Insights into the Anti-Adipogenic and Anti-Inflammatory Potentialities of Probiotics against Obesity
Abstract
1. Introduction
2. Methodology
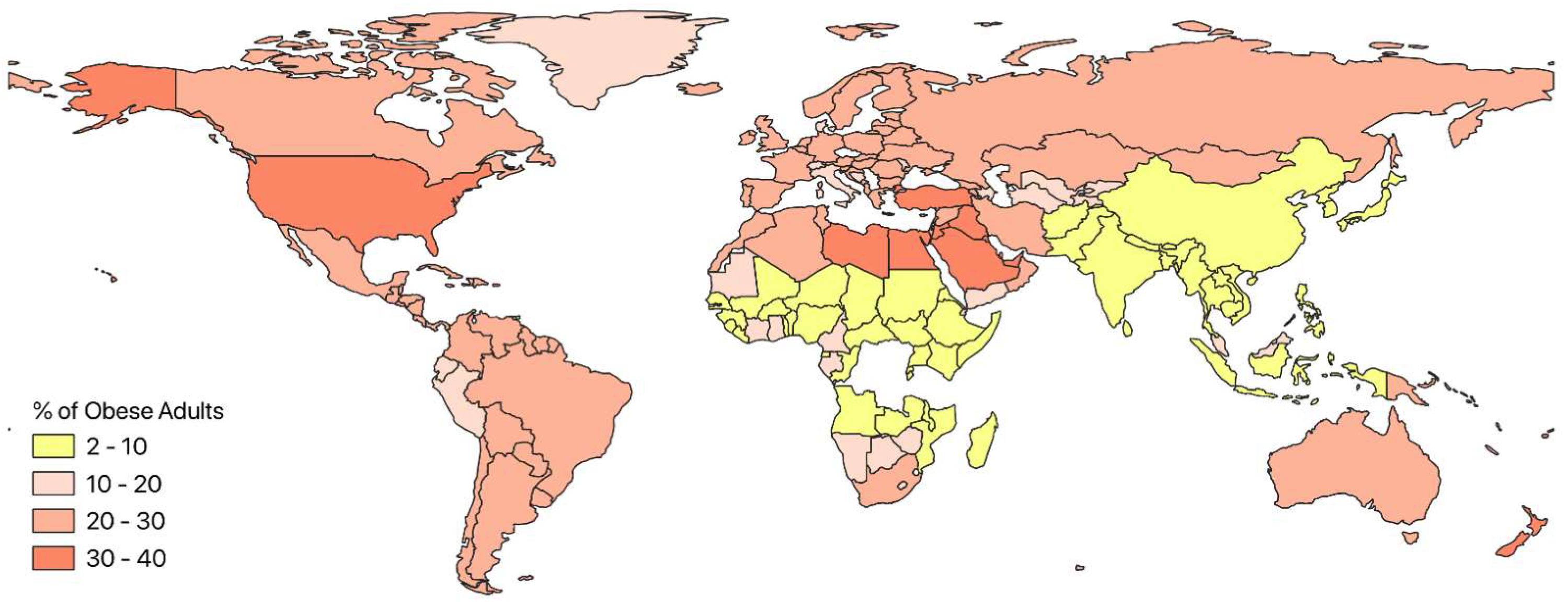
3. Global Trends in Obesity
4. Overview and Role of Adipose Tissue in Obesity
5. Role of Probiotics in Human Physiology
6. Anti-Obesity and Anti-Inflammatory Effects of Probiotics
6.1. In Vivo Studies Performed in Animal Model
6.2. In Vivo Studies Performed in Human Clinical Trials
6.3. In Vitro Studies Performed Using Different Animal Cell Lines
7. Limitations of Using Probiotics in Different Research Models (In Vitro and In Vivo)
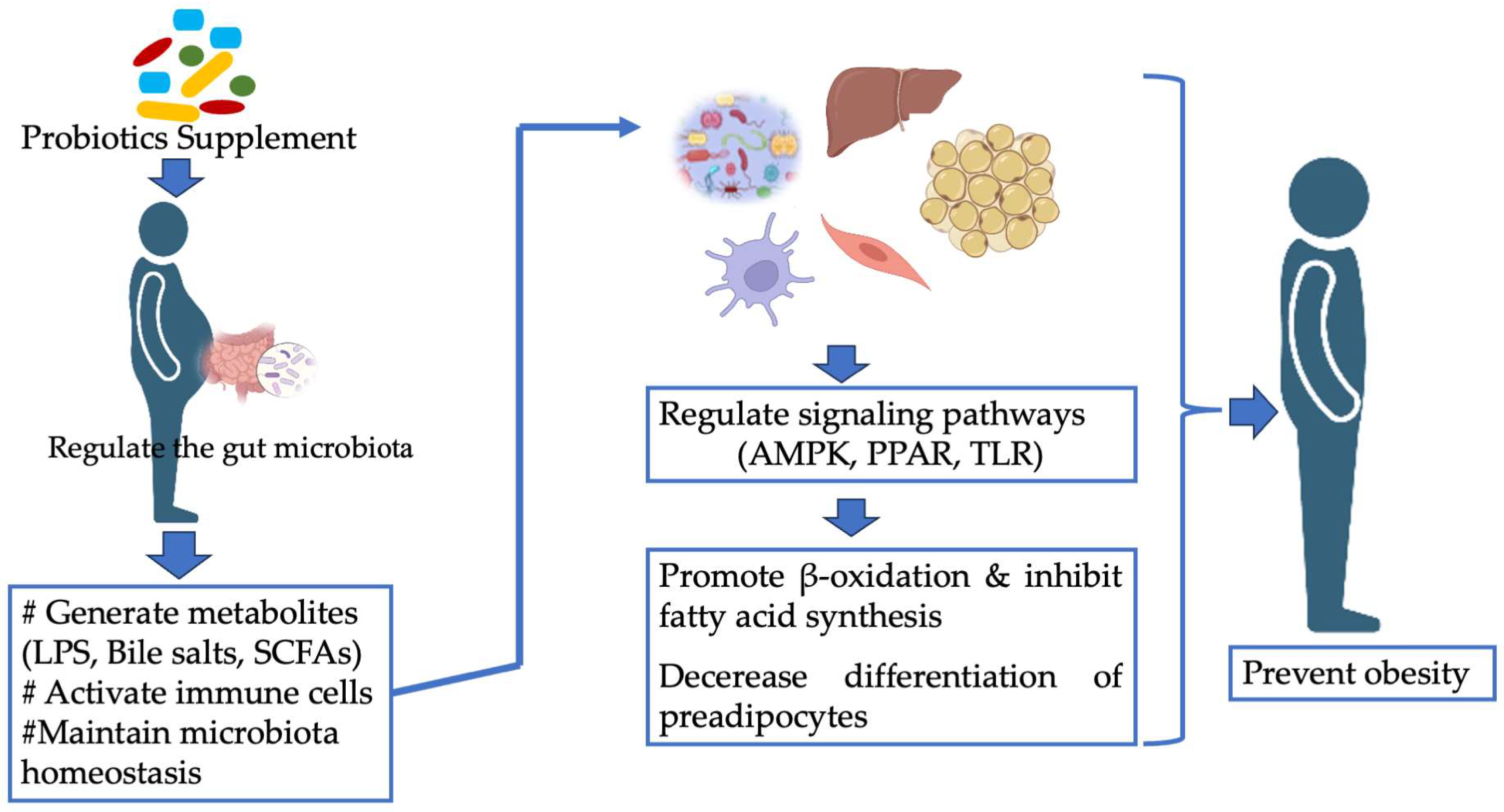
8. Mechanisms of Action of Probiotics as Anti-Obesity
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization (WHO). Obesity. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight#:~:text=In%202022%2C%202.5%20billion%20adults,age%20of%205%20were%20overweight (accessed on 10 August 2022).
- Jung, U.J.; Choi, M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [PubMed]
- Kelly, T.; Yang, W.; Chen, C.S.; Reynolds, K.; He, J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. 2008, 32, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.J.; Ding, H.J.; Deng, J.Y.; Pan, H.; Wang, L.J.; Li, N.S.; Wang, X.Q.; Shi, Y.F.; Gong, F.Y. Inhibition of preadipocyte differentiation and adipogenesis by zinc-a2-glycoprotein treatment in 3T3-L1 cells. J. Diabetes Investig. 2013, 4, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Oliver, E.; McGillicuddy, F.; Phillips, C.; Toomey, S.; Roche, H.M. The role of inflammation and macrophage accumulation in the development of obesity-induced type 2 diabetes mellitus and the possible therapeutic effects of long-chain n-3 PUFA. Proc. Nutr. Soc. 2010, 69, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, A.; Villa, C.R.; Comelli, E.M. Probiotics in early life: A preventative and treatment approach. Food Funct. 2016, 7, 1752–1768. [Google Scholar] [CrossRef] [PubMed]
- Ara, A.; Uddin, M.J.; Saha, S.; Khan, M.M.H.; Baset, M.A. Intervention of fruit juice in yoghurt preparation. Isesco J. Sci. Technol. 2015, 11, 30–35. [Google Scholar]
- Villena, J.; Kitazawa, H. Modulation of Intestinal TLR4-Inflammatory Signaling Pathways by Probiotic Microorganisms: Lessons Learned from Lactobacillus jensenii TL2937. Front. Immunol. 2014, 4, 512. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Park, K.Y.; Kim, B.; Kim, E.; Hyun, C.K. Lactobacillus rhamnosus GG improves insulin sensitivity and reduces adiposity in high-fat diet-fed mice through enhancement of adiponectin production. Biochem. Biophys. Res. Commun. 2013, 431, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Tonucci, L.B.; Dos Santos, K.M.O.; de Oliveira, L.L.; Ribeiro, S.M.R.; Martino, H.S.D. Clinical application of probiotics in type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled study. Clin. Nutr. 2017, 36, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Tada, A.; Islam, M.A.; Kober, A.H.; Fukuyama, K.; Takagi, M.; Igata, M.; Albarracin, L.; Ikeda-Ohtsubo, W.; Miyazawa, K.; Yoda, K.; et al. Transcriptome Modifications in the Porcine Intramuscular Adipocytes during Differentiation and Exogenous Stimulation with TNF-α and Serotonin. Int. J. Mol. Sci. 2020, 21, 638. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.J.; Dong, H.J.; Jeong, H.U.; Ryu, D.W.; Song, S.M.; Kim, Y.R.; Jung, H.H.; Kim, T.H.; Kim, Y.H. Lactobacillus plantarum LMT1-48 exerts anti-obesity effect in high-fat diet-induced obese mice by regulating expression of lipogenic genes. Sci. Rep. 2020, 10, 869. [Google Scholar] [CrossRef] [PubMed]
- Alard, J.; Cudennec, B.; Boutillier, D.; Peucelle, V.; Descat, A.; Decoin, R.; Kuylle, S.; Jablaoui, A.; Rhimi, M.; Wolowczuk, I.; et al. Multiple Selection Criteria for Probiotic Strains with High Potential for Obesity Management. Nutrients 2021, 13, 713. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, K.; He, F.; Yoda, K. and Hiramatsu, M. Potent effects of, and mechanisms for, modification of crosstalk between macrophages and adipocytes by lactobacilli. Microbiol. Immunol. 2012, 56, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Song, J.L.; Park, E.S.; Ju, J.; Kim, H.Y.; Park, K.Y. Anti-Obesity Effects of Starter Fermented Kimchi on 3T3-L1 Adipocytes. Prev. Nutr. Food Sci. 2015, 20, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Tada, A.; Kanmani, P.; Watanabe, H.; Aso, H.; Suda, Y.; Nochi, T.; Miyazawa, K.; Yoda, K.; He, F.; et al. Advanced application of porcine intramuscular adipocytes for evaluating anti-adipogenic and anti-inflammatory activities of immunobiotics. PLoS ONE 2015, 10, e0119644. [Google Scholar] [CrossRef] [PubMed]
- Tada, A.; Kober, A.H.; Islam, M.A.; Igata, M.; Takagi, M.; Suzuki, M.; Aso, H.; Ikeda-Ohtsubo, W.; Yoda, K.; Miyazawa, K.; et al. Evaluation of Fat Accumulation and Adipokine Production during the Long-Term Adipogenic Differentiation of Porcine Intramuscular Preadipocytes and Study of the Influence of Immunobiotics. Cells 2020, 9, 1715. [Google Scholar] [CrossRef]
- Choi, J.; Joseph, L.; Pilote, L. Obesity and C-reactive protein in various populations: A systematic review and meta-analysis. Obes. Rev. 2013, 14, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Fukuyama, K.; Debnath, M.; Namai, F.; Nishiyama, K.; Kitazawa, H. Recent Advances in the Use of Probiotics to Improve Meat Quality of Small Ruminants: A Review. Microorganisms 2023, 11, 1652. [Google Scholar] [CrossRef] [PubMed]
- Molarius, A.; Seidell, J.C.; Sans, S.; Tuomilehto, J.; Kuulasmaa, K.; WHO MONICA Project. Varying sensitivity of waist action levels to identify subjects with overweight or obesity in 19 populations of the WHO MONICA Project. J. Clin. Epidemiol. 1999, 52, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Insrem. Obesity and Overweight: Almost One in Two French People Affected. Current Situation, Prevention, and Therapeutic Solutions. 2023. Available online: https://presse.inserm.fr/en/obesite-et-surpoids-pres-dun-francais-sur-deux-concerne-etat-des-lieux-prevention-et-solutions-therapeutiques/66621/ (accessed on 5 September 2022).
- Coombes, R. Overweight children could become the “new norm” for Europe, WHO says. BMJ 2014, 348. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Lu, J.; Weng, J.; Jia, W.; Ji, L.; Xiao, J.; Shan, Z.; Liu, J.; Tian, H.; Ji, Q.; et al. Prevalence of diabetes among men and women in China. N. Engl. J. Med. 2010, 362, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Goh, W.D.Y.; Chong, L.C.; Kuan, Y.H.; Ismail, M.N.M. Increased obesity rate due to economic transition and industrialisation in Asia: A systematic review. Sains Malays. 2020, 49, 249–259. [Google Scholar] [CrossRef]
- Ng, S.W.; Zaghloul, S.; Ali, H.; Harrison, G.; Yeatts, K.; El Sadig, M.; Popkin, B.M. Nutrition transition in the united arab emirates. Eur. J. Clin. Nutr. 2011, 65, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Akter, S.; Hu, H.; Kashino, I.; Kuwahara, K.; Okazaki, H.; Sasaki, N.; Ogasawara, T.; Eguchi, M.; Kochi, T.; et al. Japan Epidemiology Collaboration on Occupational Health Study Group. Five-year cumulative incidence of overweight and obesity, and longitudinal change in body mass index in Japanese workers: The Japan Epidemiology Collaboration on Occupational Health Study. J. Occup. Health 2020, 62, e12095. [Google Scholar] [CrossRef] [PubMed]
- Fried, M.; Yumuk, V.; Oppert, J.M.; Scopinaro, N.; Torres, A.J.; Weiner, R.; Yashkov, Y.; Frühbeck, G. European Association for the Study of Obesity. International Federation for the Surgery of Obesity—European Chapter Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes. Facts 2013, 6, 449–468. [Google Scholar] [CrossRef] [PubMed]
- Cawley, J.; Meyerhoefer, C. The medical care costs of obesity: An instrumental variables approach. J. Health Econ. 2012, 31, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Haider, N.; Larose, L. Harnessing adipogenesis to prevent obesity. Adipocyte 2019, 8, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef] [PubMed]
- Vishvanath, L.; Gupta, R.K. Contribution of adipogenesis to healthy adipose tissue expansion in obesity. J. Clin. Investig. 2019, 129, 4022–4031. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Sarjeant, K.; Stephens, J.M. Adipogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008417. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.J.; Murphy, K.E.; Fernandez, M.L. Impact of Obesity and Metabolic Syndrome on Immunity. Adv. Nutr. 2016, 7, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Gan, M.; Tan, Y.; Chen, L.; Shen, L.; Niu, L.; Liu, Y.; Tang, G.; Jiang, Y.; Li, X.; et al. Mir-152 Regulates 3T3-L1 Preadipocyte Proliferation and Differentiation. Molecules 2019, 24, 3379. [Google Scholar] [CrossRef] [PubMed]
- Rogero, M.M.; Calder, P.C. Obesity, Inflammation, Toll-Like Receptor 4 and Fatty Acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef] [PubMed]
- Håversen, L.; Danielsson, K.N.; Fogelstrand, L.; Wiklund, O. Induction of proinflammatory cytokines by long-chain saturated fatty acids in human macrophages. Atherosclerosis 2009, 202, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ojeda, F.J.; Rupérez, A.I.; Gomez-Llorente, C.; Gil, A.; Aguilera, C.M. Cell Models and Their Application for Studying Adipogenic Differentiation in Relation to Obesity: A Review. Int. J. Mol. Sci. 2016, 17, 1040. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Pessin, J.E. Adipokines mediate inflammation and insulin resistance. Front. Endocrinol. 2013, 4, 71. [Google Scholar] [CrossRef] [PubMed]
- McQueen, A.E.; Koliwad, S.K.; Wang, J.C. Fighting obesity by targeting factors regulating beige adipocytes. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Aubin, K.; Safoine, M.; Proulx, M.; Audet-Casgrain, M.A.; Côté, J.F.; Têtu, F.A.; Roy, A.; Fradette, J. Characterization of In Vitro Engineered Human Adipose Tissues: Relevant Adipokine Secretion and Impact of TNF-α. PLoS ONE 2015, 10, e0137612. [Google Scholar] [CrossRef] [PubMed]
- Meikle, P.J.; Wong, G.; Barlow, C.K.; Kingwell, B.A. Lipidomics: Potential role in risk prediction and therapeutic monitoring for diabetes and cardiovascular disease. Pharmacol. Ther. 2014, 143, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Cerdó, T.; García-Santos, J.A.; Bermúdez, M.G.; Campoy, C. The role of probiotics and prebiotics in the prevention and treatment of obesity. Nutrients 2019, 11, 635. [Google Scholar] [CrossRef] [PubMed]
- Mazloom, K.; Siddiqi, I.; Covasa, M. Probiotics: How effective are they in the fight against obesity? Nutrients 2019, 11, 258. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Zhou, D.D.; Gan, R.Y.; Huang, S.Y.; Zhao, C.N.; Shang, A.; Xu, X.Y.; Li, H.B. Effects and mechanisms of probiotics, prebiotics, synbiotics, and postbiotics on metabolic diseases targeting gut microbiota: A narrative review. Nutrients 2021, 13, 3211. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.R.; Kim, Y.J.; Park, D.Y.; Jung, U.J.; Jeon, S.M.; Ahn, Y.T.; Huh, C.S.; McGregor, R.; Choi, M.S. Probiotics, L. plantarum and L. curvatus in combination alter hepatic lipid metabolism and suppress diet-induced obesity. Obesity 2013, 21, 2571–2578. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Yun, S.I.; Park, M.H.; Park, J.H.; Jeong, S.Y.; Park, H.O. Anti-obesity effect of Lactobacillus gasseri BNR17 in high-sucrose diet-induced obese mice. PLoS ONE 2013, 8, e54617. [Google Scholar] [CrossRef]
- An, H.M.; Park, S.Y.; Lee, D.K.; Kim, J.R.; Cha, M.K.; Lee, S.W.; Lim, H.T.; Kim, K.J.; Ha, N.J. Antiobesity and lipid-lowering effects of Bifidobacterium spp. in high fat diet-induced obese rats. Lipids Health Dis. 2011, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, H.; Zhang, C.; Zhao, Y.; Derrien, M.; Rocher, E.; van-Hylckama Vlieg, J.E.; Strissel, K.; Zhao, L.; Obin, M.; et al. Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. ISME J. 2015, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, R.; Li, X.F.; Wang, R.L. Bifidobacterium adolescentis supplementation ameliorates visceral fat accumulation and insulin sensitivity in an experimental model of the metabolic syndrome. Br. J. Nutr. 2012, 107, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.; Jain, S.; Sinha, P.R. Effect of Dahi containing Lactococcus lactis on the progression of diabetes induced by a high-fructose diet in rats. Biosci. Biotechnol. Biochem. 2006, 70, 1255–1258. [Google Scholar] [CrossRef] [PubMed]
- Park, D.Y.; Ahn, Y.T.; Park, S.H.; Huh, C.S.; Yoo, S.R.; Yu, R.; Sung, M.K.; McGregor, R.A.; Choi, M.S. Supplementation of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 in diet-induced obese mice is associated with gut microbial changes and reduction in obesity. PLoS ONE 2013, 8, e59470. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Higashikawa, F.; Noda, M.; Kawamura, Y.; Matoba, Y.; Kumagai, T.; Sugiyama, M. The obesity and fatty liver are reduced by plant-derived Pediococcus pentosaceus LP28 in high fat diet-induced obese mice. PLoS ONE 2012, 7, e30696. [Google Scholar] [CrossRef] [PubMed]
- Fabersani, E.; Portune, K.; Campillo, I.; López-Almela, I.; La Paz, S.M.D.; Romaní-Pérez, M.; Benítez-Páez, A.; Sanz, Y. Bacteroides uniformis CECT 7771 alleviates inflammation within the gut-adipose tissue axis involving TLR5 signaling in obese mice. Sci. Rep. 2021, 11, 11788. [Google Scholar] [CrossRef] [PubMed]
- Ritze, Y.; Bárdos, G.; Claus, A.; Ehrmann, V.; Bergheim, I.; Schwiertz, A.; Bischoff, S.C. Lactobacillus rhamnosus GG protects against non-alcoholic fatty liver disease in mice. PLoS ONE 2014, 9, e80169. [Google Scholar] [CrossRef] [PubMed]
- Park, K.Y.; Kim, B.; Hyun, C.K. Lactobacillus rhamnosus GG improves glucose tolerance through alleviating ER stress and suppressing macrophage activation in db/db mice. J. Clin. Biochem. Nutr. 2015, 56, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.N.; Yu, Q.F.; Fu, N.; Liu, X.W.; Lu, F.G. Effects of four Bifidobacteria on obesity in high-fat diet induced rats. World J. Gastroenterol. 2010, 16, 3394–3401. [Google Scholar] [CrossRef] [PubMed]
- Reichold, A.; Brenner, S.A.; Spruss, A.; Förster-Fromme, K.; Bergheim, I.; Bischoff, S.C. Bifidobacterium adolescentis protects from the development of nonalcoholic steatohepatitis in a mouse model. J. Nutr. Biochem. 2014, 25, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Gomez-Llorente, C.; Abadia-Molina, F.; Saez-Lara, M.J.; Campaña-Martin, L.; Muñoz-Quezada, S.; Romero, F.; Gil, A.; Fontana, L. Effects of Lactobacillus paracasei CNCM I-4034, Bifidobacterium breve CNCM I-4035 and Lactobacillus rhamnosus CNCM I-4036 on hepatic steatosis in Zucker rats. PLoS ONE 2014, 9, e98401. [Google Scholar] [CrossRef] [PubMed]
- Savcheniuk, O.; Kobyliak, N.; Kondro, M.; Virchenko, O.; Falalyeyeva, T.; Beregova, T. Short-term periodic consumption of multiprobiotic from childhood improves insulin sensitivity, prevents development of non-alcoholic fatty liver disease and adiposity in adult rats with glutamate-induced obesity. BMC Complement. Altern. Med. 2014, 14, 247. [Google Scholar] [CrossRef] [PubMed]
- Kondro, M.; Kobyliak, N.; Virchenko, O.; Falalyeyeva, T.; Beregova, T.; Bodnar, P. Multiprobiotic therapy from childhood prevents the development of nonalcoholic fatty liver disease in adult monosodium glutamate-induced obese rats. Curr. Issues Pharm. Med. Sci. 2014, 27, 243–245. [Google Scholar] [CrossRef]
- Park, S.Y.; Cho, S.A.; Lee, M.K.; Lim, S.D. Effect of Lactobacillus plantarum FH185 on the Reduction of Adipocyte Size and Gut Microbial Changes in Mice with Diet-induced Obesity. Korean J. Food Sci. Anim. Resour. 2015, 35, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Lo, Y.H.; Pan, T.M. Anti-obesity activity of Lactobacillus fermented soy milk products. J. Funct. Foods 2013, 5, 905–913. [Google Scholar] [CrossRef]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Ross, R.P.; O’Toole, P.W.; Shanahan, F.; Cotter, P.D. Targeting the microbiota to address diet-induced obesity: A time dependent challenge. PLoS ONE 2013, 8, e65790. [Google Scholar] [CrossRef] [PubMed]
- Kadooka, Y.; Sato, M.; Imaizumi, K.; Ogawa, A.; Ikuyama, K.; Akai, Y.; Okano, M.; Kagoshima, M.; Tsuchida, T. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur. J. Clin. Nutr. 2010, 64, 636–643. [Google Scholar] [CrossRef]
- Kadooka, Y.; Sato, M.; Ogawa, A.; Miyoshi, M.; Uenishi, H.; Ogawa, H.; Ikuyama, K.; Kagoshima, M.; Tsuchida, T. Effect of Lactobacillus gasseri SBT2055 in fermented milk on abdominal adiposity in adults in a randomised controlled trial. Br. J. Nutr. 2013, 110, 1696–1703. [Google Scholar] [CrossRef]
- Mykhal’chyshyn, H.P.; Bodnar, P.M.; Kobyliak, N.M. Effect of probiotics on proinflammatory cytokines level in patients with type 2 diabetes and nonalcoholic fatty liver disease. Lik. Sprava 2013, 2, 56–62. [Google Scholar]
- Aller, R.; De Luis, D.A.; Izaola, O.; Conde, R.; Gonzalez Sagrado, M.; Primo, D.; De La Fuente, B.; Gonzalez, J. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: A double blind randomized clinical trial. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 1090–1095. [Google Scholar] [PubMed]
- Shavakhi, A.; Minakari, M.; Firouzian, H.; Assali, R.; Hekmatdoost, A.; Ferns, G. Effect of a Probiotic and Metformin on Liver Aminotransferases in Non-alcoholic Steatohepatitis: A double blind randomized clinical trial. Int. J. Prev. Med. 2013, 4, 531–537. [Google Scholar] [PubMed]
- Malaguarnera, M.; Vacante, M.; Antic, T.; Giordano, M.; Chisari, G.; Acquaviva, R.; Mastrojeni, S.; Malaguarnera, G.; Mistretta, A.; Li Volti, G.; et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig. Dis. Sci. 2012, 57, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.S.; Wong, G.L.H.; Chim, A.M.L.; Chu, W.C.W.; Yeung, D.K.W.; Li, K.C.T.; Chan, H.L.Y. Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study. Ann. Hepatol. 2013, 12, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Mazloom, Z.; Yousefinejad, A.; Dabbaghmanesh, M.H. Effect of probiotics on lipid profile, glycemic control, insulin action, oxidative stress, and inflammatory markers in patients with type 2 diabetes: A clinical trial. Iran. J. Med. Sci. 2013, 38, 38–43. [Google Scholar] [PubMed]
- Zarrati, M.; Salehi, E.; Mofid, V.; Zadeh-Attar, M.J.H.; Nourijelyani, K.; Bidad, K.; Shidfar, F. Relationship between probiotic consumption and IL-10 and IL-17 secreted by PBMCs in overweight and obese people. Iran. J. Allergy Asthma Immunol. 2013, 12, 404–406. [Google Scholar] [PubMed]
- Zarrati, M.; Shidfar, F.; Nourijelyani, K.; Mofid, V.; Hossein zadeh-Attar, M.J.; Bidad, K.; Najafi, F.; Gheflati, Z.; Chamari, M.; Salehi, E. Lactobacillus acidophilus La5, Bifidobacterium BB12, and Lactobacillus casei DN001 modulate gene expression of subset specific transcription factors and cytokines in peripheral blood mononuclear cells of obese and overweight people. Biofactors 2013, 39, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Zarrati, M.; Salehi, E.; Nourijelyani, K.; Mofid, V.; Zadeh, M.J.H.; Najafi, F.; Ghaflati, Z.; Bidad, K.; Chamari, M.; Karimi, M.; et al. Effects of probiotic yogurt on fat distribution and gene expression of proinflammatory factors in peripheral blood mononuclear cells in overweight and obese people with or without weight-loss diet. J. Am. Coll. Nutr. 2014, 33, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.P.; Lee, K.M.; Kang, J.H.; Yun, S.I.; Park, H.O.; Moon, Y.; Kim, J.Y. Effect of Lactobacillus gasseri BNR17 on Overweight and Obese Adults: A randomized, double-blind clinical trial. Korean J. Fam. Med. 2013, 34, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Luoto, R.; Kalliomäki, M.; Laitinen, K.; Isolauri, E. The impact of perinatal probiotic intervention on the development of overweight and obesity: Follow-up study from birth to 10 years. Int. J. Obes. 2010, 34, 1531–1537. [Google Scholar] [CrossRef]
- Brahe, L.K.; Le Chatelier, E.; Prifti, E.; Pons, N.; Kennedy, S.; Blædel, T.; Håkansson, J.; Dalsgaard, T.K.; Hansen, T.; Pedersen, O.; et al. Dietary modulation of the gut microbiota--a randomised controlled trial in obese postmenopausal women. Br. J. Nutr. 2015, 114, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Ivey, K.L.; Hodgson, J.M.; Kerr, D.A.; Lewis, J.R.; Thompson, P.L.; Prince, R.L. The effects of probiotic bacteria on glycaemic control in overweight men and women: A randomised controlled trial. Eur. J. Clin. Nutr. 2014, 68, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Ivey, K.L.; Hodgson, J.M.; Kerr, D.A.; Lewis, J.R.; Thompson, P.L.; Prince, R.L. The effect of yoghurt and its probiotics on blood pressure and serum lipid profile; a randomised controlled trial. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, H.; Mahmood, N.; Kumar, M.; Varikuti, S.R.; Challa, H.R.; Myakala, S.P. Effect of probiotic (VSL#3) and omega-3 on lipid profile, insulin sensitivity, inflammatory markers, and gut colonization in overweight adults: A randomized, controlled trial. Mediators Inflamm. 2014, 2014, 348959. [Google Scholar] [PubMed]
- Tripolt, N.J.; Leber, B.; Blattl, D.; Eder, M.; Wonisch, W.; Scharnagl, H.; Stojakovic, T.; Obermayer-Pietsch, B.; Wascher, T.C.; Pieber, T.R.; et al. Short communication: Effect of supplementation with Lactobacillus casei Shirota on insulin sensitivity, β-cell function, and markers of endothelial function and inflammation in subjects with metabolic syndrome—A pilot study. J. Dairy Sci. 2013, 96, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Barreto, F.M.; Simão, A.N.C.; Morimoto, H.K.; Lozovoy, M.A.B.; Dichi, I.; da Silva Miglioranza, L.H. Beneficial effects of Lactobacillus plantarum on glycemia and homocysteine levels in postmenopausal women with metabolic syndrome. Nutrition 2014, 30, 939–942. [Google Scholar] [CrossRef] [PubMed]
- Song, E.J.; Han, K.; Lim, T.J.; Lim, S.; Chung, M.J.; Nam, M.H.; Kim, H.; Nam, Y.D. Effect of probiotics on obesity-related markers per enterotype: A double-blind, placebo-controlled, randomized clinical trial. EPMA J. 2020, 11, 31–51. [Google Scholar] [CrossRef] [PubMed]
- Sohn, M.; Na, G.Y.; Joung, H.; Kim, B.K.; Lim, S. Efficacy and safety of Lactobacillus plantarum K50 on lipids in Koreans with obesity: A Randomized, Double-Blind Controlled Clinical Trial. Front. Endocrinol. 2022, 12, 790046. [Google Scholar] [CrossRef] [PubMed]
- Igata, M.; Islam, M.A.; Takahashi, H.; Villena, J.; Kitazawa, H. Transcriptome Modifications in Porcine Adipocytes via Toll-Like Receptors Activation. Front. Immunol. 2019, 10, 1180. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.F.; Cotter, P.D.; Hogan, A.; O’Sullivan, O.; Joyce, A.; Fouhy, F.; Clarke, S.F.; Marques, T.M.; O’Toole, P.W.; Stanton, C.; et al. Divergent metabolic outcomes arising from targeted manipulation of the gut microbiota in diet-induced obesity. Gut 2013, 62, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Moon, P.D.; Kim, S.J.; Choi, I.Y.; An, H.J.; Myung, N.Y.; Jeong, H.J.; Um, J.Y.; Hong, S.H.; Kim, H.M. Lipid profile lowering effect of Soypro fermented with lactic acid bacteria isolated from Kimchi in high-fat diet-induced obese rats. Biofactors 2008, 33, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Virtue, S.; Vidal-Puig, A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome—An allostatic perspective. Biochim. Biophys. Acta 2010, 1801, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Zemel, M.B. Role of calcium and dairy products in energy partitioning and weight management. Am. J. Clin. Nutr. 2004, 79, 907S–912S. [Google Scholar] [CrossRef] [PubMed]
- Senauer, B.; Gemma, M. Why Is the Obesity Rate so Low in Japan and High in the US? Some Possible Economic Explanations. 2006. Available online: https://ageconsearch.umn.edu/record/14321?v=pdf (accessed on 7 September 2022).
- Roy, C.S.; Mukhopadhyay, A.; Bhadra, M. Preavalence of overweight and obesity among Bengalee urban Adult Men of North 24 Parganas, West Bengal, India. Int. J. Exp. Res. Rev. 2016, 4, 45–50. [Google Scholar]
- Nanditha, A.; Ma, R.C.; Ramachandran, A.; Snehalatha, C.; Chan, J.C.; Chia, K.S.; Shaw, J.E.; Zimmet, P.Z. Diabetes in Asia and the Pacific: Implications for the Global Epidemic. Diabetes Care 2016, 39, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A.C.; Isolauri, E.; Kirjavainen, P.V.; Ölkkö, S.T.; Salminen, S.J. The mucus binding of Bifidobacterium lactis Bb12 is enhanced in the presence of Lactobacillus GG and Lact. delbrueckii subsp. bulgaricus. Lett. Appl. Microbiol. 2000, 30, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guan, W.; Ma, S.; Lin, S.; Yang, N.; Liu, R.; Liang, H.; Zhou, H. Lipopolysaccharide and inflammatory cytokines levels decreased after sleeve gastrectomy in Chinese adults with obesity. Endocr. J. 2019, 66, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.H.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Park, J.H.; Seok, S.H.; Baek, M.W.; Kim, D.J.; Lee, K.E.; Paek, K.S.; Lee, Y.; Park, J.H. Human originated bacteria, Lactobacillus rhamnosus PL60, produce conjugated linoleic acid and show anti-obesity effects in diet-induced obese mice. Biochim. Biophys. Acta 2006, 1761, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Iyer, N.; Williams, M.A.; O’Callaghan, A.A.; Dempsey, E.; Cabrera-Rubio, R.; Raverdeau, M.; Crispie, F.; Cotter, P.D.; Corr, S.C. Lactobacillus salivarius UCC118™ Dampens Inflammation and Promotes Microbiota Recovery to Provide Therapeutic Benefit in a DSS-Induced Colitis Model. Microorganisms 2022, 10, 1383. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.; Wall, R.; Lisai, S.; Ross, R.P.; Dinan, T.G.; Cryan, J.F.; Fitzgerald, G.F.; Banni, S.; Quigley, E.M.; Shanahan, F.; et al. Bifidobacterium breve with α-linolenic acid alters the composition, distribution and transcription factor activity associated with metabolism and absorption of fat. Sci. Rep. 2017, 7, 43300. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- López-Ortega, O.; Moreno-Corona, N.C.; Cruz-Holguin, V.J.; Garcia-Gonzalez, L.D.; Helguera-Repetto, A.C.; Romero-Valdovinos, M.; Arevalo-Romero, H.; Cedillo-Barron, L.; León-Juárez, M. The immune response in adipocytes and their susceptibility to infection: A possible relationship with infectobesity. Int. J. Mol. Sci. 2022, 23, 6154. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Xiao, J.Z.; Satoh, T.; Odamaki, T.; Takahashi, S.; Sugahara, H.; Yaeshima, T.; Iwatsuki, K.; Kamei, A.; Abe, K. Antiobesity effects of Bifidobacterium breve strain B-3 supplementation in a mouse model with high-fat diet-induced obesity. Biosci. Biotechnol. Biochem. 2010, 74, 1656–1661. [Google Scholar] [CrossRef] [PubMed]
- Takemura, N.; Okubo, T.; Sonoyama, K. Lactobacillus plantarum strain No. 14 reduces adipocyte size in mice fed high-fat diet. Exp. Biol. Med. 2010, 235, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, F.; Zhao, P.; Zhang, R.; Zeng, Q. Effect of heat-killed Streptococcus thermophilus on type 2 diabetes rats. Peer J. 2019, 7, e7117. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Olefsky, J.M. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012, 15, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Cichoż-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082–8091. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; Cassader, M.; Pagano, G. Meta-analysis: Natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann. Med. 2011, 43, 617–649. [Google Scholar] [CrossRef] [PubMed]
- Griffin, C.; Eter, L.; Lanzetta, N.; Abrishami, S.; Varghese, M.; McKernan, K.; Muir, L.; Lane, J.; Lumeng, C.N.; Singer, K. TLR4, TRIF, and MyD88 are essential for myelopoiesis and CD11c+ adipose tissue macrophage production in obese mice. J. Biol. Chem. 2018, 293, 8775–8786. [Google Scholar] [CrossRef]
- Vaez, H.; Soraya, H.; Garjani, A.; Gholikhani, T. Toll-like receptor 4 (TLR4) and AMPK relevance in cardiovascular disease. Adv. Pharm. Bull. 2023, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, J.; Liu, M.; Zhou, Y.; Zhang, L.; Li, Y. The new role of AMP-activated protein kinase in regulating fat metabolism and energy expenditure in adipose tissue. Biomolecules 2021, 11, 1757. [Google Scholar] [CrossRef] [PubMed]

| Probiotics Species/Strains | Expt. Mode | Animal | Time | Effects with Respect to | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Weight Gain | FFA/TG/Chole-Sterol | Anti-Inflammatory Adipokines | Proinflammatory Adipokines | |||||
| Lactiplantibacillus plantarum LMT1-48 | HFD-induced | Mice | 5 wk | ↓ | ↓ | - | - | [12] |
| Bifidobacterium longum PI10, Bifidobacterium animalis and Lactobacillus gasseri LA806 | HFD-induced | Mice | 12 wk | ↓ | ↓ | - | ↓ IL-10 and Leptin | [13] |
| Lactobacillus curvatus HY7601or along with Lactiplantibacillus plantarum KY1032 | High-fat high-cholesterol diet | Mice | 9 wk | ↓ | ↓ | - | ↓TNF-α and IL-1β | [46] |
| Lactobacillus gasseri BNR17 | High-sucrose diet/standard chow-fed | Mice | 10 wk | ↓ | ↓ | - | ↓ Leptin | [47] |
| Bifidobacterium pseudocatenulatum SPM 1204, Bifidobacterium longum SPM 1205, and Bifidobacterium longum SPM | High dietary fat induced rat | Rat | 7 wk | ↓ | ↓ | - | - | [48] |
| Lacticaseibacillus rhamnosus I-3690, Lacticaseibacillus paracasei CNCM I-4270, or Bifidobacterium lactis I-2494 | HFD-induced/standard chow-fed | Mice | 12 wk | ↓ | - | - | ↓ TNF-α | [49] |
| Bifidobacterium adolescentis | HFD-induced/standard chow-fed | Rat | 12 wk | ↓ | ↓ | - | - | [50] |
| Lactococcus lactis | Diet containing high fructose/standard chow-fed | Rat | 42 d | - | ↓ | - | - | [51] |
| Lactobacillus curvatus HY7601 and Lactiplantibacillus plantarum KY1032 | HFD-induced/standard chow-fed | Mice | 10 wk | ↓ | ↓ | - | ↓ TNF-α, IL-6, IL-1β and MCP-1 | [52] |
| Akkermansia muciniphila | HFD-induced/standard chow-fed | Mice | 4 wk | ↓ | - | - | - | [53] |
| Pediococcus pentosaceus LP28/Lactiplantibacillus plantarum SN13T | HFD-induced/standard chow-fed | Mice | 6 wk | ↓ | ↓ | - | - | [54] |
| Bacteroides uniformis CECT 7771 | HFD-induced/standard diet | Mice | 14 wk | ↓ | ↓ | ↑ IL-10, IL-33, thymic stromal lymphopoietin | TSLP | [55] |
| Lacticaseibacillus rhamnosus GG | High-fructose diet | Mice | 8 wk | ↓ | ↓ | - | ↓ TNF-α, IL-8 and IL-1β | [56] |
| Lacticaseibacillus rhamnosus GG | Induced diabetic | Mice | 4 wk | ↓ | ↓ Lipotoxicity | - | ↔ TNF-α and IL-6 ↓ MCP-1 | [57] |
| Bifidobacteria L66-5, L75-4, M13-4 and FS31-12 | HFD-induced/standard chow-fed | Mice | 6 wk | ↓ B. L66-5 ↑ B.M13-4 ↔L75-4 and FS31-12 | ↓ | - | - | [58] |
| Bifidobacterium adolescentis | HFD-induced/standard chow-fed | Mice | 12 wk | ↓ | - | - | ↑ CCL2 | [59] |
| Lacticaseibacillus paracasei CNCMI-4034, Bifidobacterium breve CNCM I-4035 and Lacticaseibacillus rhamnosus CNCM | Genetically obese animal | Rat | 30 d | ↓ | ↓ | ↓ Adiponectin | ↓ TNF-α and IL-6 | [60] |
| 14 probiotics of Bifidobacterium, Lactobacillus, Lactococcus, Propionibacterium genera | Obesity created with Monosodium glutamate | Rat | 3 mon | ↓ | ↓ | ↓ Adiponectin | ↔ Leptin | [61] |
| 14 probiotics of Bifidobacterium, Lactobacillus, Lactococcus, Propionibacterium genera | Monosodium glutamate induced obesity | Rat | 3 mon | ↓ | ↓ | - | - | [62] |
| Lactiplantibacillus plantarum FH185 | High dietary fat induced mice | Mice | 6 wk | - | ↓ | - | - | [63] |
| Lacticaseibacillus paracasei subsp. paracasei NTU 101 | HFD-induced/standard chow-fed | Rat | 5 wk | ↓ | ↓ | - | ↓ Leptin | [64] |
| Ligilactobacillus salivarius UCC118 | A low fat (lean) or diet-induced obese | Mice | 8 wk | ↓ | ↓ | - | ↓TNF-α and MCP1 | [65] |
| Probiotics Species/Strains | Type of Study/Subjects | Time | Effects with respect to | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Weight Gain | FFA/TG /Cholesterol | Anti-Inflammatory Adipokines | Pro-Inflammatory Adipokines | Other Measures | ||||
| Lactobacillus gasseri SBT2055 (LG2055) | A multicenter, double-blind, randomized, placebo-controlled intervention trial | 12 wk | ↓ | ↓ | ↑ Adiponectin | - | ↓ Abdominal adiposity | [66] |
| Lactobacillus gasseri SBT2055 (LG2055) | Multicenter, double-blind, parallel-group randomized controlled trial (RCT) | 12 wk | ↓ | ↓ | - | - | ↔ Lean mass and subcutaneous fat area, ↓ BMI | [67] |
| Multiprobiotic “Symbiter” containing concentrated biomass of 14 alive probiotic bacteria | Open-label study in patients with non-alcoholic fatty liver disease | 4 wk | - | - | - | ↓ IL-6, IL-8, and TNF-α | ↓ Low-grade systemic inflammation | [68] |
| Lactobacillus bulgaricus vs. Streptococcus thermophilus | Randomized, double-blind, parallel, placebo-controlled trial | 3 mon | - | - | - | - | ↓ Liver aminotransferases levels in patients with NAFLD | [69] |
| Probiotic combination with Metformin 500 mg (Met/Pro) versus Metformin 500 mg plus placebo (Met/P) | Double-blind, randomized, placebo-controlled trial in patients with histology-proven—NASH | 6 mon | ↓ BMI | ↓ | - | - | ↓ Liver aminotransferases level and fasting blood glucose, ↑ Liver function | [70] |
| Bifidobacterium longum with fructo-oligosaccharides | Open-label study in patients with NASH | 24 wk | ↓ BMI | ↓ | - | ↓TNF-α | ↓ CRP, serum AST, HOMA-IR, serum endotoxin, steatosis, NASH activity index | [71] |
| Lepicol probiotic formula (Lactiplantibacillus plantarum, Lactobacillus deslbrueckii, Lactobacillus acidophilus, Lacticaseibacillus rhamnosus and Bifidobacterium bifidum) vs. usual care | Randomized, open-label study in patients with histology-proven NASH | 6 mon | ↔ BMI | ↔ | - | - | ↓ AST | [72] |
| Lactobacillus probiotics (Lactobacillus acidophilus, Lactobacillus bulgaricus, Lactobacillus bifidus and Lacticaseibacillus casei) | Randomized, single-blinded, placebo-controlled trial | 6 wk | - | ↔ | - | ↔ IL-6 | ↓ IR | [73] |
| Lactobacillus acidophilus La5, Bifidobacterium Bb12 and Lacticaseibacillus casei DN001 | Subjects with high BMI | 8 wk | ↓ BMI | ↓ | ↓ Leptin | ↔ TNF-α and TGF-β ↓ IFN-γ | Changes in gene expression in PBMCs | [74,75,76] |
| Lactobacillus gasseri BNR17 in capsule | Double-blind RCT placebo group | 12 wk | ↓ | ↔ | - | - | ↔ BMI, fat, waist circumference, VAT, SAT, DAT, ↑ HDL, ↓ LDL | [77] |
| Lacticaseibacillus rhamnosus GG, ATCC 53103 | Randomized, double-blind, prospective follow-up study | Mothers: 4 wk ≥ EDD Child: ≤6 mon | ↓ | - | - | - | ↓ Weight gain (1st yr) | [78] |
| Lacticaseibacillus paracasei N19 | Obese post menopausal women | 6 wk | - | - | - | - | ↑ Insulin sensitivity, ↑ Gut microbiota | [79] |
| Lactobacillus acidophilus La5 and Bifidobacterium animalis subsp. Lactis Bb12 | Overweight adults | 6 wk | ↔ BMI | ↔ | - | - | ↓ Fasting glucose, ↑ HOMA-IR | [80,81] |
| Bifidobacteria, lactobacilli, and Streptococcus thermophilus | Overweight subjects | 6 wk | - | ↓ | - | - | ↑ Insulin sensitivity, ↓ CRP, ↑ HDL | [82] |
| Lacticaseibacillus casei Shirota | Patients with IRS | 12 wk | ↑ | - | - | ↔ TNF-α and IL-6 | ↓ VCAM-1 | [83] |
| Lactiplantibacillus plantarum | PM women with IRS | 12 wk | ↔ | ↓ | - | ↓ IL-6 | ↓ Glucose, homocysteine | [84] |
| Bifidobacterium breve CBT and Lactiplantibacillus plantarum CBT LP3 | Randomized, double-blind, placebo-controlled trial | 12 wk | ↔ | ↓ | - | ↓ HDL, glucose, and insulin | [85] | |
| Lactiplantibacillus plantarum K50 (LPK) | Randomized, double-blind, placebo-controlled trial | 12 wk | ↔ | ↓ | ↓ Leptin | - | - | [86] |
| Probiotics Species/Strains | Expt. Mode | Animal | Time | Effects in Respect to | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Weight Gain | FFA/TG/Cholesterol | Anti-Inflammatory Adipokines | Pro-Inflammatory Adipokines | |||||
| Lactiplantibacillus plantarum LMT1-48 | In vitro (3T3-L1 model) | Mice | 48 h | - | ↓ | - | - | [12] |
| Bifidobacterium longum PI10, Bifidobacterium animalis, and Lactobacillus gasseri LA806 | In vitro (3T3-L1 model) | Mouse | 48 h | - | ↓ | - | ↓ Leptin | [13] |
| Lacticaseibacillus rhamnosus GG and Lactobacillus gasseri TMC0356 | In vitro (3T3-L1 model) | Mice | 24 h | - | ↓ | - | ↑ IL-6 and IL-12 | [14] |
| Leuconostoc mesenteroides and Lactiplantibacillus plantarum | In vitro (3T3 L1 model) | Mice | 3 wk | - | ↓ Adipogenesis/Lipogenesis ↑ Lipolysis | - | - | [15] |
| Lacticaseibacillus rhamnosus GG, Lactobacillus gasseri TMC0356, and Lacticaseibacillus rhamnosus LA-2 | In vitro (porcine intramuscular preadipocyte; PIP model) | Pig | 48 h | - | ↓ | - | ↑ IL-6, MCP-1, and TGF-β | [16] |
| Bifidobacterium bifidum TMC3108; LGG, Lacticaseibacillus rhamnosus GG; LA-2, Lacticaseibacillus rhamnosus LA-2; TMC0409, Lacticaseibacillus paracasei TMC0409 | In vitro (PIP model) | Pig | 48 h | - | ↓ | ↑ CCL2 | ↓ IFN-γ, IL-6, 12 | [17] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kober, A.K.M.H.; Saha, S.; Ayyash, M.; Namai, F.; Nishiyama, K.; Yoda, K.; Villena, J.; Kitazawa, H. Insights into the Anti-Adipogenic and Anti-Inflammatory Potentialities of Probiotics against Obesity. Nutrients 2024, 16, 1373. https://doi.org/10.3390/nu16091373
Kober AKMH, Saha S, Ayyash M, Namai F, Nishiyama K, Yoda K, Villena J, Kitazawa H. Insights into the Anti-Adipogenic and Anti-Inflammatory Potentialities of Probiotics against Obesity. Nutrients. 2024; 16(9):1373. https://doi.org/10.3390/nu16091373
Chicago/Turabian StyleKober, A. K. M. Humayun, Sudeb Saha, Mutamed Ayyash, Fu Namai, Keita Nishiyama, Kazutoyo Yoda, Julio Villena, and Haruki Kitazawa. 2024. "Insights into the Anti-Adipogenic and Anti-Inflammatory Potentialities of Probiotics against Obesity" Nutrients 16, no. 9: 1373. https://doi.org/10.3390/nu16091373
APA StyleKober, A. K. M. H., Saha, S., Ayyash, M., Namai, F., Nishiyama, K., Yoda, K., Villena, J., & Kitazawa, H. (2024). Insights into the Anti-Adipogenic and Anti-Inflammatory Potentialities of Probiotics against Obesity. Nutrients, 16(9), 1373. https://doi.org/10.3390/nu16091373










