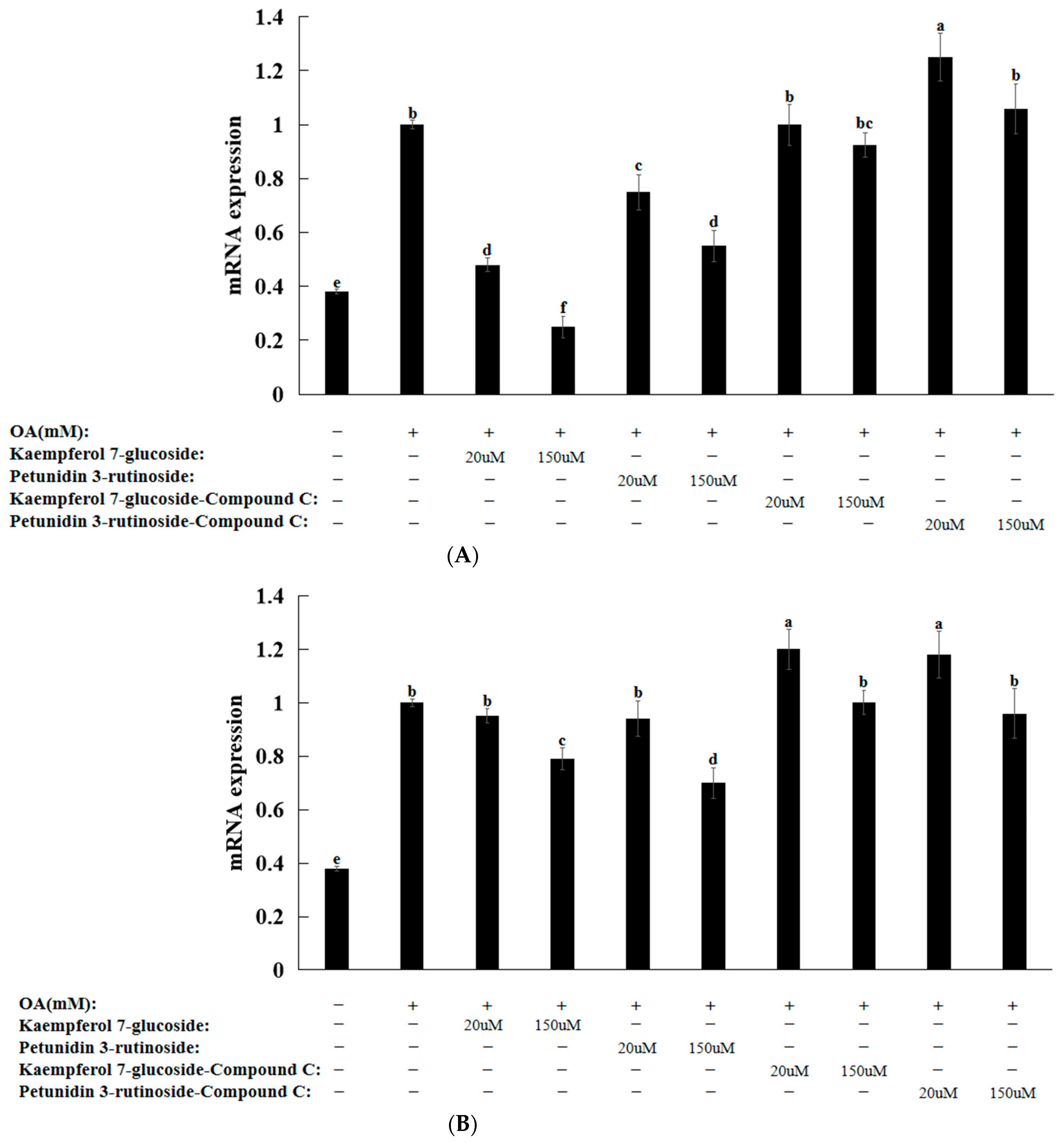
3.8. Natural Compounds in HepG2 Cells Induced by Oleic Acid
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benedict, M.; Zhang, X. Nonalcoholic fatty liver disease: An expanded review. World J. Hepatol. 2017, 9, 715. [Google Scholar] [CrossRef] [PubMed]
- Gofton, C.; Upendran, Y.; Zheng, M.H.; George, J. MAFLD: How is it different from NAFLD? Clin. Mol. Hepatol. 2023, 29, S17–S31. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.A.; Waters, O.R.; Knuiman, M.W.; Elliott, R.R.; Olynyk, J.K. NAFLD as a risk factor for the development of diabetes and the metabolic syndrome: An eleven-year follow-up study. Am. Coll. Gastroenterol. 2009, 104, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kang, S. High carbohydrate and noodle/meat-rich dietary patterns interact with the minor haplotype in the 22q13 loci to increase its association with nonalcoholic fatty liver disease risk in Koreans. Nutr. Res. 2020, 82, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Petta, S.; Targher, G.; Romeo, S.; Pajvani, U.B.; Zheng, M.H.; Aghemo, A.; Valenti, L.V.C. The first MASH drug therapy on the horizon: Current perspectives of resmetirom. Liver Int. 2024. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.-H.; Lin, H.-T.; Chung, D.-J.; Huang, C.-N.; Wang, C.-J. Mulberry Leaf Extracts prevent obesity-induced NAFLD with regulating adipocytokines, inflammation and oxidative stress. J. Food Drug Anal. 2018, 26, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Yogalakshmi, B.; Sreeja, S.; Geetha, R.; Radika, M.K.; Anuradha, C.V. Grape seed proanthocyanidin rescues rats from steatosis: A comparative and combination study with metformin. J. Lipids 2013, 2013, 153897. [Google Scholar] [CrossRef] [PubMed]
- Yari, Z.; Rahimlou, M.; Eslamparast, T.; Ebrahimi-Daryani, N.; Poustchi, H.; Hekmatdoost, A. Flaxseed supplementation in nonalcoholic fatty liver disease: A pilot randomized, open labeled, controlled study. Int. J. Food Sci. Nutr. 2016, 67, 461–469. [Google Scholar] [CrossRef]
- Oczkowski, M. Health-promoting effects of bioactive compounds in blackcurrant (Ribes nigrum L.) Berries. Roczniki Państwowego Zakładu Higieny 2021, 72, 229–238. [Google Scholar] [CrossRef]
- Day, E.A.; Ford, R.J.; Steinberg, G.R. AMPK as a therapeutic target for treating metabolic diseases. Trends Endocrinol. Metab. 2017, 28, 545–560. [Google Scholar] [CrossRef]
- Kumari, M.; Schoiswohl, G.; Chitraju, C.; Paar, M.; Cornaciu, I.; Rangrez, A.Y.; Wongsiriroj, N.; Nagy, H.M.; Ivanova, P.T.; Scott, S.A. Adiponutrin functions as a nutritionally regulated lysophosphatidic acid acyltransferase. Cell Metab. 2012, 15, 691–702. [Google Scholar] [PubMed]
- Lindén, D.; Ahnmark, A.; Pingitore, P.; Ciociola, E.; Ahlstedt, I.; Andréasson, A.-C.; Sasidharan, K.; Madeyski-Bengtson, K.; Zurek, M.; Mancina, R.M. Pnpla3 silencing with antisense oligonucleotides ameliorates nonalcoholic steatohepatitis and fibrosis in Pnpla3 I148M knock-in mice. Mol. Metab. 2019, 22, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Kumashiro, N.; Yoshimura, T.; Cantley, J.L.; Majumdar, S.K.; Guebre-Egziabher, F.; Kursawe, R.; Vatner, D.F.; Fat, I.; Kahn, M.; Erion, D.M. Role of patatin-like phospholipase domain-containing 3 on lipid-induced hepatic steatosis and insulin resistance in rats. Hepatology 2013, 57, 1763–1772. [Google Scholar] [CrossRef] [PubMed]
- Qiao, A.; Liang, J.; Ke, Y.; Li, C.; Cui, Y.; Shen, L.; Zhang, H.; Cui, A.; Liu, X.; Liu, C. Mouse patatin-like phospholipase domain-containing 3 influences systemic lipid and glucose homeostasis. Hepatology 2011, 54, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ahn, J.; Lee, B.-K. Self-rated subjective health status is strongly associated with sociodemographic factors, lifestyle, nutrient intakes, and biochemical indices, but not smoking status: KNHANES 2007–2012. J. Korean Med. Sci. 2015, 30, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Liu, M.; Kang, S. Alcohol intake interacts with CDKAL1, HHEX, and OAS3 genetic variants, associated with the risk of type 2 diabetes by lowering insulin secretion in Korean adults. Alcohol. Clin. Exp. Res. 2018, 42, 2326–2336. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Han, B.-G.; Group, K. Cohort profile: The Korean genome and epidemiology study (KoGES) consortium. Int. J. Epidemiol. 2017, 46, e20. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kang, S. A Western-style diet interacts with genetic variants of the LDL receptor to hyper-LDL cholesterolemia in Korean adults. Public. Health Nutr. 2021, 24, 2964–2974. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Jin, H.S.; Park, S. Protein and fat intake interacts with the haplotype of PTPN11_rs11066325, RPH3A_rs886477, and OAS3_rs2072134 to modulate serum HDL concentrations in middle-aged people. Clin. Nutr. 2020, 39, 942–949. [Google Scholar] [CrossRef]
- Doustmohammadian, A.; Amini, M.; Esmaillzadeh, A.; Omidvar, N.; Abtahi, M.; Dadkhah-Piraghaj, M.; Nikooyeh, B.; Neyestani, T.R. Validity and reliability of a dish-based semi-quantitative food frequency questionnaire for assessment of energy and nutrient intake among Iranian adults. BMC Res. Notes 2020, 13, 95. [Google Scholar] [CrossRef]
- Rajendran, V.; Purohit, R.; Sethumadhavan, R. In silico investigation of molecular mechanism of laminopathy caused by a point mutation (R482W) in lamin A/C protein. Amino Acids 2012, 43, 603–615. [Google Scholar] [CrossRef]
- Kumar, A.; Purohit, R. Computational screening and molecular dynamics simulation of disease associated nsSNPs in CENP-E. Mutat. Res. 2012, 738, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Rajendran, V.; Sethumadhavan, R.; Purohit, R. Evidence of colorectal cancer-associated mutation in MCAK: A computational report. Cell Biochem. Biophys. 2013, 67, 837–851. [Google Scholar] [CrossRef]
- Khodarahmi, G.; Asadi, P.; Farrokhpour, H.; Hassanzadeh, F.; Dinari, M. Design of novel potential aromatase inhibitors via hybrid pharmacophore approach: Docking improvement using the QM/MM method. RSC Adv. 2015, 5, 58055–58064. [Google Scholar] [CrossRef]
- Araya, J.; Rodrigo, R.; Videla, L.A.; Thielemann, L.; Orellana, M.; Pettinelli, P.; Poniachik, J. Increase in long-chain polyunsaturated fatty acid n− 6/n− 3 ratio in relation to hepatic steatosis in patients with nonalcoholic fatty liver disease. Clin. Sci. 2004, 106, 635–643. [Google Scholar] [CrossRef]
- Okamoto, Y.; Tanaka, S.; Haga, Y. Enhanced GLUT2 gene expression in an oleic acid-induced in vitro fatty liver model. Hepatol. Res. 2002, 23, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Janorkar, A.V.; King, K.R.; Megeed, Z.; Yarmush, M.L. Development of an in vitro cell culture model of hepatic steatosis using hepatocyte-derived reporter cells. Biotech. Bioeng. 2009, 102, 1466–1474. [Google Scholar] [CrossRef]
- Eslam, M.; George, J. Genetic contributions to NAFLD: Leveraging shared genetics to uncover systems biology. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 40–52. [Google Scholar] [CrossRef]
- Mantovani, A.; Petracca, G.; Beatrice, G.; Csermely, A.; Tilg, H.; Byrne, C.D.; Targher, G. Nonalcoholic fatty liver disease and increased risk of incident extrahepatic cancers: A meta-analysis of observational cohort studies. Gut 2022, 71, 778–788. [Google Scholar] [CrossRef]
- Estes, C.; Anstee, Q.M.; Arias-Loste, M.T.; Bantel, H.; Bellentani, S.; Caballeria, J.; Colombo, M.; Craxi, A.; Crespo, J.; Day, C.P. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, united kingdom, and united states for the period 2016–2030. J. Hepatol. 2018, 69, 896–904. [Google Scholar] [CrossRef]
- Byrne, C.D.; Patel, J.; Scorletti, E.; Targher, G. Tests for diagnosing and monitoring nonalcoholic fatty liver disease in adults. BMJ 2018, 362, k2734. [Google Scholar] [CrossRef] [PubMed]
- Molina-Molina, E.; Krawczyk, M.; Stachowska, E.; Lammert, F.; Portincasa, P. Nonalcoholic fatty liver disease in non-obese individuals: Prevalence, pathogenesis and treatment. Clin. Res. Hepatol. Gastroenterol. 2019, 43, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Guo, X.; Loomba, R.; Goodarzi, M.O.; Haritunians, T.; Kwon, S.; Cui, J.; Taylor, K.D.; Wilson, L.; Cummings, O.W. Genome-wide association study identifies variants associated with histologic features of nonalcoholic fatty liver disease. Gastroenterology 2010, 139, 1567–1576.e1566. [Google Scholar] [CrossRef]
- Sookoian, S.; Pirola, C.J. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology 2011, 53, 1883–1894. [Google Scholar] [CrossRef]
- Huang, Y.; Cohen, J.C.; Hobbs, H.H. Expression and characterization of a PNPLA3 protein isoform (I148M) associated with nonalcoholic fatty liver disease. J. Biol. Chem. 2011, 286, 37085–37093. [Google Scholar] [CrossRef] [PubMed]
- BasuRay, S.; Wang, Y.; Smagris, E.; Cohen, J.C.; Hobbs, H.H. Accumulation of PNPLA3 on lipid droplets is the basis of associated hepatic steatosis. Proc. Natl. Acad. Sci. USA 2019, 116, 9521–9526. [Google Scholar] [CrossRef]
- Pirazzi, C.; Valenti, L.; Motta, B.M.; Pingitore, P.; Hedfalk, K.; Mancina, R.M.; Burza, M.A.; Indiveri, C.; Ferro, Y.; Montalcini, T. PNPLA3 has retinyl-palmitate lipase activity in human hepatic stellate cells. Hum. Mol. Genet. 2014, 23, 4077–4085. [Google Scholar] [CrossRef]
- Bruschi, F.V.; Claudel, T.; Tardelli, M.; Caligiuri, A.; Stulnig, T.M.; Marra, F.; Trauner, M. The PNPLA3 I148M variant modulates the fibrogenic phenotype of human hepatic stellate cells. Hepatology 2017, 65, 1875–1890. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, B.E.; Rajagopal, V.; Smith, C.; Cohick, E.; Whissell, G.; Gamboa, M.; Pai, R.; Sigova, A.; Grossman, I.; Bumcrot, D. Discovery and targeting of the signaling controls of PNPLA3 to effectively reduce transcription, expression, and function in pre-clinical NAFLD/NASH settings. Cells 2020, 9, 2247. [Google Scholar] [CrossRef]
- Jung, E.-J.; Kwon, S.-W.; Jung, B.-H.; Oh, S.-H.; Lee, B.-H. Role of the AMPK/SREBP-1 pathway in the development of orotic acid-induced fatty liver. J. Lipid Res. 2011, 52, 1617–1625. [Google Scholar] [CrossRef]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Z.; Huang, Y.; Karaman, R.; Ivanova, P.T.; Brown, H.A.; Roddy, T.; Castro-Perez, J.; Cohen, J.C.; Hobbs, H.H. Chronic overexpression of PNPLA3 I148M in mouse liver causes hepatic steatosis. J. Clin. Investig. 2012, 122, 4130–4144. [Google Scholar] [CrossRef] [PubMed]
- Pingitore, P.; Pirazzi, C.; Mancina, R.M.; Motta, B.M.; Indiveri, C.; Pujia, A.; Montalcini, T.; Hedfalk, K.; Romeo, S. Recombinant PNPLA3 protein shows triglyceride hydrolase activity and its I148M mutation results in loss of function. Biochim. Biophys. Acta\Mol. Cell Biol. Lipids 2014, 1841, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Basantani, M.K.; Sitnick, M.T.; Cai, L.; Brenner, D.S.; Gardner, N.P.; Li, J.Z.; Schoiswohl, G.; Yang, K.; Kumari, M.; Gross, R.W. Pnpla3/Adiponutrin deficiency in mice does not contribute to fatty liver disease or metabolic syndrome. J. Lipid Res. 2011, 52, 318–329. [Google Scholar] [PubMed]
- Sinha, R.A.; Yen, P.M. Thyroid hormone-mediated autophagy and mitochondrial turnover in NAFLD. Cell Biosci. 2016, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.E.; Ramos-Roman, M.A.; Browning, J.D.; Parks, E.J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 2014, 146, 726–735. [Google Scholar] [CrossRef]
- Xu, B.; Shen, J.; Li, D.; Ning, B.; Guo, L.; Bing, H.; Chen, J.; Li, Y. Overexpression of microRNA-9 inhibits 3T3-L1 cell adipogenesis by targeting PNPLA3 via activation of AMPK. Gene 2020, 730, 144260. [Google Scholar]


| Non-NAFLD (n = 48,999) | NAFLD (n = 2089) | Adjusted OR (95% CI) | |
|---|---|---|---|
| Age (years) 1 | 53.77 ± 8.033 | 54.90 ± 7.886 *** | 1.040 (0.945~1.144) |
| Genders (men: N, %) | 14,908 (30.4) | 918 (43.9) *** | 1.343 (1.176~1.535) *** |
| BMI (kg/m2) 2 | 23.65 ± 2.775 | 25.56 ± 3.403 *** | 2.581 (2.357~2.827) *** |
| Waist circumference 3 | 79.93 ± 8.35 | 85.66 ± 9.26 *** | 1.479 (1.304~1.678) *** |
| Plasma total cholesterol (mg/dL) 4 | 197 ± 35.22 | 200 ± 39.97 ** | 1.259 (1.126~1.408) *** |
| Plasma triglyceride (mg/dL) 5 | 119 ± 76.22 | 161 ± 126 *** | 1.831 (1.635~2.049) *** |
| Hypertension (N, %) 6 | 12,997 (26.5) | 849 (40.7) *** | 1.317 (1.192~1.456) *** |
| Type 2 diabetes (N, %) 7 | 3641 (7.4) | 407 (19.5) *** | 2.330 (2.062~2.633) *** |
| Education (N, %) 8 | |||
| <High school | 14,907 (30.7) | 703 (33.9) ** | |
| High school | 21,027 (43.3) | 837 (40.4) | 1.021 (0.910~1.146) |
| College more | 12,616 (26.0) | 534 (25.7) | 1.002 (0.869~1.155) |
| Income (N, %) 9 | |||
| <$2000/month | 14,379 (31.1) | 656 (33.4) | |
| $2000–4000/month | 27,969 (60.5) | 1152 (58.7) | 0.996 (0.891~1.113) |
| >$4000/month | 3888 (8.4) | 154 (7.8) | 0.876 (0.694~1.106) |
| Energy intake (EER %) 10 | 98.80 ± 31.66 | 99.01 ± 32.59 | 1.093 (0.986~1.211) |
| CHO (EER %) 11 | 71.79 ± 6.93 | 71.80 ± 7.23 | 1.029 (0.936~1.131) |
| Protein (EER %) 12 | 13.39 ± 2.56 | 13.45 ± 2.65 | 1.033 (0.942~1.133) |
| Fat (EER %) 13 | 13.83 ± 5.38 | 13.72 ± 5.56 | 1.077 (0.938~1.237) |
| Cholesterol intake 14 | 169 ± 124 | 174 ± 138 | 1.083 (0.951~1.233) |
| Na intake (mg) 15 | 2421 ± 1364 | 2545 ± 1496 *** | 1.001 (0.896~1.118) |
| Fiber intake(g) 16 | 14.61 ± 9.26 | 15.45 ± 10.51 *** | 1.045 (0.936~1.166) |
| Alcohol (g) 17 | 2.09 ± 4.77 | 3.37 ± 6.35 *** | 1.406 (1.116~1.770) ** |
| KBD (%) 18 | 15,710 (32.1) | 756 (36.2) *** | 1.039 (0.938~1.150) |
| PBD (%) 18 | 16,963 (34.6) | 638 (30.5) *** | 0.948 (0.851~1.056) |
| WSD (%) 18 | 19,151 (39.1) | 859 (41.1) | 0.940 (0.847~1.043) |
| RMD (%) 18 | 16,276 (33.2) | 673 (32.2) | 1.014 (0.916~1.121) |
| Smoking (Number, %) | 11,458 (23.5) | 717 (34.4) *** | 1.167 (1.013~1.345) * |
| CHR | SNP | Position | Mi | Ma | OR | Adjust p Value | MAF | HWE_P | GENE | Function |
|---|---|---|---|---|---|---|---|---|---|---|
| 22 | rs738409 | 44324727 | G | C | 1.487 | 1.482 × 10−33 | 0.417 | 0.819 | PNPLA3 | Missense variant |
| Major (n = 17,448) | Hetero (n = 24,821) | Minor (n = 8819) | Gene–Nutrient Interaction p Value | |
|---|---|---|---|---|
| Low energy 1 High energy | 1 | 1.330 (1.167~1.516) 1.360 (1.109~1.669) | 2.342 (2.019~2.716) 1.941 (1.524~2.473) | 0.218 |
| Non-smoke Former + current smokers | 1 | 1.310 (1.148~1.495) 1.386 (1.139~1.686) | 1.892 (1.619~2.210) 3.121 (2.519~3.867) | <0.0001 |
| Low KBD 2 High KBD | 1 | 1.355 (1.181~1.556) 1.308 (1.090~1.570) | 2.206 (1.883~2.584) 2.247 (1.823~2.771) | 0.360 |
| Low PBD 2 High PBD | 1 | 1.367 (1.197~1.562) 1.285 (1.056~1.564) | 2.404 (2.066~2.798) 1.896 (1.509~2.383) | 0.019 |
| Low WSD 2 High WSD | 1 | 1.256 (1.091~1.446) 1.475 (1.235~1.760) | 1.955 (1.660~2.304) 2.696 (2.208~3.291) | 0.017 |
| Low RMD 2 High RMD | 1 | 1.388 (1.214~1.588) 1.236 (1.020~1.497) | 2.299 (1.970~2.682) 2.050 (1.644~2.555) | 0.462 |
| No exercise 3 Exercise | 1 | 1.348 (1.150~1.580) 1.329 (1.141~1.549) | 2.394 (2.000~2.865) 2.070 (1.732~2.473) | 0.072 |
| Compound Name | Effective Food | Residues Involved in Hydrogen Bond | Residues Involved in Hydrophobic Interactions | Wild Type | Mutant Type | ||
|---|---|---|---|---|---|---|---|
| Wild-Type | Mutant-Type | Wild-Type | Mutant-Type | Docking Energy, ΔG (kcal mol−1) | |||
| Delphinidin 3-caffeoyl-glucoside | grape | His204, Tyr188, Ser152, Cys146, Arg74 | Tyr188, Thr200, Ser199, Ser47, Asp166, Arg74 | Leu72, Leu51, Pro229, Phe150, Ile148 | Phe150, Leu203, Met148, Leu154 | −7.6 | −9.4 |
| Pyranocyanin A | blackcurrant | Arg74, Ser152, Val197, Ser199, Lys198, Pro195, Tyr191 | Val197, Lys198, Ser199, Pro195, Asn201, Tyr188, His204, Cys146 | His204, Phe150, Pro228, Pro229 | Cys15, Met148, Lys198, Tyr188, Phe150, Pro229 | −9.1 | −10.2 |
| Delta-viniferin | grape | His204, Pro229, Asn201, Pro195 | Cys15, Cys146, Arg74, Ser152, Ser199 | Pro228, Phe150, Tyr191 | Met148, Pro228, Phe150, Pro229 | −9 | −10 |
| Kaempferol 7-glucoside | flaxseed | Asp166, Ser152, Asn201, Ser199 | Cys146, Arg74, Ser152, Tyr191, Pro195, Asn201, His204 | Cys15, Tyr188, Ile148, Pro229, Phe150, Pro228 | Met148, Phe150, Pro228, Pro229 | −8.2 | −9.6 |
| Petunidin 3-rutinoside | mulberry | His204, Asn201, Ser199, Lys198 | Ser199, Asn201, Tyr191, Arg74, Pro228, Pro22, Ser78, Pro195, Ser152 | Phe150, Tyr191, Pro195 | Tyr191, Leu154, Met148 | −7.9 | −9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Park, S. The Role of PNPLA3_rs738409 Gene Variant, Lifestyle Factors, and Bioactive Compounds in Nonalcoholic Fatty Liver Disease: A Population-Based and Molecular Approach towards Healthy Nutrition. Nutrients 2024, 16, 1239. https://doi.org/10.3390/nu16081239
Liu M, Park S. The Role of PNPLA3_rs738409 Gene Variant, Lifestyle Factors, and Bioactive Compounds in Nonalcoholic Fatty Liver Disease: A Population-Based and Molecular Approach towards Healthy Nutrition. Nutrients. 2024; 16(8):1239. https://doi.org/10.3390/nu16081239
Chicago/Turabian StyleLiu, Meiling, and Sunmin Park. 2024. "The Role of PNPLA3_rs738409 Gene Variant, Lifestyle Factors, and Bioactive Compounds in Nonalcoholic Fatty Liver Disease: A Population-Based and Molecular Approach towards Healthy Nutrition" Nutrients 16, no. 8: 1239. https://doi.org/10.3390/nu16081239
APA StyleLiu, M., & Park, S. (2024). The Role of PNPLA3_rs738409 Gene Variant, Lifestyle Factors, and Bioactive Compounds in Nonalcoholic Fatty Liver Disease: A Population-Based and Molecular Approach towards Healthy Nutrition. Nutrients, 16(8), 1239. https://doi.org/10.3390/nu16081239








