Human Milk Oligosaccharides, Growth, and Body Composition in Very Preterm Infants
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
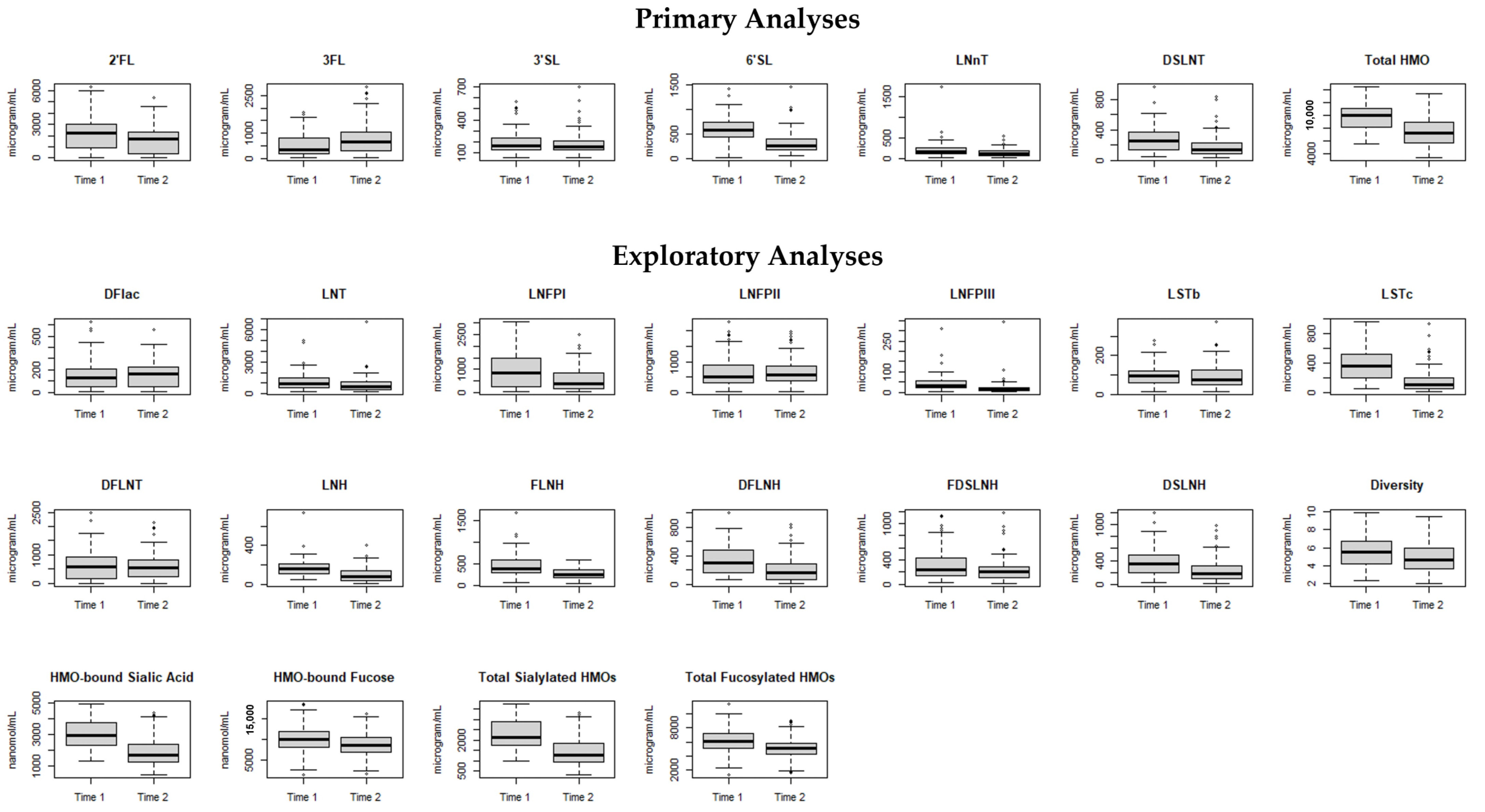
2.2. Milk Collection and HMO Analysis
2.3. Growth and Body Composition Outcomes
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cooke, R.; Foulder-Hughes, L. Growth impairment in the very preterm and cognitive and motor performance at 7 year. Arch. Dis. Child. 2003, 88, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Serenius, F.; Kallen, K.; Blennow, M.; Ewald, U.; Fellman, V.; Holmstrom, G.; Lindberg, E.; Lundqvist, P.; Marsal, K.; Norman, M.; et al. Neurodevelopmental outcome in extremely preterm infants at 2.5 years after active perinatal care in Sweden. JAMA 2013, 309, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; Tonkin, E.; Damarell, R.A.; McPhee, A.J.; Suganuma, M.; Suganuma, H.; Middleton, P.F.; Makrides, M.; Collins, C.T. A Systematic Review and Meta-Analysis of Human Milk Feeding and Morbidity in Very Low Birth Weight Infants. Nutrients 2018, 10, 707. [Google Scholar] [CrossRef] [PubMed]
- Thai, J.D.; Gregory, K.E. Bioactive Factors in Human Breast Milk Attenuate Intestinal Inflammation during Early Life. Nutrients 2020, 12, 581. [Google Scholar] [CrossRef]
- Gregory, K.E.; Samuel, B.S.; Houghteling, P.; Shan, G.; Ausubel, F.M.; Sadreyev, R.I.; Walker, W.A. Influence of maternal breast milk ingestion on aquisition of the intestinal microbiome in preterm infants. Microbiome 2016, 4, 68. [Google Scholar] [CrossRef] [PubMed]
- Belfort, M.B.; Anderson, P.J.; Nowak, V.A.; Lee, K.J.; Molesworth, C.; Thompson, D.K.; Doyle, L.W.; Inder, T.E. Breast Milk Feeding, Brain Development, and Neurocognitive Outcomes: A 7-Year Longitudinal Study in Infants Born at Less Than 30 Weeks’ Gestation. J. Pediatr. 2016, 177, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Ottolini, K.M.; Andescavage, N.; Kapse, K.; Jacobs, M.; Liperopoulos, C. Improved brain growth and microstructural development in breast milk–fed very low birth weight premature infants. Acta Paediatr. 2020, 109, 1580–1587. [Google Scholar] [CrossRef]
- Blesa, M.; Sullivan, G.; Anblagan, D.; Telford, E.J.; Quigley, A.J.; Sparrow, S.A.; Serag, A.; Semple, S.I.; Bastin, M.E.; Boardman, J.P. Early breast milk exposure modifies brain connectivity in preterm infants. NeuroImage 2019, 184, 431–439. [Google Scholar] [CrossRef]
- Belfort, M.B.; Knight, E.; Chandarana, S.; Ikem, E.; Gould, J.F.; Collins, C.T.; Makrides, M.; Gibson, R.A.; Anderson, P.J.; Simmer, K.; et al. Associations of Maternal Milk Feeding with Neurodevelopmental Outcomes at 7 Years of Age in Former Preterm Infants. JAMA Netw. Open 2022, 5, e2221608. [Google Scholar] [CrossRef]
- Belfort, M.B.; Inder, T.E. Human Milk and Preterm Infant Brain Development: A Narrative Review. Clin. Ther. 2022, 44, 612–621. [Google Scholar] [CrossRef]
- Autran, C.A.; Benjamin, K.P.; Kim, J.H.; Asztalos, E.; Blood, A.B.; Spence, E.C.H.; Patel, A.L.; Hou, J.; Lewis, N.E.; Bode, L. Human milk oligosaccharide composition predicts risk of necrotising enterocolitis in preterm infants. Gut 2018, 67, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Van Niekerk, E.; Autran, C.A.; Nel, D.G.; Kirsten, G.F.; Blaauw, R.; Bode, L. Human Milk Oligosaccharides Differ between HIV-Infected and HIV-Uninfected Mothers and Are Related to Necrotizing Enterocolitis Incidence in Their Preterm Very-Low-Birth-Weight Infants. J. Nutr. 2014, 144, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Jantscher-Krenn, E.; Zheretsov, M.; Nissan, C.; Goth, K.; Guner, Y.S.; Naidu, N.; Choudhury, B.; Grishin, A.V.; Ford, H.R.; Bode, L. The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats. Gut 2012, 61, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Masi, A.C.; Embleton, N.D.; Lamb, C.A.; Young, G.; Granger, C.L.; Najera, J.; Smith, D.P.; Hoffman, K.L.; Petrosino, J.F.; Bode, L.; et al. Human milk oligosaccharide DSLNT and gut microbiome in preterm infants predicts necrotising enterocolitis. Gut 2021, 70, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Gila-Diaz, A.; Arribas, S.M.; Algara, A.; Martin-Cabrejas, M.A.; Lopez de Pablo, A.L.; Saenz de Pipaon, M.; Ramiro-Cortijo, D. A Review of Bioactive Factors in Human Breastmilk: A Focus on Prematurity. Nutrients 2019, 11, 1307. [Google Scholar] [CrossRef] [PubMed]
- Mank, E.; Naninck, E.F.G.; Limpens, J.; van Toledo, L.; van Goudoever, J.B.; van den Akker, C.H.P. Enteral Bioactive Factor Supplementation in Preterm Infants: A Systematic Review. Nutrients 2020, 12, 2916. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Rubio, R.; Kunz, C.; Rudloff, S.; Garcia-Mantrana, I.; Crehua-Gaudiza, E.; Martinez-Costa, C.; Collado, M.C. Association of Maternal Secretor Status and Human Milk Oligosaccharides with Milk Microbiota: An Observational Pilot Study. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 256–263. [Google Scholar] [CrossRef]
- Kunz, C.; Meyer, C.; Collado, M.C.; Geiger, L.; Garcia-Mantrana, I.; Bertua-Rios, B.; Martinez-Costa, C.; Borsch, C.; Rudloff, S. Influence of Gestational Age, Secretor, and Lewis Blood Group Status on the Oligosaccharide Content of Human Milk. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Robertson, B.; Atakora, F.; Becker, A.B.; Subbarao, P.; Moraes, T.J.; Mandhane, P.J.; Turvey, S.E.; Lefbvre, D.L.; Sears, M.R.; et al. Human Milk Oligosaccharide Concentrations Are Associated with Multiple Fixed and Modifiable Maternal Characteristics, Environmental Factors, and Feeding Practices. J. Nutr. 2018, 148, 1733–1742. [Google Scholar] [CrossRef]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef]
- Reverri, E.J.; Devitt, A.A.; Kajzer, J.A.; Baggs, G.E.; Borschel, M.W. Review of the Clinical Experiences of Feeding Infants Formula Containing the Human Milk Oligosaccharide 2′-Fucosyllactose. Nutrients 2018, 10, 1346. [Google Scholar] [CrossRef] [PubMed]
- Lagstrom, H.; Rautava, S.; Ollila, H.; Kaljonen, A.; Turta, O.; Makela, J.; Yonemitsu, C.; Gupta, J.; Bode, L. Associations between human milk oligosaccharides and growth in infancy and early childhood. Am. J. Clin. Nutr. 2020, 111, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M.W.; Lind, M.V.; Laursen, R.P.; Yonemitsu, C.; Lamjaer, A.; Molgaard, C.; Michaelsen, K.F.; Bode, L. Human Milk Oligosaccharide Composition Is Associated with Excessive Weight Gain During Exclusive Breastfeeding—An Explorative Study. Front. Pediatr. 2019, 7, 279. [Google Scholar] [CrossRef]
- Alderete, T.L.; Autran, C.; Brekke, B.E.; Knight, R.; Bode, L.; Goran, M.I.; Fields, D.A. Associations between human milk oligosaccharides and infant body composition in the first 6 mo of life. Am. J. Clin. Nutr. 2015, 102, 1381–1388. [Google Scholar] [CrossRef]
- Schönknecht, Y.B.; Tovar, M.V.M.; Jensen, S.R.; Parschat, K. Clinical Studies on the Supplementation of Manufactured Human Milk Oligosaccharides: A Systematic Review. Nutrients 2023, 15, 3622. [Google Scholar] [CrossRef]
- Jochum, F.; Meyer-Krott, M.; Hübler, T.; Lorenz, M.; Bedikian, R.; Zakarian, J.; Litzka, A.; Judex, G.; Hertzberg, H.; Klee, D.; et al. Real-world evidence study on tolerance and growth in infants fed an infant formula with two human milk oligosaccharides vs. mixed fed and exclusively breastfed infants. Mol. Cell Pediatr. 2023, 10, 7. [Google Scholar] [CrossRef]
- Puccio, G.; Alliet, P.; Cajozzo, C.; Janssens, E.; Corsello, G.; Sprenger, N.; Wernimont, S.; Egli, D.; Gosoniu, L.; Steenhout, P. Effects of Infant Formula with Human Milk Oligosaccharides on Growth and Morbidity: A Randomized Multicenter Trial. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 624–631. [Google Scholar] [CrossRef]
- Lasekan, J.; Choe, Y.; Dvoretskiy, S.; Devitt, A.; Zhang, S.; Mackey, A.; Wulf, K.; Buck, R.; Steele, C.; Johnson, M.; et al. Growth and Gastrointestinal Tolerance in Healthy Term Infants Fed Milk-Based Infant Formula Supplemented with Five Human Milk Oligosaccharides (HMOs): A Randomized Multicenter Trial. Nutrients 2022, 14, 2625. [Google Scholar] [CrossRef]
- Ehrenkranz, R.A.; Dusick, A.M.; Vohr, B.R.; Wright, L.L.; Wrage, L.A.; Poole, K. Network ftNIoCHaHDNR: Growth in the Neonatal Intensive Care Unit Influences Neurodevelopmental and Growth Outcomes of Extremely Low Birth Weight Infants. Pediatrics 2006, 117, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Wejryd, E.; Marti, M.; Marchini, G.; Werme, A.; Jonsson, B.; Landberg, E.; Abrahamsson, T.R. Low Diversity of Human Milk Oligosaccharides is Associated with Necrotising Enterocolitis in Extremely Low Birth Weight Infants. Nutrients 2018, 10, 1556. [Google Scholar] [CrossRef]
- Roze, J.-C.; Hartweg, M.; Simon, L.; Billard, H.; Chen, Y.; Austin, S.; Boscher, C.; Moyon, T.; Darmaun, D.; Garcia Rodenas, C.I.; et al. Human milk oligosaccharides in breast milk and 2-year outcome in preterm infants: An exploratory analysis. Clin. Nutr. 2022, 41, 1896–1905. [Google Scholar] [CrossRef] [PubMed]
- Cordova, E.G.; Cherkerzian, S.; Bell, K.; Joung, K.E.; Collins, C.T.; Makrides, M.; Gould, J.; Anderson, P.J.; Belfort, M.B. Association of Poor Postnatal Growth with Neurodevelopmental Impairment in Infancy and Childhood: Comparing the Fetus and the Healthy Preterm Infant References. J. Pediatr. 2020, 225, 37–43.e5. [Google Scholar] [CrossRef] [PubMed]
- Pfister, K.M.; Zhang, L.; Miller, N.C.; Ingolfsland, E.C.; Demerath, E.W.; Ramel, S.E. Early body composition changes are associated with neurodevelopmental and metabolic outcomes at 4 years of age in very preterm infants. Pediatr. Res. 2018, 84, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Ramel, S.E.; Gray, H.L.; Christiansen, E.; Boys, C.; Georgieff, M.K.; Demerath, E.W. Greater Early Gains in Fat-Free Mass, but Not Fat Mass, Are Associated with Improved Neurodevelopment at 1 Year Corrected Age for Prematurity in Very Low Birth Weight Preterm Infants. J. Pediatr. 2016, 173, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Belfort, M.; Cherkerzian, S.; Bell, K.; Soldateli, B.; Ramos, E.C.; Palmer, C.; Steele, T.; Pepin, H.; Ellard, D.; Drouin, K.; et al. Macronutrient Intake from Human Milk, Infant Growth, and Body Composition at Term Equivalent Age: A Longitudinal Study of Hospitalized Very Preterm Infants. Nutrients 2020, 12, 2249. [Google Scholar] [CrossRef] [PubMed]
- Berger, P.K.; Hampson, H.E.; Schmidt, K.A.; Alderete, T.L.; Furst, A.; Yonemitsu, C.; Demerath, E.; Goran, M.I.; Fields, D.A.; Bode, L. Stability of Human-Milk Oligosaccharide Concentrations Over 1 Week of Lactation and Over 6 Hours Following a Standard Meal. J. Nutr. 2023, 152, 2727–2733. [Google Scholar] [CrossRef] [PubMed]
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Norris, T.; Ramel, S.E.; Catalano, P.; Caoimh, C.N.; Roggero, P.; Murray, D.; Fields, D.A.; Demerath, E.W.; Johnson, W. New charts for the assessment of body composition, according to air-displacement plethysmography, at birth and across the first 6 mo of life. Am. J. Clin. Nutr. 2019, 109, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Bakdash, J.Z.; Marusich, L.R. Corrigendum: Repeated Measures Correlation. Front. Psychol. 2019, 10, 1201. [Google Scholar] [CrossRef]
- Cheema, A.S.; Trevenen, M.L.; Turlack, B.A.; Furst, A.J.; Roman, A.S.; Bode, L.; Grideneva, Z.; Lai, C.T.; Stinson, L.F.; Payne, M.S.; et al. Exclusively Breastfed Infant Microbiota Develops over Time and Is Associated with Human Milk Oligosaccharide Intakes. Int. J. Mol. Sci. 2022, 23, 2804. [Google Scholar] [CrossRef]
- Plows, J.F.; Berger, P.K.; Jones, R.B.; Alderete, T.L.; Yonemitsu, C.; Najera, J.A.; Khwajazada, S.; Bode, L.; Goran, M.I. Longitudinal Changes in Human Milk Oligosaccharides (HMOs) Over the Course of 24 Months of Lactation. J. Nutr. 2021, 151, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Friend, L.L.; Perrin, M.T. Fat and Protein Variability in Donor Human Milk and Associations with Milk Banking Processes. Breastfeed. Med. 2020, 15, 370–376. [Google Scholar] [CrossRef]
- Fu, T.T.; Schroder, P.E.; Poindexter, B.B. Macronutrient Analysis of Target-Pooled Donor Breast Milk and Corresponding Growth in Very Low Birth Weight Infants. Nutrients 2019, 11, 1884. [Google Scholar] [CrossRef] [PubMed]
- Brockway, M.; Daniel, A.I.; Reyes, S.M.; Gauglitz, J.M.; Granger, M.; McDermid, J.M.; Chan, D.; Refvik, R.; Sidhu, K.; Musse, S.; et al. Human Milk Bioactive Components and Child Growth and Body Composition in the First 2 Years: A Systematic Review. Adv. Nutr. 2024, 15, 100127. [Google Scholar] [CrossRef]
- Saben, J.L.; Sims, C.R.; Abraham, A.; Bode, L.; Andres, A. Human Milk Oligosaccharide Concentrations and Infant Intakes Are Associated with Maternal Overweight and Obesity and Predict Infant Growth. Nutrients 2021, 13, 446. [Google Scholar] [CrossRef] [PubMed]
- Cheema, A.S.; Gridneva, Z.; Furst, A.J.; Roman, A.S.; Trevenen, M.L.; Turlack, B.A.; Lai, C.T.; Stinson, L.F.; Bode, L.; Payne, M.S.; et al. Human Milk Oligosaccharides and Bacterial Profile Modulate Infant Body Composition during Exclusive Breastfeeding. Int. J. Mol. Sci. 2022, 23, 2865. [Google Scholar] [CrossRef] [PubMed]
- Gildrewicz, D.A.; Fenton, T.R. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatr. 2014, 14, 216. [Google Scholar]
- Austin, S.; De Castro, C.A.; Sprenger, N.; Binia, A.; Affolter, M.; Garcia-Rodenas, C.L.; Beauport, L.; Tolsa, J.-F.; Fumeaux, C.J.F. Human Milk Oligosaccharides in the Milk of Mothers Delivering Term versus Preterm Infants. Nutrients 2019, 11, 1282. [Google Scholar] [CrossRef] [PubMed]
- Roldan, V.D.T.; Uretecho, M.S.; Gupta, J.; Yonemitsu, C.; Carcamo, C.P.; Bode, L.; Ochoa, T.J. Human milk oligosaccharides and their association with late-onset neonatal sepsis in Peruvian very-low-birth-weight infants. Am. J. Clin. Nutr. 2020, 112, 106–112. [Google Scholar] [CrossRef]
- Chleilat, F.; Klancic, T.; Ma, K.; Schick, A.; Nettleton, J.E.; Reimer, R.A. Human Milk Oligosaccharide Supplementation Affects Intestinal Barrier Function and Microbial Composition in the Gastrointestinal Tract of Young Sprague Dawley Rats. Nutrients 2020, 12, 1532. [Google Scholar] [CrossRef]
- Lee, S.; Goodson, M.L.; Vang, W.; Rutkowsky, J.; Kalanetra, K.; Bhattacharya, M.; Barile, D.; Raybould, H.E. Human milk oligosaccharide 2′-fucosyllactose supplementation improves gut barrier function and signaling in the vagal afferent pathway in mice. Food Funct. 2021, 12, 8507–8521. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.O.; Martin, L.; Ostergaard, M.V.; Rudloff, S.; Roggenbuck, M.; Nguyen, D.N.; Sangild, P.T.; Bering, S.B. Human milk oligosaccharide effects on intestinal function and inflammation after preterm birth in pigs. J. Nutr. Biochem. 2017, 40, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Beukema, M.; Wang, M.; de Haan, B.J.; de Vos, P. Human milk oligosaccharides and non-digestible carbohydrates prevent adhesion of specific pathogens via modulating glycosylation or inflammatory genes in intestinal epithelial cells. Food Funct. 2021, 12, 8100–8119. [Google Scholar] [CrossRef] [PubMed]
- Belfort, M.B.; Ramel, S.E.; Martin, C.R.; Fichorova, R.; Kuban, K.C.K.; Heeren, T.; Fry, R.C.; O’Shea, T.M. Systemic Inflammation in the First 2 Weeks after Birth as a Determinant of Physical Growth Outcomes in Hospitalized Infants with Extremely Low Gestational Age. J. Pediatr. 2022, 240, 37–43. [Google Scholar] [CrossRef]
- Christian, P.; Smith, E.R.; Lee, S.E.; Vargas, A.J.; Bremer, A.A.; Raiten, D.J. The need to study human milk as a biological system. Am. J. Clin. Nutr. 2021, 113, 1063–1072. [Google Scholar] [CrossRef]
- Mainardi, F.; Binia, A.; Rajhans, P.; Austin, S.; Deoni, S.; Schneider, N. Human milk oligosaccharide composition and associations with growth: Results from an observational study in the US. Front. Nutr. 2023, 10. [Google Scholar] [CrossRef]
| Maternal Characteristics (n = 76) | |
| Maternal age, years | 32.0 (6.4) |
| Race | |
| Asian | 5 (7%) |
| Black | 23 (33%) |
| Multiple | 3 (4%) |
| Other | 3 (4%) |
| White | 35 (51%) |
| Ethnicity—Hispanic | 12 (16%) |
| Parity | 1.6 (0.9) |
| Mode of delivery—Cesarean | 50 (67%) |
| Infant Characteristics (n = 82) | |
| Gestational age, weeks | 28.2 (2.2) |
| Male | 46 (56%) |
| Multiple gestation | 16 (20%) |
| Maternal milk at DOL 28—% | 99 (4) |
| Postnatal steroid treatment | 13 (16%) |
| NEC | 2 (2%) |
| Late-onset sepsis | 6 (8%) |
| IVH grade 3 or 4 | 3 (4%) |
| Infant Birth Measurements | |
| Weight (g) | 1063 (386) |
| Weight z-score | −0.21 (1.05) |
| Length (cm) | 36.2 (4.0) |
| Length z-score | −0.14 (1.22) |
| Head circumference (cm) | 25.1 (2.7) |
| Head circumference z-score | −0.40 (1.10) |
| Outcome | n | Raw | Z-Score |
|---|---|---|---|
| Weight (g) | 82 | 2792 ± 610 | −1.07 ± 1.30 |
| Length (cm) | 79 | 46.8 ± 2.9 | −1.20 ± 1.36 |
| Head circumference (cm) | 78 | 33.4 ± 1.5 | −0.47 ± 1.15 |
| Fat mass (kg) | 65 | 0.55 ± 0.21 | 1.79 ± 1.12 |
| Fat-free mass (kg) | 65 | 2.28 ± 0.46 | −1.30 ± 1.56 |
| Body fat percentage (%) | 65 | 18.5 ± 4.7 | 2.23 ± 1.11 |
| HMO | Weight (n = 82) | Length (n = 73) | Head Circumference (n = 74) | |||
|---|---|---|---|---|---|---|
| High vs. Low | Intermediate vs. Low | High vs. Low | Intermediate vs. Low | High vs. Low | Intermediate vs. Low | |
| Primary Analyses | ||||||
| 2′FL | −0.02 (−0.55, 0.51) | 0.22 (−0.28, 0.73) | −0.11 (−0.54, 0.32) | 0.10 (−0.32, 0.51) | −0.13 (−0.59, 0.33) | 0.29 (−0.16, 0.73) |
| 3FL | −0.04 (−0.57, 0.49) | 0.05 (−0.48, 0.58) | 0.12 (−0.32, 0.56) | 0.25 (−0.20, 0.70) | 0.30 (−0.15, 0.76) | 0.61 (0.15, 1.07) * |
| 3′SL | 0.04 (−0.47, 0.55) | −0.15 (−0.67, 0.38) | −0.25 (−0.67, 0.18) | −0.16 (−0.59, 0.26) | −0.03 (−0.48, 0.43) | 0.10 (−0.37, 0.57) |
| 6′SL | 0.23 (−0.27, 0.73) | −0.29 (−0.77, 0.20) | −0.11 (−0.52, 0.31) | 0.03 (−0.39, 0.45) | 0.01 (−0.46, 0.47) | 0.10 (−0.36, 0.55) |
| LNnT | −0.40 (−0.90, 0.10) | −0.45 (−0.94, 0.05) | −0.23 (−0.65, 0.19) | −0.19 (−0.60, 0.23) | −0.39 (−0.83, 0.05) | −0.01 (−0.46, 0.45) |
| DSLNT | −0.06 (−0.59, 0.46) | −0.32 (−0.84, 0.20) | −0.25 (−0.70, 0.20) | −0.18 (−0.61, 0.25) | −0.05 (−0.55, 0.44) | 0.11 (−0.37, 0.60) |
| Total HMO | −0.04 (−0.56, 0.48) | −0.06 (−0.58, 0.47) | −0.14 (−0.56, 0.29) | −0.21 (−0.64, 0.22) | −0.13 (−0.60, 0.34) | 0.01 (−0.47, 0.50) |
| Exploratory Analyses | ||||||
| DFlac | −0.27 (−0.79, 0.26) | −0.09 (−0.61, 0.43) | −0.01 (−0.43, 0.41) | −0.07 (−0.50, 0.36) | 0.20 (−0.27, 0.67) | 0.27 (−0.21, 0.75) |
| LNT | 0.08 (−0.44, 0.60) | 0.002 (−0.51, 0.51) | 0.12 (−0.30, 0.54) | 0.21 (−0.22, 0.65) | 0.05 (−0.42, 0.53) | 0.15 (−0.31, 0.61) |
| LNFPI | −0.01 (−0.55, 0.54) | −0.01 (−0.53, 0.50) | −0.14 (−0.60, 0.32) | 0.14 (−0.26, 0.54) | −0.23 (−0.72, 0.26) | 0.07 (−0.38, 0.51) |
| LNFPII | 0.20 (−0.32, 0.71) | 0.11 (−0.41, 0.63) | 0.07 (−0.35, 0.49) | −0.14 (−0.57, 0.29) | −0.004 (−0.44, 0.44) | 0.38 (−0.09, 0.85) |
| LNFPIII | −0.01 (−0.56, 0.53) | −0.21 (−0.74, 0.31) | 0.13 (−0.30, 0.56) | 0.43 (−0.003, 0.86) | −0.02 (−0.52, 0.48) | 0.07 (−0.40, 0.54) |
| LSTb | 0.08 (−0.42, 0.59) | −0.42 (−0.93, 0.09) | −0.35 (−0.78, 0.08) | −0.17 (−0.59, 0.26) | −0.33 (−0.80, 0.14) | −0.53 (−0.99, −0.08) * |
| LSTc | −0.03 (-0.55, 0.48) | −0.03 (−0.56, 0.50) | −0.15 (−0.56, 0.25) | −0.48 (−0.90, −0.05) * | −0.33 (−0.78, 0.13) | −0.16 (−0.65, 0.33) |
| DFLNT | −0.19 (−0.71, 0.33) | −0.11 (−0.65, 0.43) | −0.09 (−0.54, 0.36) | −0.13 (−0.57, 0.30) | 0.26 (−0.22, 0.74) | 0.25 (−0.23, 0.72) |
| LNH | −0.21 (−0.72, 0.30) | −0.30 (−0.81, 0.21) | 0.21 (−0.22, 0.64) | −0.04 (−0.49, 0.42) | −0.11 (−0.58, 0.37) | −0.01 (−0.48, 0.45) |
| FLNH | −0.03 (−0.57, 0.51) | −0.14 (−0.66, 0.38) | 0.05 (−0.38, 0.48) | −0.21 (−0.64, 0.21) | −0.04 (−0.51, 0.43) | 0.21 (−0.27, 0.69) |
| DFLNH | −0.09 (−0.61, 0.42) | −0.46 (−0.96, 0.03) | 0.28 (−0.13, 0.70) | −0.13 (−0.53, 0.27) | 0.06 (−0.41, 0.53) | 0.20 (−0.27, 0.66) |
| FDSLNH | 0.27 (−0.24, 0.77) | 0.20 (−0.31, 0.71) | 0.01 (−0.41, 0.43) | −0.26 (−0.69, 0.17) | 0.19 (−0.26, 0.65) | −0.12 (−0.58, 0.35) |
| DSLNH | 0.13 (−0.39, 0.65) | −0.31 (−0.81, 0.18) | 0.01 (−0.42, 0.44) | 0.01 (−0.42, 0.44) | 0.11 (−0.34, 0.57) | −0.08 (−0.54, 0.39) |
| Diversity | 0.23 (−0.28, 0.74) | 0.16 (−0.35, 0.66) | 0.07 −0.34, 0.49) | 0.02 (−0.39, 0.43) | 0.28 (−0.17, 0.73) | 0.08 (−0.37, 0.54) |
| HMO-bound sialic acid | 0.35 (−0.16, 0.87) | −0.25 (−0.75, 0.24) | −0.15 (−0.56, 0.27) | 0.03 (−0.38, 0.44) | 0.05 (−0.43, 0.53) | −0.15 (−0.61, 0.31) |
| HMO-bound fucose | −0.02 (−0.56, 0.52) | 0.09 (−0.44, 0.62) | −0.07 (−0.50, 0.35) | −0.08 (−0.52, 0.36) | −0.20 (−0.66, 0.26) | −0.10 (−0.58, 0.38) |
| Total sialylated | 0.36 (−0.17, 0.88) | −0.15 (−0.64, 0.35) | −0.10 (−0.51, 0.32) | 0.02 (−0.39, 0.44) | 0.09 (−0.39, 0.57) | −0.12 (−0.58, 0.34) |
| Total fucosylated | 0.21 (−0.33, 0.75) | 0.18 (−0.35, 0.71) | 0.13 (−0.31, 0.58) | 0.06 (−0.38, 0.51) | −0.11 (−0.58, 0.36) | 0.02 (−0.44, 0.49) |
| HMO | Fat Mass | Fat-Free Mass | Body Fat Percentage | |||
|---|---|---|---|---|---|---|
| High vs. Low | Intermediate vs. Low | High vs. Low | Intermediate vs. Low | High vs. Low | Intermediate vs. Low | |
| Primary Analyses | ||||||
| 2′FL | 0.005 (−0.73, 0.74) | 0.16 (−0.52, 0.83) | 0.08 (−0.49, 0.64) | −0.01 (−0.54, 0.51) | 0.14 (−0.62, 0.89) | 0.11 (−0.59, 0.81) |
| 3FL | 0.18 (−0.59, 0.95) | 0.45 (−0.25, 1.15) | 0.10 (−0.50, 0.69) | 0.30 (−0.24, 0.85) | 0.09 (−0.72, 0.89) | 0.29 (−0.44, 1.02) |
| 3′SL | −0.07 (−0.75, 0.60) | −0.39 (−1.08, 0.31) | 0.23 (−0.30, 0.75) | −0.12 (−0.66, 0.42) | −0.15 (−0.83, 0.54) | −0.53 (−1.25, 0.19) |
| 6′SL | 0.06 (−0.65, 0.78) | −0.26 (−0.93, 0.40) | 0.03 (−0.53, 0.59) | 0.01 (−0.51, 0.53) | −0.13 (−0.87, 0.61) | −0.37 (−1.06, 0.32) |
| LNnT | −0.64 (−1.31, 0.03) | −0.59 (−1.25, 0.06) | −0.03 (−0.58, 0.51) | −0.09 (−0.62, 0.44) | −0.66 (−1.36, 0.03) | −0.59 (−1.26, 0.07) |
| DSLNT | −0.53 (−1.24, 0.19) | −0.12 (−0.79, 0.56) | −0.27 (−0.83, 0.29) | −0.24 (−0.77, 0.29) | −0.55 (−1.27, 0.18) | −0.23 (−0.92, 0.46) |
| Total HMO | −0.21 (−0.92, 0.51) | 0.10 (−0.58, 0.77) | −0.06 (−0.61, 0.49) | −0.13 (−0.66, 0.39) | −0.26 (−1.00, 0.47) | 0.02 (−0.67, 0.72) |
| Exploratory Analyses | ||||||
| DFlac | −0.21 (−0.90, 0.47) | −0.51 (−1.19, 0.18) | 0.09 (−0.43, 0.60) | −0.44 (−0.96, 0.08) | −0.28 (−0.99, 0.42) | −0.51 (−1.22, 0.21) |
| LNT | −0.42 (−1.13, 0.29) | −0.41 (−1.09, 0.27) | −0.0001 (−0.56, 0.56) | −0.11 (−0.64, 0.43) | −0.63 (−1.34, 0.08) | −0.73 (−1.41, −0.06) * |
| LNFPI | −0.32 (−1.07, 0.44) | 0.20 (−0.46, 0.85) | −0.10 (−0.69, 0.50) | 0.18 (−0.33, 0.69) | −0.25 (−1.04, 0.53) | 0.16 (−0.53, 0.84) |
| LNFPII | −0.12 (−0.82, 0.58) | −0.15 (−0.86, 0.56) | 0.10 (−0.44, 0.64) | 0.25 (−0.30, 0.79) | −0.32 (−1.04, 0.40) | −0.47 (−1.18, 0.25) |
| LNFPIII | −0.06 (−0.80, 0.68) | −0.10 (−0.85, 0.64) | −0.17 (−0.74, 0.40) | −0.02 (−0.60, 0.55) | 0.03 (−0.74, 0.79) | −0.07 (−0.84, 0.70) |
| LSTb | −0.29 (−0.98, 0.40) | −0.54 (−1.21, 0.13) | −0.44 (−0.97, 0.08) | −0.47 (−0.98, 0.04) | −0.18 (−0.90, 0.53) | −0.57 (−1.26, 0.12) |
| LSTc | −0.41 (−1.07, 0.24) | −0.43 (−1.12, 0.26) | −0.15 (−0.66, 0.36) | 0.13 (−0.40, 0.67) | −0.45 (−1.13, 0.23) | −0.34 (−1.05, 0.36) |
| DFLNT | −0.33 (−1.03, 0.37) | 0.19 (−0.56, 0.95) | −0.11 (−0.64, 0.43) | −0.50 (−1.08, 0.08) | −0.40 (−1.10, 0.31) | 0.28 (−0.48, 1.05) |
| LNH | −0.27 (−0.98, 0.43) | −0.10 (−0.80, 0.59) | −0.06 (−0.61, 0.49) | 0.02 (−0.52, 0.56) | −0.44 (−1.16, 0.29) | −0.37 (−1.07, 0.34) |
| FLNH | −0.01 (−0.76, 0.74) | −0.28 (−0.95, 0.39) | 0.09 (−0.49, 0.67) | −0.23 (−0.74, 0.29) | −0.20 (−0.97, 0.57) | −0.39 (−1.06, 0.29) |
| DFLNH | 0.37 (−0.36, 1.11) | 0.12 (−0.52, 0.76) | 0.07 (−0.51, 0.64) | −0.03 (−0.53, 0.47) | 0.25 (−0.51, 1.02) | 0.02 (−0.64, 0.69) |
| FDSLNH | 0.04 (−0.64, 0.72) | 0.03 (−0.66, 0.71) | 0.28 (−0.24, 0.81) | 0.03 (−0.50, 0.56) | −0.25 (−0.96, 0.45) | −0.13 (−0.84, 0.58) |
| DSLNH | 0.11 (−0.62, 0.85) | −0.09 (−0.74, 0.56) | −0.31 (−0.88, 0.26) | −0.14 (−0.64, 0.35) | 0.07 (−0.69, 0.83) | −0.23 (−0.90, 0.43) |
| Diversity | −0.38 (−1.07, 0.31) | −0.20 (−0.87, 0.47) | −0.16 (−0.68, 0.36) | 0.27 (−0.23, 0.78) | −0.59 (−1.28, 0.11) | −0.50 (−1.18, 0.17) |
| HMO-bound sialic acid | −0.10 (−0.80, 0.60) | −0.29 (−0.93, 0.35) | 0.01 (−0.53, 0.55) | −0.27 (−0.76, 0.22) | −0.27 (−0.98, 0.45) | −0.47 (−1.11, 0.17) |
| HMO-bound fucose | −0.01 (−0.71, 0.69) | 0.13 (−0.57, 0.84) | 0.11 (−0.43, 0.65) | 0.04 (−0.51, 0.59) | 0.001 (−0.72, 0.72) | 0.11 (−0.62, 0.85) |
| Total sialylated | −0.08 (−0.77, 0.62) | −0.18 (−0.83, 0.48) | 0.02 (−0.52, 0.55) | −0.20 (−0.70, 0.30) | −0.27 (−0.98, 0.44) | −0.38 (−1.04, 0.27) |
| Total fucosylated | −0.03 (−0.73, 0.67) | 0.11 (−0.60, 0.81) | 0.12 (−0.42, 0.65) | 0.32 (−0.22, 0.86) | −0.12 (−0.84, 0.60) | −0.12 (−0.85, 0.60) |
| Outcome Z-Score | n | Beta Estimate (95% CI) |
|---|---|---|
| Weight | 82 | −0.14 (−0.64, 0.36) |
| Length | 73 | −0.06 (−0.46, 0.34) |
| Head Circumference | 74 | 0.13 (−0.31, 0.58) |
| Fat Mass | 65 | −0.14 (−0.81, 0.53) |
| Fat-Free Mass | 65 | −0.04 (−0.56, 0.48) |
| Body Fat Percentage | 65 | −0.11 (−0.81, 0.59) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong, M.L.; Cherkerzian, S.; Bell, K.A.; Berger, P.K.; Furst, A.; Sejane, K.; Bode, L.; Belfort, M.B. Human Milk Oligosaccharides, Growth, and Body Composition in Very Preterm Infants. Nutrients 2024, 16, 1200. https://doi.org/10.3390/nu16081200
Ong ML, Cherkerzian S, Bell KA, Berger PK, Furst A, Sejane K, Bode L, Belfort MB. Human Milk Oligosaccharides, Growth, and Body Composition in Very Preterm Infants. Nutrients. 2024; 16(8):1200. https://doi.org/10.3390/nu16081200
Chicago/Turabian StyleOng, Margaret L., Sara Cherkerzian, Katherine A. Bell, Paige K. Berger, Annalee Furst, Kristija Sejane, Lars Bode, and Mandy B. Belfort. 2024. "Human Milk Oligosaccharides, Growth, and Body Composition in Very Preterm Infants" Nutrients 16, no. 8: 1200. https://doi.org/10.3390/nu16081200
APA StyleOng, M. L., Cherkerzian, S., Bell, K. A., Berger, P. K., Furst, A., Sejane, K., Bode, L., & Belfort, M. B. (2024). Human Milk Oligosaccharides, Growth, and Body Composition in Very Preterm Infants. Nutrients, 16(8), 1200. https://doi.org/10.3390/nu16081200





