Combination of Parenteral Amino Acid Infusion and Intermittent Loading Exercise Ameliorates Progression of Postoperative Sarcopenia in Rat Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design and Operative Procedures
2.2.1. Models of Postoperative Sarcopenia and Intermittent Loading
2.2.2. Nutritional Management
2.2.3. Tissue and Blood Sampling
2.3. Measurements
2.3.1. Body Weight and Composition
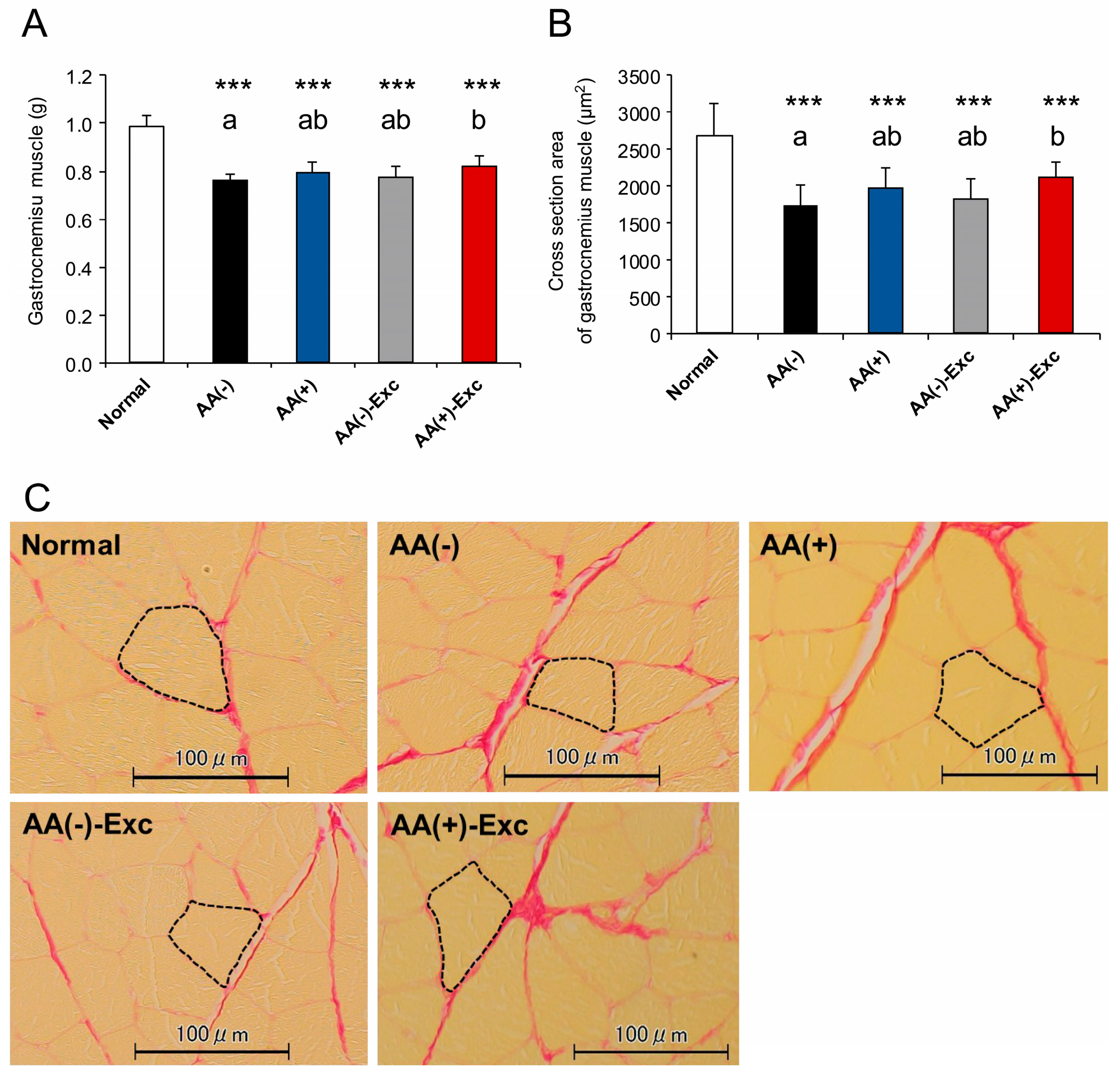
2.3.2. Muscle Weight and Gastrocnemius Myofiber Cross-Sectional Area
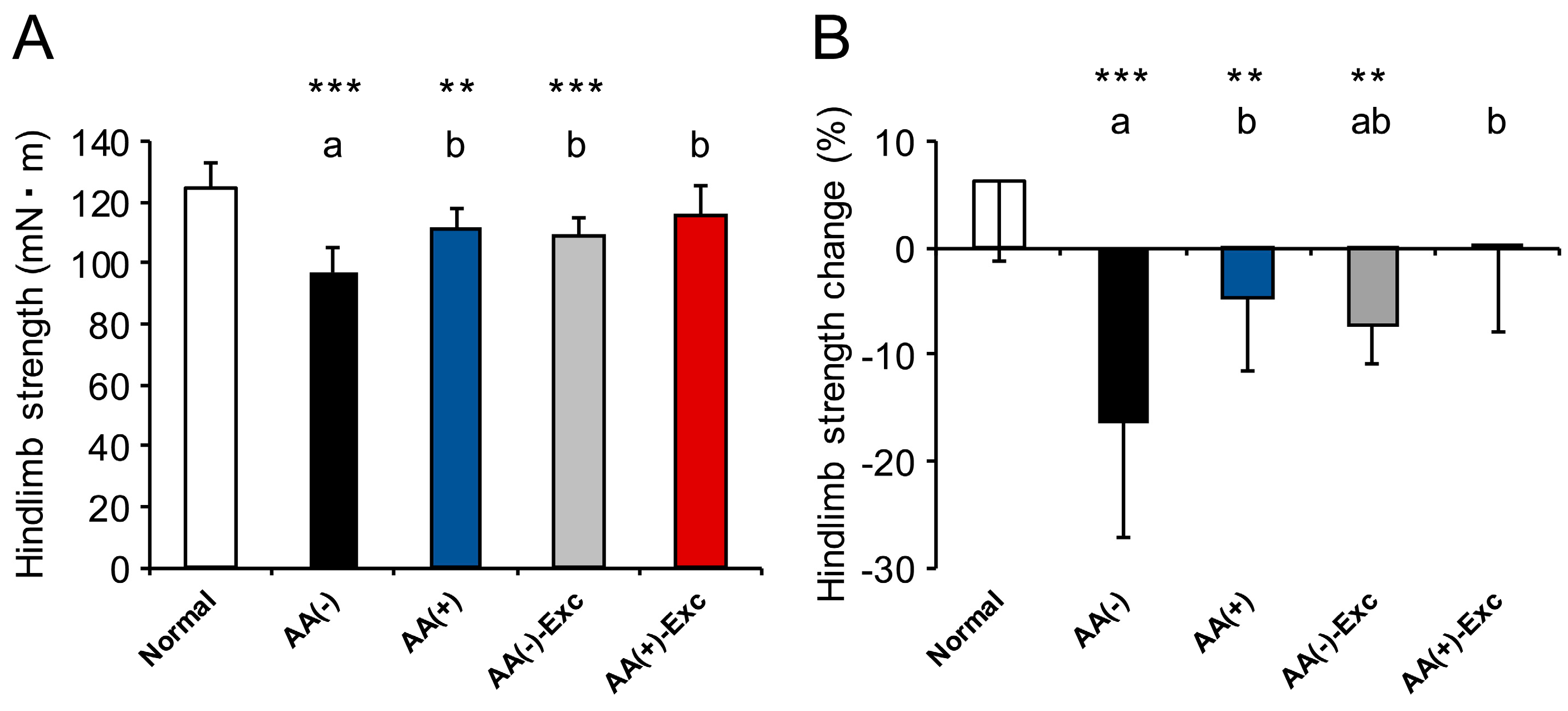
2.3.3. Hindlimb Muscle Strength
2.3.4. Quantitative Real-Time Polymerase Chain Reaction
2.3.5. Western Blot Analysis
2.3.6. Plasma AA Analysis
2.4. Statistical Analyses
3. Results
3.1. Body Weight and Lean Mass
3.2. Skeletal Muscle Mass and Gastrocnemius Myofiber CSA
3.3. Hindlimb Muscle Strength
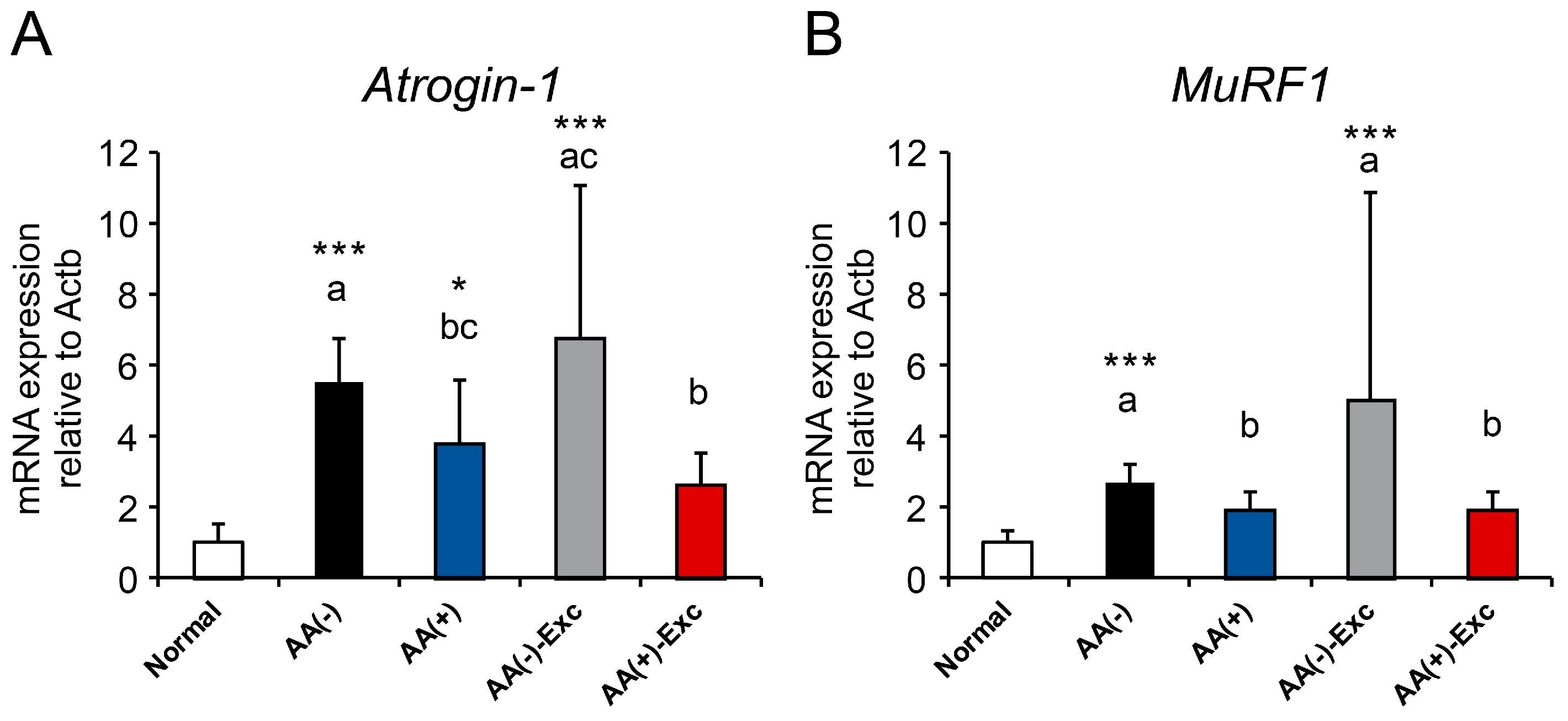
3.4. mRNA Expression of Atrogenes
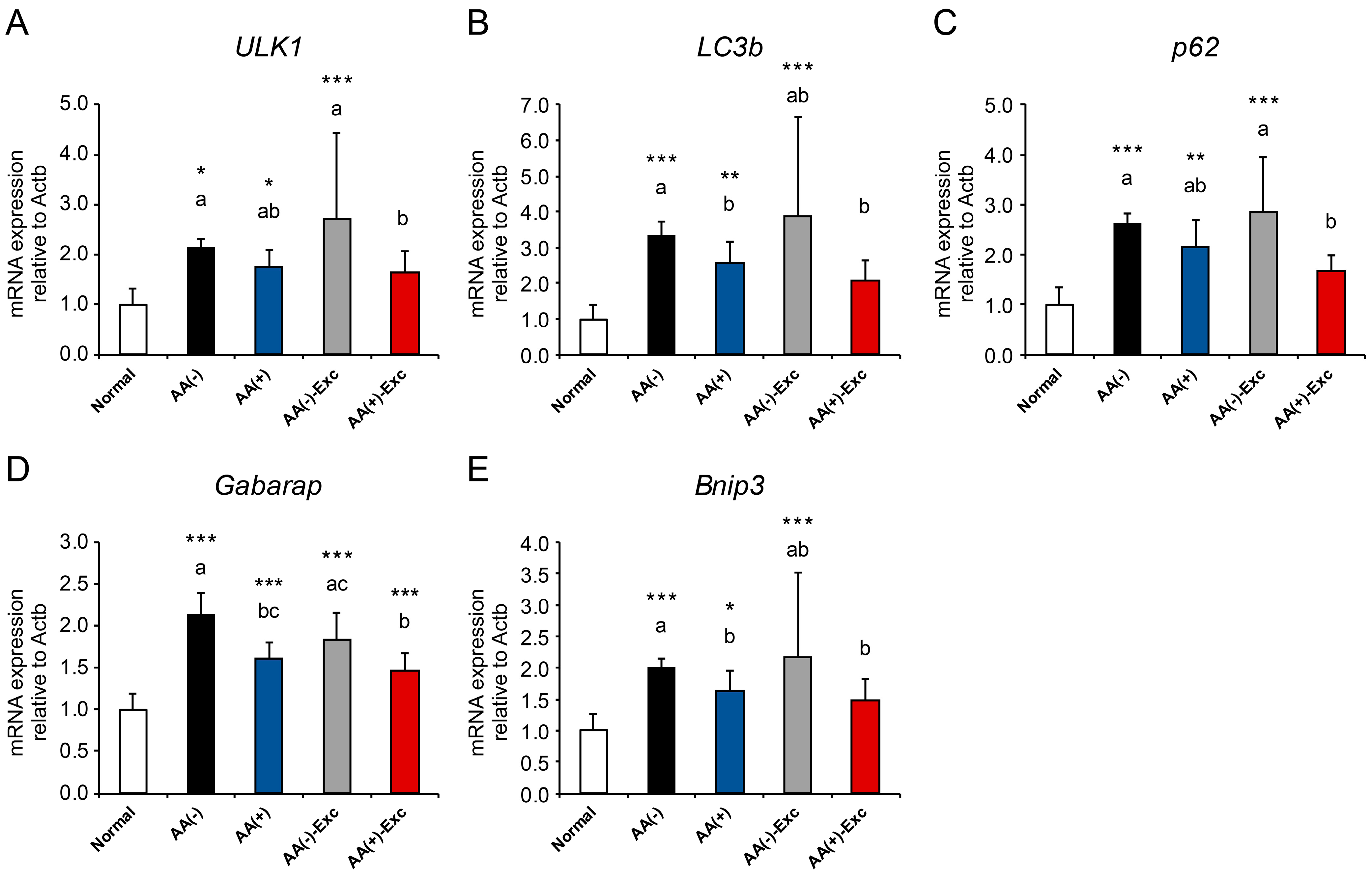
3.5. mRNA Expression of Autophagy-Related Genes
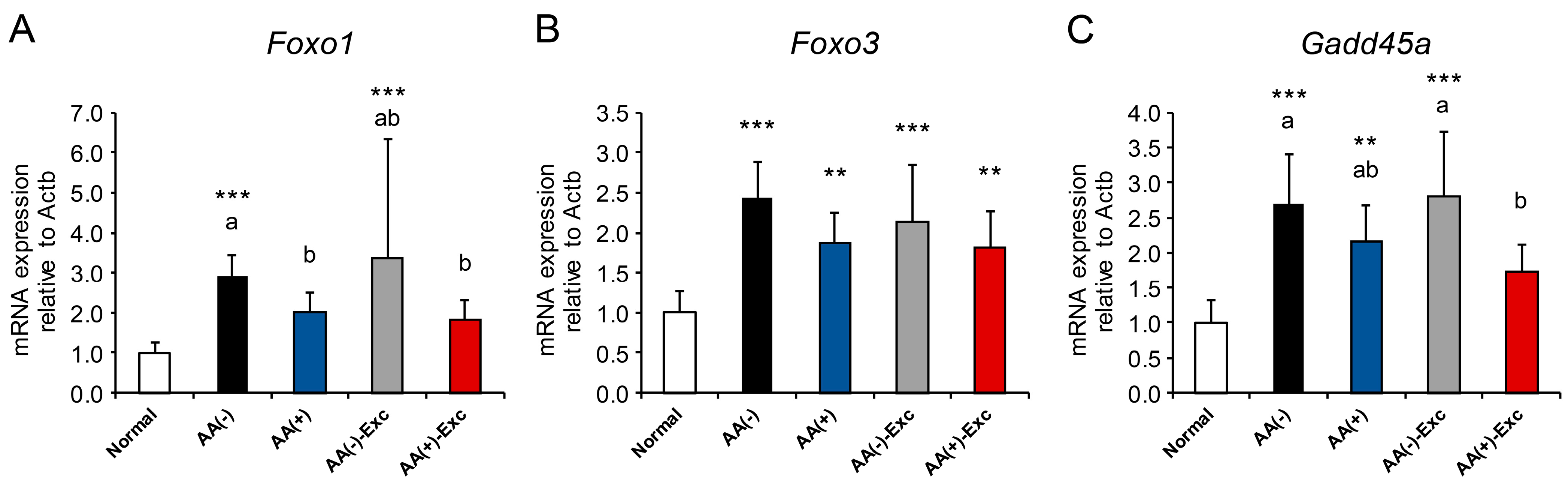
3.6. mRNA Expression of FOXO Transcription Factors and Its Target Gene
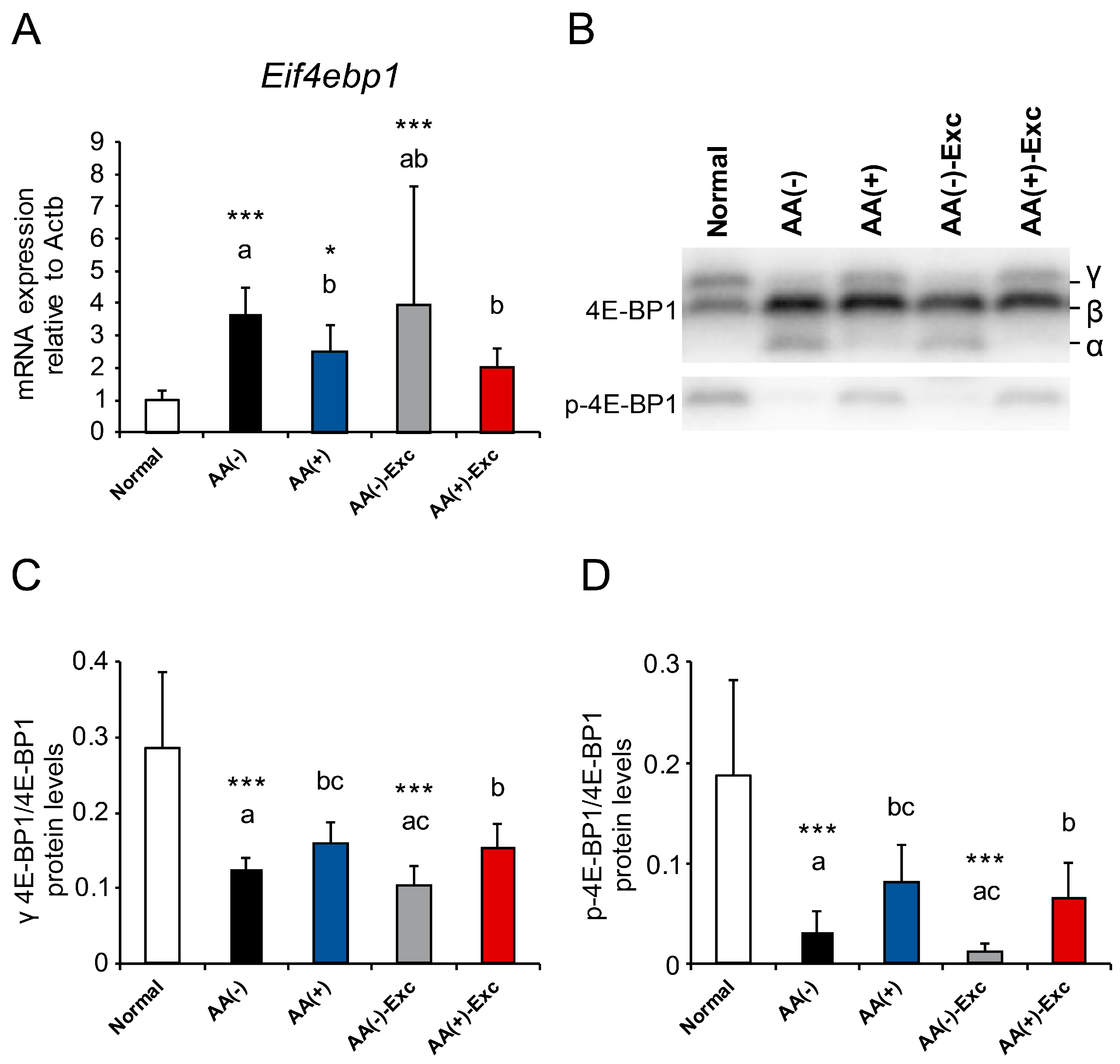
3.7. mRNA Expression of Eif4ebp1 and Phosphorylation of 4E-BP1
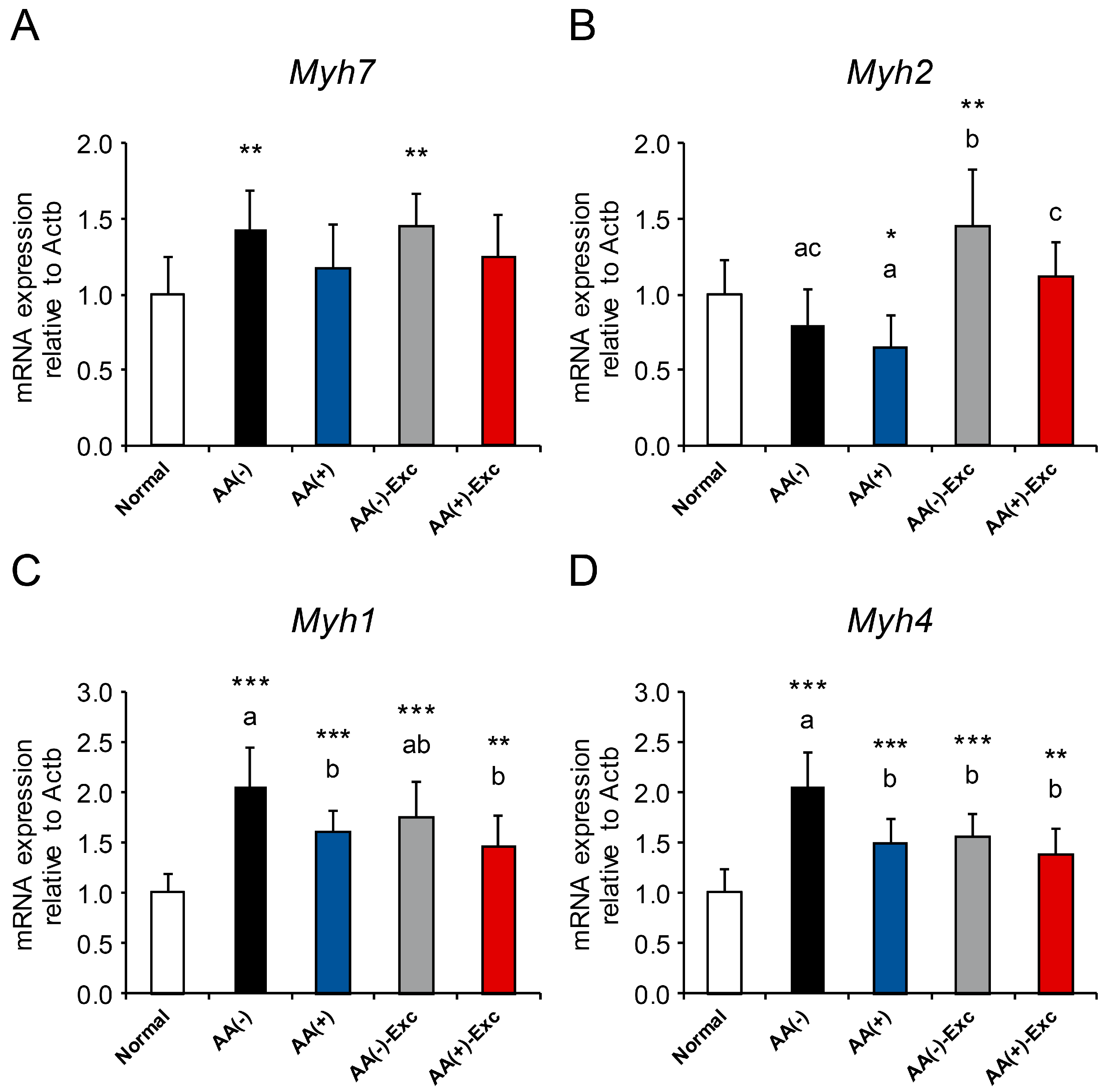
3.8. mRNA Expression of Myosin Heavy Chain Isoforms
3.9. Plasma AA Concentrations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Welch, C.; Hassan-Smith, Z.K.; Greig, C.A.; Lord, J.M.; Jackson, T.A. Acute sarcopenia secondary to hospitalisation—An emerging condition affecting older adults. Aging Dis. 2018, 9, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.S.; Guerra, R.S.; Fonseca, I.; Pichel, F.; Amaral, T.F. Sarcopenia among hospitalized patients—A cross-sectional study. Clin. Nutr. 2015, 34, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.; Abete, P.; Bellelli, G.; Bo, M.; Cherubini, A.; Corica, F.; Di Bari, M.; Maggio, M.; Manca, G.M.; Rizzo, M.R.; et al. Prevalence and clinical correlates of sarcopenia, identified according to the EWGSOP definition and diagnostic algorithm, in hospitalized older people: The GLISTEN study. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Aliberti, M.J.R.; Szlejf, C.; Covinsky, K.E.; Lee, S.J.; Jacob-Filho, W.; Suemoto, C.K. Prognostic value of a rapid sarcopenia measure in acutely ill older adults. Clin. Nutr. 2020, 39, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Bhat, S.; Xia, W.; Barazanchi, A.W.H.; Frampton, C.; Hill, A.G.; MacCormick, A.D. Consensus-defined sarcopenia predicts adverse outcomes after elective abdominal surgery: Meta-analysis. BJS Open. 2023, 10, zrad065. [Google Scholar] [CrossRef] [PubMed]
- Terayama, M.; Ohashi, M.; Ri, M.; Makuuchi, R.; Hayami, M.; Ida, S.; Kumagai, K.; Sano, T.; Nunobe, S. Long-term impact of sarcopenia in older patients undergoing gastrectomy for gastric cancer: A systematic review and meta-analysis. Transl. Gastroenterol. Hepatol. 2023, 26, 35. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Karvellas, C.J.; Baracos, V.; Williams, D.C.; Khadaroo, R.G.; Acute Care and Emergency Surgery (ACES) Group. Sarcopenia is a predictor of outcomes in very elderly patients undergoing emergency surgery. Surgery 2014, 156, 521–527. [Google Scholar] [CrossRef]
- Rangel, E.L.; Rios-Diaz, A.J.; Uyeda, J.W.; Castillo-Angeles, M.; Cooper, Z.; Olufajo, O.A.; Salim, A.; Sodickson, A.D. Sarcopenia increases risk of long-term mortality in elderly patients undergoing emergency abdominal surgery. J. Trauma Acute Care Surg. 2017, 83, 1179–1186. [Google Scholar] [CrossRef]
- Welch, C.; Greig, C.; Lewis, D.; Majid, Z.; Masud, T.; Moorey, H.; Pinkney, T.; Stanley, B.; Jackson, T. Trajectories of muscle quantity, quality and function measurements in hospitalized older adults. Geriatr. Gerontol. Int. 2022, 22, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Martone, A.M.; Bianchi, L.; Abete, P.; Bellelli, G.; Bo, M.; Cherubini, A.; Corica, F.; Di Bari, M.; Maggio, M.; Manca, G.M.; et al. The incidence of sarcopenia among hospitalized older patients: Results from the Glisten study. J. Cachexia Sarcopenia Muscle 2017, 8, 907–914. [Google Scholar] [CrossRef]
- Matsui, K.; Kawakubo, H.; Hirata, Y.; Matsuda, S.; Mayanagi, S.; Irino, T.; Fukuda, K.; Nakamura, R.; Wada, N.; Kitagawa, Y. Relationship between early postoperative change in total psoas muscle area and long-term prognosis in esophagectomy for patients with esophageal cancer. Ann. Surg. Oncol. 2021, 28, 6378–6387. [Google Scholar] [CrossRef]
- Schiaffino, S.; Dyar, K.A.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Ozdemir, M.; Hyatt, H. Redox control of proteolysis during inactivity-induced skeletal muscle atrophy. Antioxid. Redox Signal. 2020, 33, 559–569. [Google Scholar] [CrossRef]
- Milan, G.; Romanello, V.; Pescatore, F.; Armani, A.; Paik, J.H.; Frasson, L.; Seydel, A.; Zhao, J.; Abraham, R.; Goldberg, A.L.; et al. Regulation of autophagy and the ubiquitin-proteasome system by the FoxO transcriptional network during muscle atrophy. Nat. Commun. 2015, 6, 6670. [Google Scholar] [CrossRef]
- Wilburn, D.; Ismaeel, A.; Machek, S.; Fletcher, E.; Koutakis, P. Shared and distinct mechanisms of skeletal muscle atrophy: A narrative review. Ageing Res. Rev. 2021, 71, 101463. [Google Scholar] [CrossRef] [PubMed]
- Ciciliot, S.; Rossi, A.C.; Dyar, K.A.; Blaauw, B.; Schiaffino, S. Muscle type and fiber type specificity in muscle wasting. Int. J. Biochem. Cell Biol. 2013, 45, 2191–2199. [Google Scholar] [CrossRef]
- Rus, G.E.; Porter, J.; Brunton, A.; Crocker, M.; Kotsimbos, Z.; Percic, J.; Polzella, L.; Willet, N.; Huggins, C.E. Nutrition interventions implemented in hospital to lower risk of sarcopenia in older adults: A systematic review of randomised controlled trials. Nutr. Diet. 2020, 77, 90–102. [Google Scholar] [CrossRef]
- Tomassini, S.; Abbasciano, R.; Murphy, G.J. Interventions to prevent and treat sarcopenia in a surgical population: A systematic review and meta-analysis. BJS Open 2021, 5, zraa069. [Google Scholar] [CrossRef]
- Min, J.; An, K.Y.; Park, H.; Cho, W.; Jung, H.J.; Chu, S.H.; Cho, M.; Yang, S.Y.; Jeon, J.Y.; Kim, N.K. Postoperative inpatient exercise facilitates recovery after laparoscopic surgery in colorectal cancer patients: A randomized controlled trial. BMC Gastroenterol. 2023, 23, 127. [Google Scholar] [CrossRef]
- Senkal, M.; Bonavina, L.; Reith, B.; Caruso, R.; Matern, U.; Duran, M. Perioperative peripheral parenteral nutrition to support major gastrointestinal surgery: Expert opinion on treating the right patients at the right time. Clin. Nutr. ESPEN 2021, 43, 16–24. [Google Scholar] [CrossRef]
- Berlana, D. Parenteral nutrition overview. Nutrients 2022, 14, 4480. [Google Scholar] [CrossRef]
- Kikuchi, S.; Takata, N.; Kuroda, S.; Umeda, H.; Tanabe, S.; Maeda, N.; Takagi, K.; Noma, K.; Hasegawa, Y.; Nawachi, K.; et al. Impact of amino acids nutrition following gastrectomy in gastric cancer patients. Anticancer Res. 2022, 42, 3637–3643. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Wada, A.; Nakamura, M.; Kobayashi, K.; Kuroda, A.; Harada, D.; Kido, S.; Kuwahata, M. Effects of amino acids and albumin administration on albumin metabolism in surgically stressed rats: A basic nutritional study. JPEN J. Parenter. Enteral. Nutr. 2023, 47, 399–407. [Google Scholar] [CrossRef]
- Morey-Holton, E.R.; Globus, R.K. Hindlimb unloading rodent model: Technical aspects. J. Appl. Physiol. 2002, 92, 1367–1377. [Google Scholar] [CrossRef]
- Miyatake, S.; Hino, K.; Natsui, Y.; Ebisu, G.; Fujita, S. Protein supplementation enhances the effects of intermittent loading on skeletal muscles by activating the mTORC1 signaling pathway in a rat model of disuse atrophy. Nutrients 2020, 12, 2729. [Google Scholar] [CrossRef]
- Kanazashi, M.; Tanaka, M.; Murakami, S.; Kondo, H.; Nagatomo, F.; Ishihara, A.; Roland, R.R.; Fujino, H. Amelioration of capillary regression and atrophy of the soleus muscle in hindlimb-unloaded rats by astaxanthin supplementation and intermittent loading. Exp. Physiol. 2014, 99, 1065–1077. [Google Scholar] [CrossRef]
- Nakayama, M.; Motoki, T.; Kuwahata, T.; Onodera, R. The optimal nitrogen proportion to non-protein calories in normal rats receiving hypocaloric parenteral nutrition. Nutr. Res. 2002, 22, 1091–1099. [Google Scholar] [CrossRef]
- Yoshizawa, F.; Mochizuki, S.; Sugahara, K. Differential dose response of mTOR signaling to oral administration of leucine in skeletal muscle and liver of rats. Biosci. Biotechnol. Biochem. 2013, 77, 839–842. [Google Scholar] [CrossRef]
- Mantuano, P.; Boccanegra, B.; Bianchini, G.; Cappellari, O.; Tulimiero, L.; Conte, E.; Cirmi, S.; Sanarica, F.; De Bellis, M.; Mele, A.; et al. Branched-chain amino acids and di-alanine supplementation in aged mice: A translational study on sarcopenia. Nutrients 2023, 15, 330. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307. [Google Scholar] [CrossRef]
- Wall, B.T.; Dirks, M.L.; Snijders, T.; Senden, J.M.; Dolmans, J.; van Loon, L.J. Substantial skeletal muscle loss occurs during only 5 days of disuse. Acta Physiol. 2014, 210, 600–611. [Google Scholar] [CrossRef]
- O’Leary, M.F.; Vainshtein, A.; Iqbal, S.; Ostojic, O.; Hood, D.A. Adaptive plasticity of autophagic proteins to denervation in aging skeletal muscle. Am. J. Physiol. Cell Physiol. 2013, 304, C422–C430. [Google Scholar] [CrossRef]
- Trevino, M.B.; Zhang, X.; Standley, R.A.; Wang, M.; Han, X.; Reis, F.C.G.; Periasamy, M.; Yu, G.; Kelly, D.P.; Goodpaster, B.H.; et al. Loss of mitochondrial energetics is associated with poor recovery of muscle function but not mass following disuse atrophy. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E899–E910. [Google Scholar] [CrossRef]
- Brocca, L.; Cannavino, J.; Coletto, L.; Biolo, G.; Sandri, M.; Bottinelli, R.; Pellegrino, M.A. The time course of the adaptations of human muscle proteome to bed rest and the underlying mechanisms. J. Physiol. 2012, 590, 5211–5230. [Google Scholar] [CrossRef]
- Xia, Q.; Huang, X.; Huang, J.; Zheng, Y.; March, M.E.; Li, J.; Wei, Y. The role of autophagy in skeletal muscle diseases. Front. Physiol. 2021, 12, 638983. [Google Scholar] [CrossRef]
- Chen, K.; Gao, P.; Li, Z.; Dai, A.; Yang, M.; Chen, S.; Su, J.; Deng, Z.; Li, L. Forkhead Box O signaling pathway in skeletal muscle atrophy. Am. J. Pathol. 2022, 192, 1648–1657. [Google Scholar] [CrossRef]
- Salles, J.; Chanet, A.; Berry, A.; Giraudet, C.; Patrac, V.; Domingues-Faria, C.; Rocher, C.; Guillet, C.; Denis, P.; Pouyet, C.; et al. Fast digestive, leucine-rich, soluble milk proteins improve muscle protein anabolism, and mitochondrial function in undernourished old rats. Mol. Nutr. Food Res. 2017, 61, 1700287. [Google Scholar] [CrossRef] [PubMed]
- Mortimore, G.E.; Schworer, C.M. Induction of autophagy by amino-acid deprivation in perfused rat liver. Nature 1977, 270, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Ito, Y.; Nagasawa, T. Dietary L-lysine suppresses autophagic proteolysis and stimulates Akt/mTOR signaling in the skeletal muscle of rats fed a low-protein diet. J. Agric. Food Chem. 2015, 63, 8192–8198. [Google Scholar] [CrossRef] [PubMed]
- Kamata, S.; Yamamoto, J.; Kamijo, K.; Ochiai, T.; Morita, T.; Yoshitomi, Y.; Hagiya, Y.; Kubota, M.; Ohkubo, R.; Kawaguchi, M.; et al. Dietary deprivation of each essential amino acid induces differential systemic adaptive responses in mice. Mol. Nutr. Food Res. 2014, 58, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- Atherton, P.J.; Greenhaff, P.L.; Phillips, S.M.; Bodine, S.C.; Adams, C.M.; Lang, C.H. Control of skeletal muscle atrophy in response to disuse: Clinical/preclinical contentions and fallacies of evidence. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E594–E604. [Google Scholar] [CrossRef]
- Stevenson, E.J.; Giresi, P.G.; Koncarevic, A.; Kandarian, S.C. Global analysis of gene expression patterns during disuse atrophy in rat skeletal muscle. J. Physiol. 2003, 551, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Mirzoev, T.M.; Tyganov, S.A.; Petrova, I.O.; Shenkman, B.S. Acute recovery from disuse atrophy: The role of stretch-activated ion channels in the activation of anabolic signaling in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E86–E95. [Google Scholar] [CrossRef] [PubMed]
- Tyganov, S.A.; Mochalova, E.; Belova, S.; Sharlo, K.; Rozhkov, S.; Kalashnikov, V.; Turtikova, O.; Mirzoev, T.; Shenkman, B. Plantar mechanical stimulation attenuates protein synthesis decline in disused skeletal muscle via modulation of nitric oxide level. Sci. Rep. 2021, 11, 9806. [Google Scholar] [CrossRef] [PubMed]
- Theilen, N.T.; Jeremic, N.; Weber, G.J.; Tyagi, S.C. Exercise preconditioning diminishes skeletal muscle atrophy after hindlimb suspension in mice. J. Appl. Physiol. 2018, 125, 999–1010. [Google Scholar] [CrossRef]
- Vilchinskaya, N.; Altaeva, E.; Lomonosova, Y. Gaining insight into the role of FoxO1 in the progression of disuse-induced skeletal muscle atrophy. Adv. Biol. Regul. 2022, 85, 100903. [Google Scholar] [CrossRef]
- Adachi, Y.; Ono, N.; Imaizumi, A.; Muramatsu, T.; Andou, T.; Shimodaira, Y.; Nagao, K.; Kageyama, Y.; Mori, M.; Noguchi, Y.; et al. Plasma amino acid profile in severely frail elderly patients in Japan. Int. J. Gerontol. 2018, 12, 290–293. [Google Scholar] [CrossRef]
- Toyoshima, K.; Nakamura, M.; Adachi, Y.; Imaizumi, A.; Hakamada, T.; Abe, Y.; Kaneko, E.; Takahashi, S.; Shimokado, K. Increased plasma proline concentrations are associated with sarcopenia in the elderly. PLoS ONE 2017, 12, e0185206. [Google Scholar] [CrossRef] [PubMed]
- Staron, R.S.; Kraemer, W.J.; Hikida, R.S.; Fry, A.C.; Murray, J.D.; Campos, G.E. Fiber type composition of four hindlimb muscles of adult Fisher 344 rats. Histochem Cell Biol. 1999, 111, 117–123. [Google Scholar] [CrossRef] [PubMed]

| AA (−) | AA (+) | AA (−)-Exc | AA (+)-Exc | |
|---|---|---|---|---|
| Composition | ||||
| Glucose (%, w/v) | 10.0 | 7.5 | 10.0 | 7.5 |
| Amino acids (%, w/v) | 0 | 3.0 | 0 | 3.0 |
| Total calories (kcal) | 400 | 420 | 400 | 420 |
| Daily Dosage | ||||
| Volume (mL/kg/d) | 245 | 233.3 | 245 | 233.3 |
| Total energy (kcal/kg/d) | 98 | 98 | 98 | 98 |
| Glucose (g/kg/d) | 24.5 | 17.5 | 24.5 | 17.5 |
| Amino acids (g/kg/d) | 0 | 7.0 | 0 | 7.0 |
| Normal | AA (−) | AA (+) | AA (−)-Exc | AA (+)-Exc | |
|---|---|---|---|---|---|
| BCAA | 524.7 ± 104.9 | 338.6 ± 25.5 **, a | 525.7 ± 39.7 b | 318.9 ± 32.5 ***, a | 489.4 ± 37.8 b |
| EAA | 1608.1 ± 193.2 | 1364.1 ± 71.4 *, a | 1757.2 ± 91.0 b | 1311.7 ± 83.8 **, a | 1683.3 ± 88.3 b |
| NEAA | 2580.1 ± 211.5 | 2826.2 ± 174.3 **, a | 2205.6 ± 98.5 ***, b | 2620.2 ± 158.9 c | 2097.0 ± 108.9 ***, b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wada, A.; Yamashita, H.; Togashi, A.; Ogawa, S.; Muroi, A.; Kido, S.; Furuya, S. Combination of Parenteral Amino Acid Infusion and Intermittent Loading Exercise Ameliorates Progression of Postoperative Sarcopenia in Rat Model. Nutrients 2024, 16, 1218. https://doi.org/10.3390/nu16081218
Wada A, Yamashita H, Togashi A, Ogawa S, Muroi A, Kido S, Furuya S. Combination of Parenteral Amino Acid Infusion and Intermittent Loading Exercise Ameliorates Progression of Postoperative Sarcopenia in Rat Model. Nutrients. 2024; 16(8):1218. https://doi.org/10.3390/nu16081218
Chicago/Turabian StyleWada, Akira, Hayato Yamashita, Ayaka Togashi, Shunsuke Ogawa, Arashi Muroi, Satoshi Kido, and Shigeki Furuya. 2024. "Combination of Parenteral Amino Acid Infusion and Intermittent Loading Exercise Ameliorates Progression of Postoperative Sarcopenia in Rat Model" Nutrients 16, no. 8: 1218. https://doi.org/10.3390/nu16081218
APA StyleWada, A., Yamashita, H., Togashi, A., Ogawa, S., Muroi, A., Kido, S., & Furuya, S. (2024). Combination of Parenteral Amino Acid Infusion and Intermittent Loading Exercise Ameliorates Progression of Postoperative Sarcopenia in Rat Model. Nutrients, 16(8), 1218. https://doi.org/10.3390/nu16081218





