Effects of Wheat Biscuits Enriched with Plant Proteins Incorporated into an Energy-Restricted Dietary Plan on Postprandial Metabolic Responses of Women with Overweight/Obesity
Abstract
1. Introduction
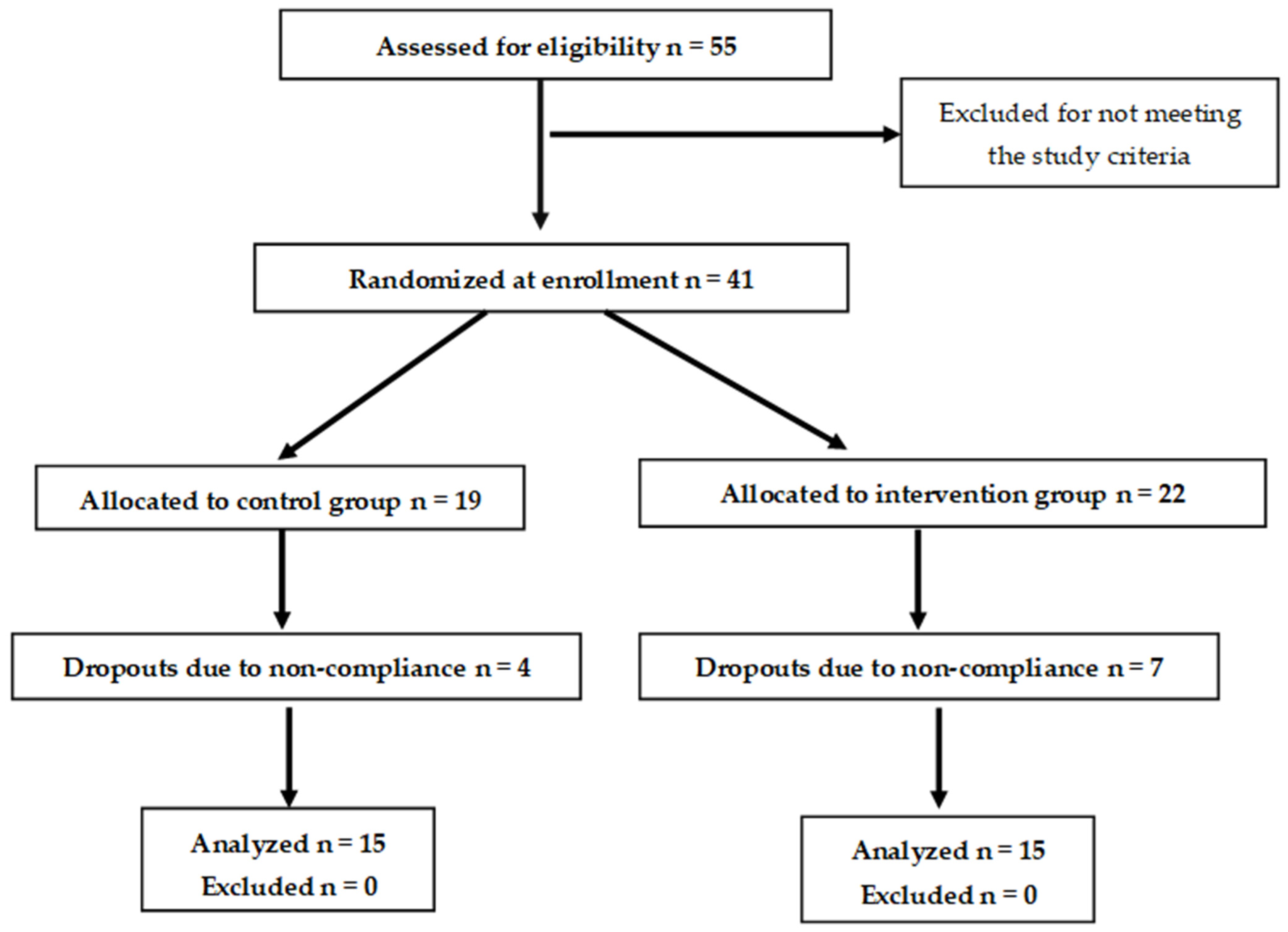
2. Materials and Methods
2.1. Study Subjects
2.2. Study Design
2.3. Blood Analyses
2.4. Subjective Satiety Measurements
2.5. Test Biscuits
2.6. Calculations and Statistical Analysis
3. Results
3.1. Anthropometric and Biochemical Characteristics
3.2. Mixed Meal Tolerance Test
3.2.1. Glucose and Hormone Responses
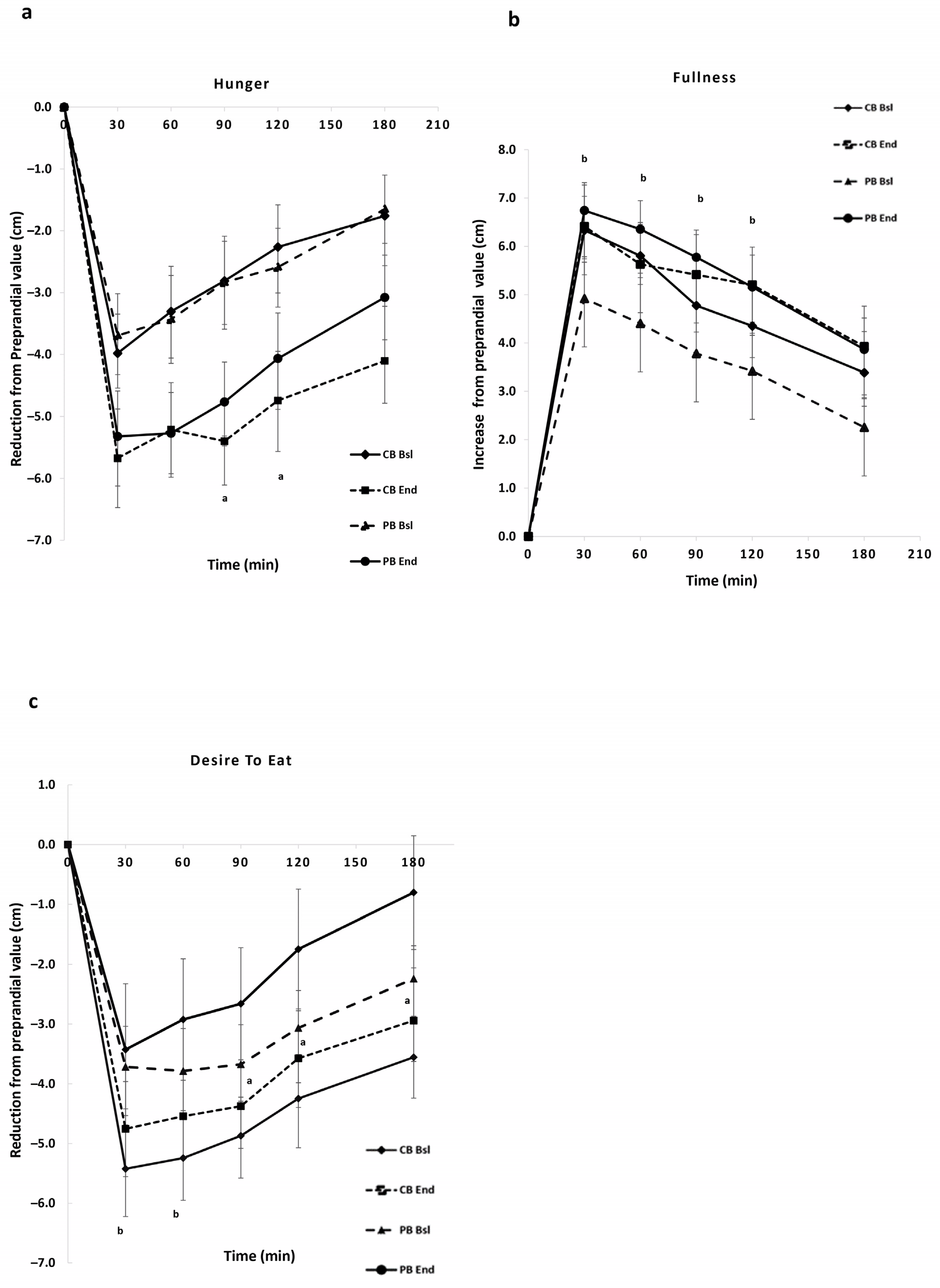
3.2.2. Subjective Appetite Ratings (VAS)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mayo Clinic. Available online: https://www.mayoclinic.org/diseases-consitions/obesity/symptoms-causes/syn-20375742 (accessed on 10 January 2024).
- Koliaki, C.; Liatis, S.; Dalamaga, M.; Kokkinos, A. The Implication of Gut Hormones in the Regulation of Energy Homeostasis and Their Role in the Pathophysiology of Obesity. Curr. Obes. Rep. 2020, 9, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Wharton, S.; Lau, D.C.W.; Vallis, M.; Sharma, A.M.; Biertho, L.; Campbell-Scherer, D.; Adamo, K.; Alberga, A.; Bell, R.; Boulé, N.; et al. Obesity in adults: A clinical practice guideline. CMAJ 2020, 192, 875–891. [Google Scholar] [CrossRef] [PubMed]
- Harvard Medical School. Available online: https://www.healthharvard.edu/blog/a-practical-guise-to-the-mediterranean-diet-2019032116194 (accessed on 15 January 2024).
- Yanni, A.E.; Iakovidi, S.; Vasilikopoulou, E.; Karathanos, V.T. Legumes: A Vehicle for Transition to Sustainability. Nutrients 2023, 16, 98. [Google Scholar] [CrossRef] [PubMed]
- Food Agriculture Organization (FAO). Available online: http://www.fao.org/3/a-i5528e.pdf (accessed on 10 January 2024).
- Papanikolaou, Y.; Fulgoni, V.L. Bean Consumption Is Associated with Greater Nutrient Intake, Reduced Systolic Blood Pressure, Lower Body Weight, and a Smaller Waist Circumference in Adults: Results from the National Health and Nutrition Examination Survey 1999–2002. J. Am. Coll. Nutr. 2008, 27, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Hafiz, M.S.; Campbell, M.D.; Orsi, N.M.; Mappa, G.; Orfila, C.; Boesch, C. Impact of food processing on postprandial glycaemic and appetite responses in healthy adults: A randomized, controlled trial. Food Funct. 2022, 13, 1280–1290. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Kendall, C.W.C.; De Souza, R.J.; Jayalath, V.H.; Cozma, A.I.; Ha, V.; Mirrahimi, A.; Chiavaroli, L.; Augustin, L.S.A.; Mejia, S.B.; et al. Dietary pulses, satiety and food intake: A systematic review and meta-analysis of acute feeding trials. Obesity 2014, 22, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Monnet, A.F.; Laleg, K.; Michon, C.; Micard, V. Legume enriched cereal products: A generic approach derived from material science to predict their structuring by the process and their final properties. Trends Food Sci. Technol. 2019, 86, 131–143. [Google Scholar] [CrossRef]
- Yanni, A.E.; Kokkinos, A.; Binou, P.; Papaioannou, V.; Halabalaki, M.; Konstantopoulos, P.; Simati, S.; Karathanos, V.T. Postprandial Glucose and Gastrointestinal Hormone Responses of Healthy Subjects to Wheat Biscuits Enriched with L-Arginine or Branched-Chain Amino Acids of Plant Origin. Nutrients 2022, 14, 4381. [Google Scholar] [CrossRef]
- Ropelle, E.R.; Pauli, J.R.; Fernandes, M.F.A.; Rocco, S.A.; Marin, R.M.; Morari, J.; Souza, K.K.; Dias, M.M.; Gomes-Marcondes, M.C.; Gontijo, J.A.R.; et al. A central role for neuronal AMP-activated protein kinase (AMPK) and mammalian target of rapamyein (mTOR) in high-protein diet-induced weight loss. Diabetes 2008, 57, 594–605. [Google Scholar] [CrossRef]
- Pathak, K.; Zhao, Y.; Calton, E.K.; James, A.P.; Newsholme, P.; Sherriff, J.; Soares, M.J. The impact of leucine supplementation on body composition and glucose tolerance following energy restriction: An 8-week RCT in adults at risk of the metabolic syndrome, The impact of leucine supplementation on body composition and glucose tolerance following energy restriction: An 8-week RCT in adults at risk of the metabolic syndrome. Eur. J. Clin. Nutr. 2024, 78, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Bolster, D.R.; Rahn, M.; Kamil, A.G.; Bristol, L.T.; Goltz, S.R.; Leidy, H.J.; Blaze, M.T.M.; Nunez, M.A.; Guo, E.; Wang, J.; et al. Consuming lower-protein nutrition bars with added leucine elicits postprandial changes in appetite sensations in healthy women. J. Nutr. 2018, 148, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Blouet, C.; Jo, Y.H.; Li, X.; Schwartz, G.J. Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus-brainstem circuit. J. Neurosci. 2009, 29, 8302–8311. [Google Scholar] [CrossRef]
- Purpera, M.N.; Shen, L.; Taghavi, M.; Münzberg, H.; Martin, R.J.; Hutson, S.M.; Morrison, C.D. Impaired branched-chain amino acid metabolism alters feeding behavior and increases orexigenic neuropeptide expression in the hypothalamus. J. Endocrinol. 2012, 212, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Monti, L.D.; Casiraghi, M.C.; Setola, E.; Galluccio, E.; Pagani, M.A.; Quaglia, L.; Bosi, E.; Piatti, P. L-Arginine enriched biscuits improve endothelial function and glucose metabolism: A pilot study in healthy subjects and a cross-over study in subjects with impaired glucose tolerance and metabolic syndrome. Metabolism 2013, 62, 255–264. [Google Scholar] [CrossRef]
- Oya, M.; Kitaguchi, T.; Pais, R.; Reimann, F.; Gribble, F.; Tsuboi, T. The G protein-coupled receptor family C group 6 subtype a (GPRC6A) receptor is involved in amino acid-induced glucagon-like peptide-1 secretion from GLUTag cells. J. Biol. Chem. 2013, 288, 4513–4521. [Google Scholar] [CrossRef]
- Wang, C.; Kang, C.; Xian, Y.; Zhang, M.; Chen, X.; Pei, M.; Zhu, W.; Hang, S. Sensing of L-Arginine by Gut-Expressed Calcium Sensing Receptor Stimulates Gut Satiety Hormones Cholecystokinin and Glucose-Dependent Insulinotropic Peptide Secretion in Pig Model. J. Food Sci. 2018, 83, 2394–2401. [Google Scholar] [CrossRef]
- Amin, A.; Neophytou, C.; Thein, S.; Martin, N.M.; Alamshah, A.; Spreckley, E.; Bloom, S.R.; Murphy, K.G. L-Arginine Increases Postprandial Circulating GLP-1 and PYY Levels in Humans. Obesity 2018, 26, 1721–1726. [Google Scholar] [CrossRef] [PubMed]
- Binou, P.; Yanni, A.E.; Kartsioti, K.; Barmpagianni, A.; Konstantopoulos, P.; Karathanos, V.T.; Kokkinos, A. Wheat Biscuits Enriched with Plant-Based Protein Contribute to Weight Loss and Beneficial Metabolic Effects in Subjects with Overweight/Obesity. Nutrients 2022, 14, 2516. [Google Scholar] [CrossRef]
- Stamataki, N.S.; Nikolidaki, E.K.; Yanni, A.E.; Stoupaki, M.; Konstantopoulos, P.; Tsigkas, A.P.; Perrea, D.; Tentolouris, N.; Karathanos, V.T. Evaluation of a high nutritional quality snack based on oat flakes and inulin: Effects on postprandial glucose, insulin and ghrelin responses of healthy subjects. Food Funct. 2016, 7, 3295–3303. [Google Scholar] [CrossRef]
- Brouns, F.; Bjorck, I.; Frayn, K.N.; Gibbs, A.L.; Lang, V.; Slama, G.; Wolever, T.M.S. Glycaemic index methodology. Nutr. Res. Rev. 2005, 18, 145–171. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Obesity and Overweight. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on 10 January 2024).
- Esposito, K.; Kastorini, C.M.; Panagiotakos, D.B.; Giugliano, D. Mediterranean Diet and Weight Loss: Meta-Analysis of Randomized Controlled Trials. Metab. Syndr. Relat. Disord. 2011, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gioxari, A.; Grammatikopoulou, M.G.; Katsarou, C.; Panagiotakos, D.B.; Toutouza, M.; Kavouras, S.A.; Sidossis, L.S.; Maraki, M.I. A Modified Mediterranean Diet Improves Fasting and Postprandial Glucoregulation in Adults with Overweight and Obesity: A Pilot Study. Int. J. Environ. Res. Public Health 2022, 19, 15347. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; De Souza, R.J.; Choo, V.L.; Ha, V.; Cozma, A.I.; Chiavaroli, L.; Mirrahimi, A.; Mejia, S.B.; Di Buono, M.; Bernstein, A.M.; et al. Effects of dietary pulse consumption on body weight: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2016, 103, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Pittaway, J.K.; Robertson, I.K.; Ball, M.J. Chickpeas May Influence Fatty Acid and Fiber Intake in an Ad Libitum Diet, Leading to Small Improvements in Serum Lipid Profile and Glycemic Control. J. Am. Diet. Assoc. 2008, 108, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Hafiz, M.S.; Campbell, M.D.; O’Mahoney, L.L.; Holmes, M.; Orfila, C.; Boesch, C. Pulse consumption improves indices of glycemic control in adults with and without type 2 diabetes: A systematic review and meta-analysis of acute and long-term randomized controlled trials. Eur. J. Nutr. 2022, 61, 809–824. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, H.; Vasconcelos, M.; Gil, A.M.; Pinto, E. Benefits of pulse consumption on metabolism and health: A systematic review of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2021, 61, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Clamp, L.D.; Hume, D.J.; Lambert, E.V.; Kroff, J. Enhanced insulin sensitivity in successful, long-term weight loss maintainers compared with matched controls with no weight loss history. Nutr. Diabetes 2017, 7, e282. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Moghadam, S.K.; Bahadoran, Z.; Ghasemi, A.; Azizi, F. Dietary L-Arginine Intakes and the Risk of Metabolic Syndrome: A 6-Year Follow-Up in Tehran Lipid and Glucose Study. Prev. Nutr. Food Sci. 2017, 22, 263–270. [Google Scholar] [CrossRef]
- Alamshah, A.; Mcgavigan, A.K.; Spreckley, E.; Kinsey-Jones, J.S.; Amin, A.; Tough, I.R.; O’Hara, H.C.; Moolla, A.; Banks, K.; France, R.; et al. L-arginine promotes gut hormone release and reduces food intake in rodents. Diabetes Obes. Metab. 2016, 18, 508–518. [Google Scholar] [CrossRef]
- Clemmensen, C.; Smajilovic, S.; Smith, E.P.; Woods, S.C.; Bräuner-Osborne, H.; Seeley, R.J.; D’Alessio, D.A.; Ryan, K.K. Oral L-arginine stimulates GLP-1 secretion to improve glucose tolerance in male mice. Endocrinology 2013, 154, 3978–3983. [Google Scholar] [CrossRef]
- Hall, R.S.; Thomas, S.J.; Johnson, S.K. Australian sweet lupin flour addition reduces the glycaemic index of a white bread breakfast without affecting palatability in healthy human volunteers. Asia Pac. J. Clin. Nutr. 2005, 14, 91–97. [Google Scholar] [PubMed]
- Lages, M.; Barros, R.; Moreira, P.; Guarino, M.P. Metabolic Effects of an Oral Glucose Tolerance Test Compared to the Mixed Meal Tolerance Tests: A Narrative Review. Nutrients 2022, 14, 2932. [Google Scholar] [CrossRef]
- Albarazanji, K.; Nawrocki, A.R.; Gao, B.; Wang, X.; Wang, Y.; Xiao, Y.F. Effects of mixed meal tolerance test on gastric emptying, glucose and lipid homeostasis in obese nonhuman primates. Sci. Rep. 2021, 11, 11866. [Google Scholar] [CrossRef] [PubMed]
- Kuhle, E.; Deacon, C.F.; Holst, J.J.; Petersen, N. What Is ab L-Cell and How Do we Study the Secretory Mechanisms of the L-Cell? Front. Endocrinol. 2021, 12, 694284. [Google Scholar] [CrossRef]
- Eelderink, C.; Noort, M.W.J.; Sozer, N.; Koehorst, M.; Holst, J.J.; Deacon, C.F.; Rehfeld, J.F.; Poutanen, K.; Vonk, R.J.; Oudhuis, L.; et al. Difference in postprandial GLP-1 response despite similar glucose kinetics after consumption of wheat breads with different particle size in healthy men. Eur. J. Nutr. 2017, 56, 1063–1076. [Google Scholar] [CrossRef]
- Stefanakis, K.; Kokkinos, A.; Simati, S.; Argyrakopoulou, G.; Konstantinidou, S.K.; Kouvari, M.; Kumar, A.; Kalra, B.; Mantzoros, C.S. Circulating levels of all proglucagon-derived peptides are differentially regulated postprandially by obesity status and in response to high-fat meals vs. high-carbohydrate meals. Clin. Nutr. 2023, 42, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- D’Alessio, D. Is GLP-1 a hormone: Whether and When? J. Diabetes Investig. 2016, 7, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Ohneda, A.; Kobayashi, T.; Nihei, J.; Takahashi, H. Effect of intraluminal administration of amino acids upon plasma glicentin. Diabetes Res. Clin. Pract. 1988, 5, 265–270. [Google Scholar] [CrossRef]
- Raffort, J.; Lareyre, F.; Massalou, D.; Fénichel, P.; Panaïa-Ferrari, P.; Chinetti, G. Insights on glicentin, a promising peptide of the proglucagon family. Biochem. Med. 2017, 27, 308–324. [Google Scholar] [CrossRef]
- Lafferty, R.A.; O’Harte, F.P.M.; Irwin, N.; Gault, V.A.; Flatt, P.R. Proglucagon-Derived Peptides as Therapeutics. Front. Endocrinol. 2021, 12, 689678. [Google Scholar] [CrossRef] [PubMed]
- Perakakis, N.; Mantzoros, C.S. The Role of Glicentin and Oxyntomodulin in Human Metabolism: New Evidence and New Directions. J. Clin. Endocrinol. Metab. 2020, 105, 3003–3005. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.S.; Ritz, C.; Albrechtsen, N.J.W.; Holst, J.J.; le Roux, C.W.; Sjödin, A. Oxyntomodulin and glicentin may predict the effect of bariatric surgery on food preferences and weight loss. J. Clin. Endocrinol. Metab. 2020, 105, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- Desroches, S.; Lapointe, A.; Ratté, S.; Gravel, K.; Légaré, F.; Turcotte, S. Interventions to enhance adherence to dietary advice for preventing and managing chronic diseases in adults. Cochrane Database Syst. Rev. 2013, 2, CD008722. [Google Scholar] [CrossRef]
- Alhassan, S.; Kim, S.; Bersamin, A.; King, A.C.; Gardner, C.D. Dietary adherence and weight loss success among overweight women: Results from the A to Z weight loss study. Int. J. Obes. 2008, 32, 985–991. [Google Scholar] [CrossRef]

| Variable | CB Baseline | CB End | p-Value * | PB Baseline | PB End | p-Value * |
|---|---|---|---|---|---|---|
| Age (years) | 38.3 ± 8.0 | 37.8± 9.4 | ||||
| Weight (kg) | 80.4 ± 2.5 | 77.0 ± 2.8 | 0.001 | 80.2 ± 3.2 | 75.9 ± 3.2 | <0.001 |
| Body fat mass (kg) | 31.4 ±1.6 | 27.9 ± 1.8 | 0.001 | 29.6 ± 2.9 | 25.8 ± 2.7 | <0.001 |
| Body fat (%) | 38.7± 1.0 | 35.8 ± 1.2 | 0.005 | 37.7 ± 1.1 | 35.2 ± 1.3 | 0.015 |
| BMI (kg/m2) | 30.5 ± 0.9 | 29.0 ± 1.0 | 0.006 | 29.4 ± 1.0 | 27.9 ± 1.0 | <0.001 |
| WC (cm) | 93.6 ± 1.9 | 85.8 ± 2.2 | <0.001 | 89.5 ± 2.9 | 80.0 ± 2.5 | <0.001 |
| Lean mass (kg) | 49.0 ± 1.2 | 49.1 ± 1.3 | 0.902 | 50.5 ± 2.2 | 50.8 ± 2.3 | 0.452 |
| Glucose (mg/dL) | 92.9 ± 1.5 | 90.5 ± 2.0 | 0.146 | 91.4 ± 2.8 | 92.6 ± 2.5 | 0.453 |
| Cholesterol (mg/dL) | 183.5 ± 10.9 | 179.7 ± 7.3 | 0.565 | 179.3 ± 7.2 | 176.9 ± 6.6 | 0.709 |
| HDL-c (mg/dL) | 49.9 ± 2.6 | 49.1 ± 2.4 | 0.331 | 54.3 ± 3.1 | 55.0 ± 2.9 | 0.367 |
| LDL-c (mg/dL) | 114.4 ± 9.8 | 114.7 ± 6.7 | 0.968 | 111.20 ± 6.5 | 108.5 ± 7.1 | 0.662 |
| Triacylglycerols (mg/dL) | 83.7 ± 9.9 | 80.3 ± 9.5 | 0.654 | 67.7 ± 7.1 | 68.4 ± 6.4 | 0.871 |
| AST (U/L) | 16.7 ± 1.1 | 15.8 ± 1.1 | 0.380 | 15.8 ± 1.2 | 14.9 ± 0.7 | 0.337 |
| ALT (U/L) | 17.0 ± 1.8 | 16.3 ± 1.6 | 0.694 | 15.8 ± 1.2 | 14.9 ± 0.7 | 0.596 |
| γ-GT (U/L) | 18.1 ± 1.3 | 18.3 ± 1.2 | 0.902 | 17.2 ± 0.7 | 16.2 ± 0.9 | 0.149 |
| Variable | CB Baseline | PB Baseline | p-Value * | CB End | PB End | p-Value * |
|---|---|---|---|---|---|---|
| Energy (Kcal) | 2356.0 ± 396.3 | 2372.5 ± 416.8 | 0.912 | 1965.0 ± 153.0 | 1703.2 ± 39.5 | 0.015 |
| Protein (g) | 97.2 ± 26.2 | 97.8 ± 4.5 | 0.940 | 79.8 ± 22.3 | 81.6 ± 2.2 | 0.772 |
| Carbohydrate (g) | 247.4 ± 41.4 | 227.3 ± 61.7 | 0.306 | 232.8 ± 42.3 | 191.1 ± 19.6 | 0.002 |
| Fat (g) | 109.5 ± 26.1 | 117.3 ± 26.3 | 0.421 | 81.4 ± 16.9 | 69.8 ± 11.2 | 0.036 |
| CB Baseline | CB End | p-Value | PB Baseline | PB End | p-Value * | p-Value ** | |
|---|---|---|---|---|---|---|---|
| Insulin (μU/mL) | 10.12 ± 1.60 | 8.97 ± 1.30 | 0.264 | 10.10 ± 1.56 | 7.30 ± 1.06 | 0.020 | 0.498 |
| HOMA-IR | 2.38 ± 0.49 | 2.08 ± 0.53 | 0.263 | 2.38 ± 0.43 | 1.71 ± 0.28 | 0.018 | 0.556 |
| iAUC-180 min | CB Baseline | CB End | p-Value * | PB Baseline | PB End | p-Value * | p-Value ** |
|---|---|---|---|---|---|---|---|
| Glucose (mg·min·dL−1) | 1837.06 ± 267.33 | 1728.45 ± 325.65 | 0.683 | 1283.42 ± 502.07 | 1248.00 ± 1004.00 | 0.900 | 0.264 |
| Insulin (μU·min·mL−1) | 10,629.00 ± 1770.76 | 7947.28 ± 1103.96 | 0.004 | 8786.55 ± 781.65 | 7178.30 ± 580.00 | 0.009 | 0.543 |
| Ghrelin (pg·min·mL−1) | −25,106.36 ± 5138.34 | −37,926.42 ± 7551.38 | 0.057 | −35,666.4 7± 8473.14 | −35,575.36 ± 6643.95 | 0.981 | 0.820 |
| GLP-1 (pg·min·mL−1) | 2976.00 ± 534.05 | 3262.00 ± 336.10 | 0.394 | 2878.00 ± 379.00 | 3185.86 ± 461.34 | 0.557 | 0.895 |
| Glicentin (pg·min·mL−1) | 20,873.72 ± 3113.35 | 25,034.95 ± 2734.00 | 0.138 | 17,809.83 ± 2634.46 | 24,086.62 ± 2966.63 | 0.040 | 0.484 |
| iAUC (cmx180 min) | CB Baseline | CB End | p-Value * | PB Baseline | PB End | p-Value * |
|---|---|---|---|---|---|---|
| Hunger | −561.27 ± 86.62 | −828.06 ± 120.85 | 0.036 | −545.66 ± 97 | −750.20 ± 112.61 | 0.072 |
| Fullness | 806.62 ± 109.01 | 873.94 ± 128.00 | 0.504 | 680.37 ± 97 | 911.87 ± 93.54 | 0.019 |
| Desire to eat | −587.52 ± 87.55 | −891.36 ± 134.22 | 0.021 | −598.28 ± 102.44 | −736.34 ± 115.78 | 0.226 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanata, M.-C.; Yanni, A.E.; Koliaki, C.; Pateras, I.; Anastasiou, I.A.; Kokkinos, A.; Karathanos, V.T. Effects of Wheat Biscuits Enriched with Plant Proteins Incorporated into an Energy-Restricted Dietary Plan on Postprandial Metabolic Responses of Women with Overweight/Obesity. Nutrients 2024, 16, 1229. https://doi.org/10.3390/nu16081229
Kanata M-C, Yanni AE, Koliaki C, Pateras I, Anastasiou IA, Kokkinos A, Karathanos VT. Effects of Wheat Biscuits Enriched with Plant Proteins Incorporated into an Energy-Restricted Dietary Plan on Postprandial Metabolic Responses of Women with Overweight/Obesity. Nutrients. 2024; 16(8):1229. https://doi.org/10.3390/nu16081229
Chicago/Turabian StyleKanata, Maria-Christina, Amalia E. Yanni, Chrysi Koliaki, Irene Pateras, Ioanna A. Anastasiou, Alexander Kokkinos, and Vaios T. Karathanos. 2024. "Effects of Wheat Biscuits Enriched with Plant Proteins Incorporated into an Energy-Restricted Dietary Plan on Postprandial Metabolic Responses of Women with Overweight/Obesity" Nutrients 16, no. 8: 1229. https://doi.org/10.3390/nu16081229
APA StyleKanata, M.-C., Yanni, A. E., Koliaki, C., Pateras, I., Anastasiou, I. A., Kokkinos, A., & Karathanos, V. T. (2024). Effects of Wheat Biscuits Enriched with Plant Proteins Incorporated into an Energy-Restricted Dietary Plan on Postprandial Metabolic Responses of Women with Overweight/Obesity. Nutrients, 16(8), 1229. https://doi.org/10.3390/nu16081229







