Consumption of Feed Supplemented with Oat Beta-Glucan as a Chemopreventive Agent against Colon Cancerogenesis in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Characterization of Low Molar Mass Oat Beta-Glucan
2.2. Animals and In Vivo Experimental Design
2.3. Blood and Tissues Sampling
2.4. Hematological Analysis and Blood Plasma Separation
2.5. Histopathological and Histomorphometry Evaluation
2.6. Blood Plasma Biochemical Parameters
2.7. Methods for RNA Isolation, Conversion to cDNA, and Gene Expression Analysis
2.8. Immunohistochemical Analysis
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
3.1. Chemical Characterization of Low Molar Mass Oat Beta-Glucan
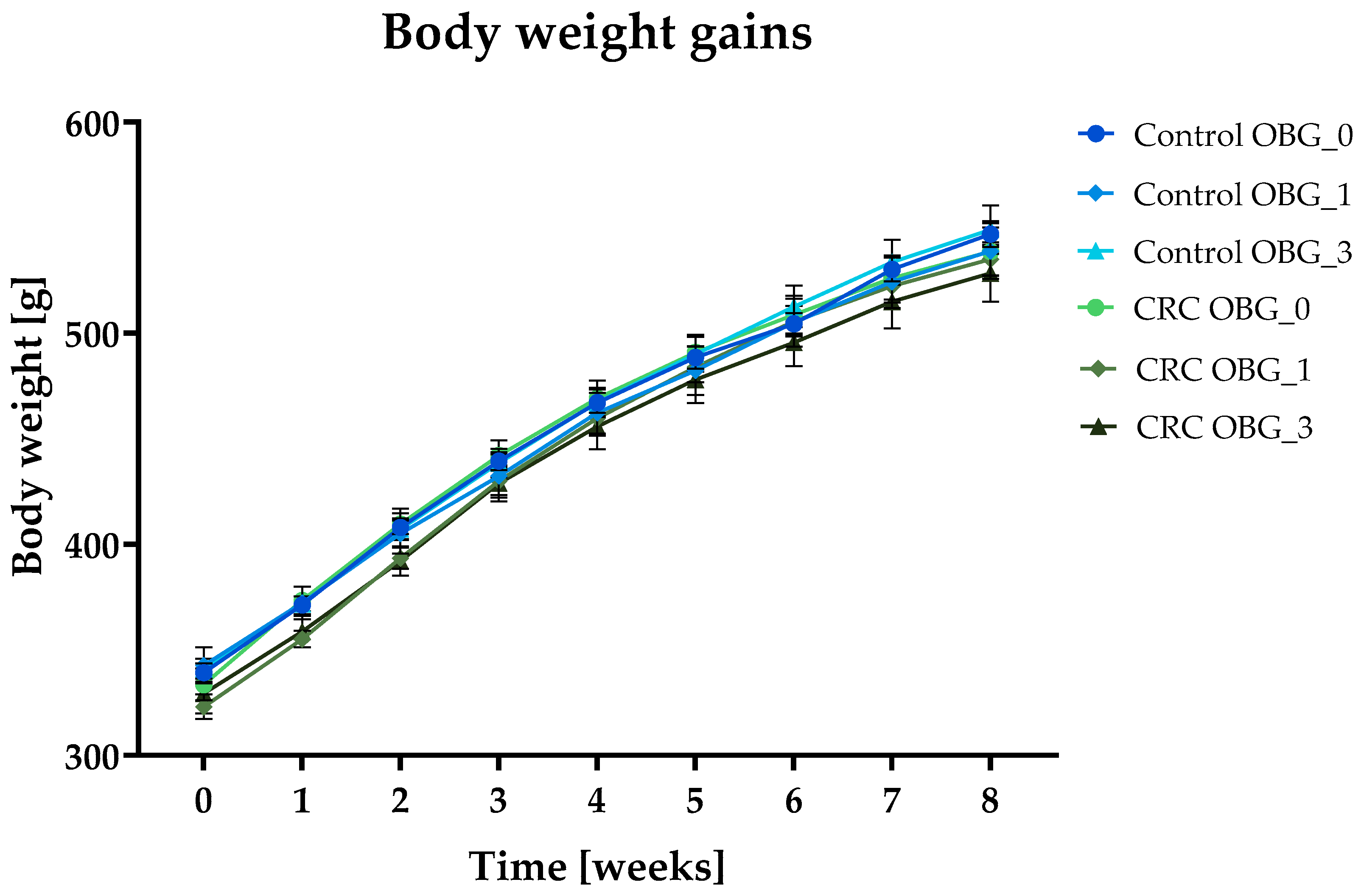
3.2. Feeds Nutritional Characteristic, Feed Intake, and Body Weight Gain
3.3. Clinical Signs, Necropsy Observations, and Macroscopic Large Intestine Evaluation
3.4. Histopathological Changes in the Large Intestine
3.5. Red Blood Cells and Platelet Parameters
3.6. White Blood Cells Parameters
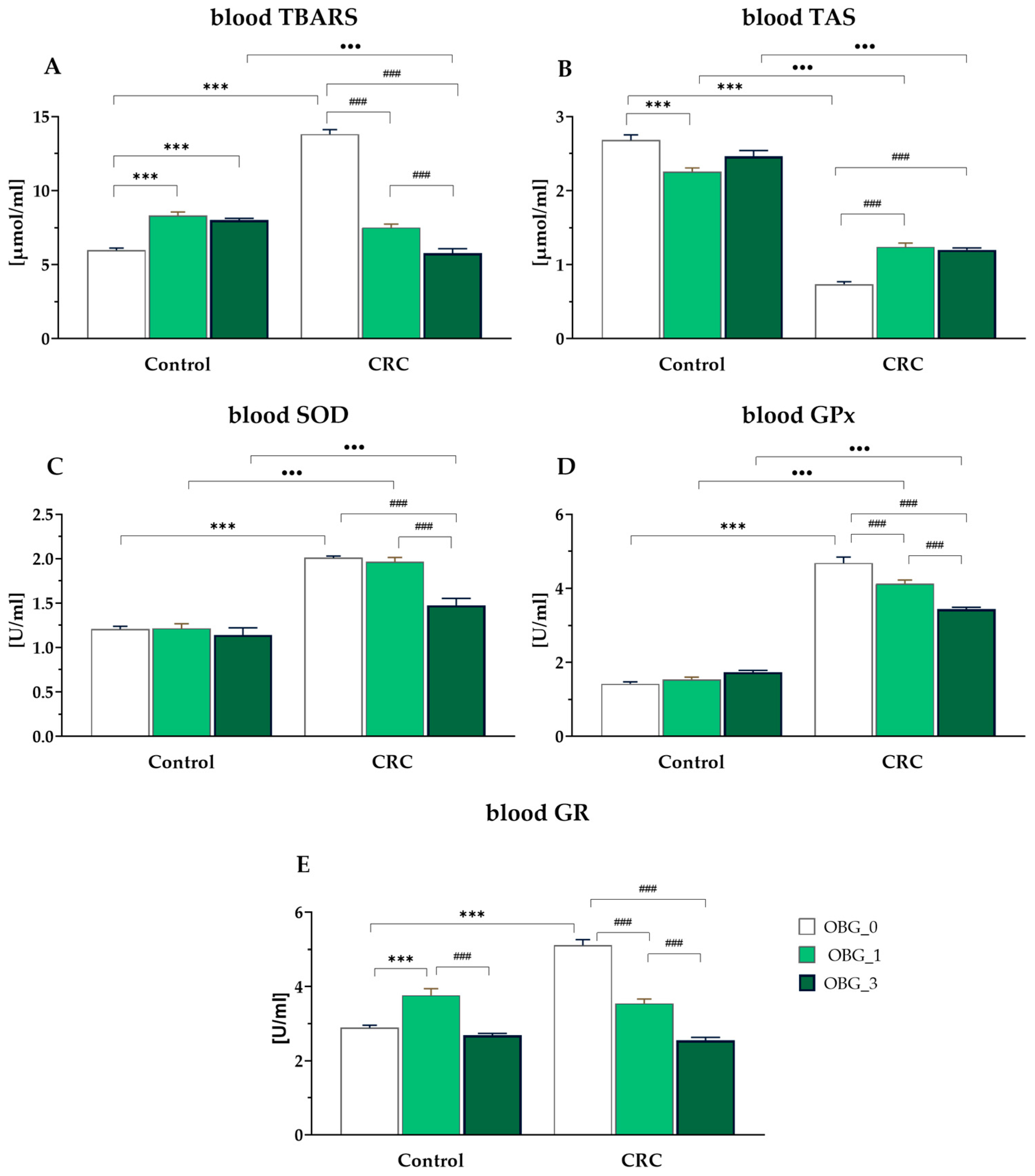
3.7. Peripheral Blood Plasma Redox Status Parameters
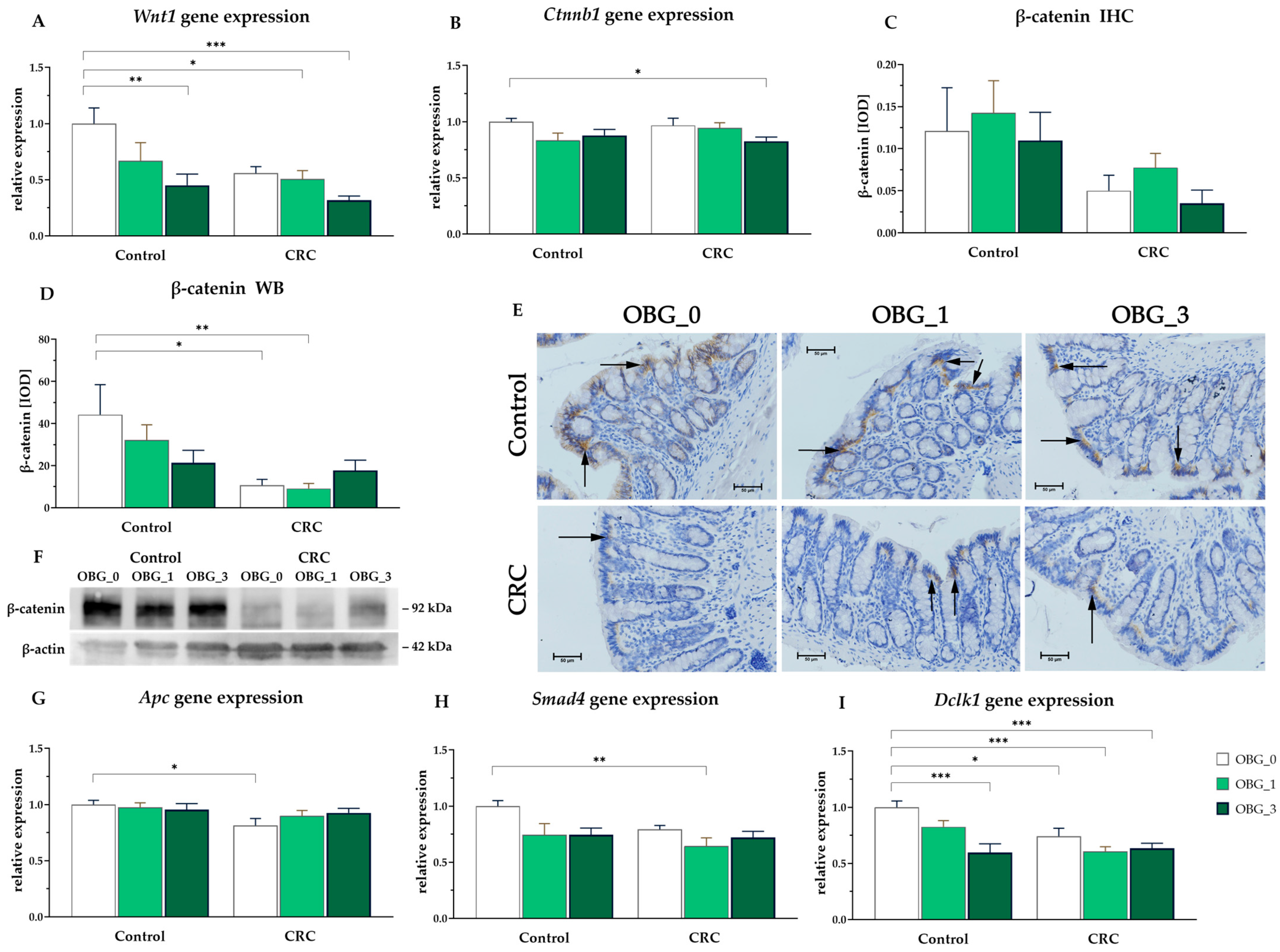
3.8. CRC Signaling Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakashima, A.; Yamada, K.; Iwata, O.; Sugimoto, R.; Atsuji, K.; Ogawa, T.; Ishibashi-Ohgo, N.; Suzuki, K. β-Glucan in foods and its physiological functions. J. Nutr. Sci. Vitaminol. (Tokyo) 2018, 64, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Wilczak, J.; Błaszczyk, K.; Kamola, D.; Gajewska, M.; Harasym, J.P.; Jałosińska, M.; Gudej, S.; Suchecka, D.; Oczkowski, M.; Gromadzka-Ostrowska, J. The effect of low or high molecular weight oat beta-glucans on the inflammatory and oxidative stress status in the colon of rats with LPS-induced enteritis. Food Funct. 2015, 6, 590–603. [Google Scholar] [CrossRef]
- Żyła, E.; Dziendzikowska, K.; Kamola, D.; Wilczak, J.; Sapierzyński, R.; Harasym, J.; Gromadzka-Ostrowska, J. Anti-inflammatory activity of oat beta-glucans in a crohn’s disease model: Time- and molar mass-dependent effects. Int. J. Mol. Sci. 2021, 22, 4485. [Google Scholar] [CrossRef]
- Choromańska, A.; Kulbacka, J.; Harasym, J.; Oledzki, R.; Szewczyk, A.; Saczko, J. High- and low-molecular weight oat beta-glucan reveals antitumor activity in human epithelial lung cancer. Pathol. Oncol. Res. 2017, 24, 583–592. [Google Scholar] [CrossRef]
- Choromańska, A.; Kulbacka, J.; Rembiałkowska, N.; Piłat, J.; Olędzki, R.; Harasym, J.; Saczko, J. Anticancer properties of low molecular weight oat beta-glucan—An in vitro study. Int. J. Biol. Macromol. 2015, 80, 23–28. [Google Scholar] [CrossRef]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef]
- Jaroszyńska, Z.; Wiśniewska, K. Epidemiology of colorectal cancer (C18-C21) in Poland. J. Educ. Health Sport 2021, 11, 143–156. [Google Scholar] [CrossRef]
- Shen, J.; Li, P.; Liu, S.; Liu, Q.; Li, Y.; Zhang, Z.; Yang, C.; Hu, M.; Sun, Y.; He, C.; et al. The chemopreventive effects of Huangqin-tea against AOM-induced preneoplastic colonic aberrant crypt foci in rats and omics analysis. Food Funct. 2020, 11, 9634–9650. [Google Scholar] [CrossRef] [PubMed]
- Sahebi, Z.; Emtyazjoo, M.; Mostafavi, P.G.; Bonakdar, S. Promising Chemoprevention of Colonic Aberrant Crypt Foci by Portunus segnis Muscle and Shell Extracts in Azoxymethane-Induced Colorectal Cancer in Rats. Anticancer Agents Med. Chem. 2020, 20, 2041–2052. [Google Scholar] [CrossRef]
- Clapper, M.L.; Chang, W.C.L.; Cooper, H.S. Dysplastic Aberrant Crypt Foci: Biomarkers of Early Colorectal Neoplasia and Response to Preventive Intervention. Cancer Prev. Res. 2020, 13, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Ezuka, A.; Sakai, E.; Kawana, K.; Nagase, H.; Kakuta, Y.; Uchiyama, S.; Ohkubo, H.; Higurashi, T.; Nonaka, T.; Endo, H.; et al. Association between factors associated with colorectal cancer and rectal aberrant crypt foci in humans. Oncol. Lett. 2015, 10, 3689–3695. [Google Scholar] [CrossRef] [PubMed]
- Kobaek-Larsen, M.; Nielsen, D.S.; Kot, W.; Krych, Ł.; Christensen, L.P.; Baatrup, G. Effect of the dietary polyacetylenes falcarinol and falcarindiol on the gut microbiota composition in a rat model of colorectal cancer. BMC Res. Notes 2018, 11, 411. [Google Scholar] [CrossRef] [PubMed]
- Madka, V.; Kumar, G.; Pathuri, G.; Zhang, Y.; Lightfoot, S.; Asch, A.S.; Mohammed, A.; Steele, V.E.; Rao, C.V. Bisphosphonates zometa and fosamax synergize with metformin to prevent AOM-induced colon cancer in f344 rat model. Cancer Prev. Res. 2020, 13, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Perše, M.; Cerar, A. Morphological and molecular alterations in 1,2 dimethylhydrazine and azoxymethane induced colon carcinogenesis in rats. J. Biomed. Biotechnol. 2011, 2011, 473964. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wei, H.; Zhou, Y.; Szeto, C.H.; Li, C.; Lin, Y.; Coker, O.O.; Lau, H.C.H.; Chan, A.W.H.; Sung, J.J.Y.; et al. High-Fat Diet Promotes Colorectal Tumorigenesis through Modulating Gut Microbiota and Metabolites. Gastroenterology 2022, 162, 135–149.e2. [Google Scholar] [CrossRef]
- Chen, J.; Huang, X.F. The signal pathways in azoxymethane-induced colon cancer and preventive implications. Cancer Biol. Ther. 2009, 8, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Im, S.A.; Kim, J.W.; Kim, H.S.; Park, C.S.; Shin, E.; Do, S.G.; Park, Y.I.; Lee, C.K. Prevention of azoxymethane/dextran sodium sulfate-induced mouse colon carcinogenesis by processed Aloe vera gel. Int. Immunopharmacol. 2016, 40, 428–435. [Google Scholar] [CrossRef]
- Saki, E.; Saiful Yazan, L.; Mohd Ali, R.; Ahmad, Z. Chemopreventive Effects of Germinated Rough Rice Crude Extract in Inhibiting Azoxymethane-Induced Aberrant Crypt Foci Formation in Sprague-Dawley Rats. Biomed Res. Int. 2017, 2017, 9517287. [Google Scholar] [CrossRef]
- Raju, J. Azoxymethane-induced rat aberrant crypt foci: Relevance in studying chemoprevention of colon cancer. World J. Gastroenterol. 2008, 14, 6632–6635. [Google Scholar] [CrossRef] [PubMed]
- Harasym, J.; Suchecka, D.; Gromadzka-Ostrowska, J. Effect of size reduction by freeze-milling on processing properties of beta-glucan oat bran. J. Cereal Sci. 2015, 61, 119–125. [Google Scholar] [CrossRef]
- Harasym, J.; Żyła, E.; Dziendzikowska, K.; Gromadzka-Ostrowska, J. Proteinaceous residue removal from oat β-glucan. Molecules 2019, 24, 1729. [Google Scholar] [CrossRef]
- Harasym, J.; Olędzki, R. The Mutual Correlation of Glucose, Starch, and Beta-Glucan Release During Microwave Heating and Antioxidant Activity of Oat Water Extracts. Food Bioprocess Technol. 2018, 11, 874–884. [Google Scholar] [CrossRef]
- Harasym, J.; Satta, E.; Kaim, U. Ultrasound treatment of buckwheat grains impacts important functional properties of resulting flour. Molecules 2020, 25, 3012. [Google Scholar] [CrossRef]
- Kopiasz, Ł.; Dziendzikowska, K.; Oczkowski, M.; Harasym, J.; Gromadzka-Ostrowska, J. Low-molar-mass oat beta-glucan impacts autophagy and apoptosis in early stages of induced colorectal carcinogenesis in rats. Int. J. Biol. Macromol. 2024, 254, 127832. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Dell, R.B.; Holleran, S.; Ramakrishnan, R. Sample size determination. ILAR J. 2002, 43, 207–212. [Google Scholar] [CrossRef]
- Aguilar Diaz De Leon, J.; Borges, C.R. Evaluation of Oxidative Stress in Biological Samples Using the Thiobarbituric Acid Reactive Substances Assay. JoVE 2020, 159, e61122. [Google Scholar]
- Nair, A.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Bielohuby, M.; Bodendorf, K.; Brandstetter, H.; Bidlingmaier, M.; Kienzle, E. Predicting metabolisable energy in commercial rat diets: Physiological fuel values may be misleading. Br. J. Nutr. 2010, 103, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Orlando, F.A.; Tan, D.; Baltodano, J.D.; Khoury, T.; Gibbs, J.F.; Hassid, V.J.; Ahmed, B.H.; Alrawi, S.J. Aberrant crypt foci as precursors in colorectal cancer progression. J. Surg. Oncol. 2008, 98, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, B.; Singh, R.P.; Tyagi, A.; Agarwal, R. Inhibition of azoxymethane-induced colonic aberrant crypt foci formation by silibinin in male Fisher 344 rats. Cancer Prev. Res. 2008, 1, 376–384. [Google Scholar] [CrossRef]
- Suzuki, R.; Kohno, H.; Sugie, S.; Tanaka, T. Sequential observations on the occurrence of preneoplastic and neoplastic lesions in mouse colon treated with azoxymethane and dextran sodium sulfate. Cancer Sci. 2004, 95, 721–727. [Google Scholar] [CrossRef]
- Raju, J.; Swamy, M.V.; Cooma, I.; Patlolla, J.M.R.; Pittman, B.; Reddy, B.S.; Steele, V.E.; Rao, C.V. Low doses of β-carotene and lutein inhibit AOM-induced rat colonic ACF formation but high doses augment ACF incidence. Int. J. Cancer 2005, 113, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, M.; Orłowski, M.; Klepacki, Ł.; Zinkiewicz, K.; Kurpiewski, W.; Kaczerska, D.; Pesta, W.; Zieliński, E.; Siermontowski, P. Rectal aberrant crypt foci (ACF) as a predictor of benign and malignant neoplastic lesions in the large intestine. BMC Cancer 2020, 20, 133. [Google Scholar] [CrossRef] [PubMed]
- Corpet, D.E.; Taché, S. Most Effective Colon Cancer Chemopreventive Agents in Rats: A Systematic Review of Aberrant Crypt Foci and Tumor Data, Ranked by Potency. Nutr. Cancer 2002, 43, 1–21. [Google Scholar] [CrossRef]
- Cheng, L.; Lai, M. De Aberrant crypt foci as microscopic precursors of colorectal cancer. World J. Gastroenterol. 2003, 9, 2642–2649. [Google Scholar] [CrossRef] [PubMed]
- Macharia, J.M.; Ngure, V.; Emődy, B.; Király, B.; Káposztás, Z.; Rozmann, N.; Erdélyi, A.; Raposa, B. Pharmacotherapeutic Potential of Aloe secundiflora against Colorectal Cancer Growth and Proliferation. Pharmaceutics 2023, 15, 1558. [Google Scholar] [CrossRef] [PubMed]
- Gigola, G.; Carriere, P.; Díaz, M.B.N.; Perdigon, G.; Zwenger, A.O.; Gentili, C. Survival effect of probiotics in a rat model of colorectal cancer treated with capecitabine. World J. Gastrointest. Oncol. 2021, 13, 1518–1531. [Google Scholar] [CrossRef]
- Bi, W.; Liu, H.; Shen, J.; Zhang, L.H.; Li, P.; Peng, B.; Cao, L.; Zhang, P.; He, C.; Xiao, P. Chemopreventive effects of Ku-jin tea against AOM-induced precancerous colorectal lesions in rats and metabolomic analysis. Sci. Rep. 2017, 7, 15893. [Google Scholar] [CrossRef]
- Wang, K.S.; Yu, G.; Xu, C.; Meng, X.H.; Zhou, J.; Zheng, C.; Deng, Z.; Shang, L.; Liu, R.; Su, S.; et al. Accurate diagnosis of colorectal cancer based on histopathology images using artificial intelligence. BMC Med. 2021, 19, 76. [Google Scholar] [CrossRef]
- Wu, R.; Feng, J.; Yang, Y.; Dai, C.; Lu, A.; Li, J.; Liao, Y.; Xiang, M.; Huang, Q.; Wang, D.; et al. Significance of Serum Total Oxidant/Antioxidant Status in Patients with Colorectal Cancer. PLoS ONE 2017, 12, e0170003. [Google Scholar] [CrossRef]
- Janion, K.; Szczepańska, E.; Nowakowska-zajdel, E.; Strzelczyk, J.; Copija, A. Selected Oxidative Stress Markers in Colorectal Cancer Patients in Relation to Primary Tumor Location—A Preliminary Research. Medicina 2020, 56, 47. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Li, B.; Xiang, L.; Xu, Z.; Tang, Y.; Zhu, Z.; Jiang, Y.; Peng, L.; He, H.; Wang, Y. Association between oxidative stress exposure and colorectal cancer risk in 98,395 participants: Results from a prospective study. Front. Nutr. 2023, 10, 1284066. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-León, D.; Gómez-Abril, S.Á.; Sanz-García, P.; Estañ-Capell, N.; Bañuls, C.; Sáez, G. The role of oxidative stress, tumor and inflammatory markers in colorectal cancer patients: A one-year follow-up study. Redox Biol. 2023, 62, 102662. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ding, K.; Wang, Y.; Yan, T.; Xu, Y.; Deng, Z.; Lin, W.; Zhang, L.; Zhu, W.; Zhao, R.; et al. Wumei Pill Ameliorates AOM/DSS-Induced Colitis-Associated Colon Cancer through Inhibition of Inflammation and Oxidative Stress by Regulating S-Adenosylhomocysteine Hydrolase- (AHCY-) Mediated Hedgehog Signaling in Mice. Oxid. Med. Cell. Longev. 2022, 2022, 4061713. [Google Scholar] [CrossRef]
- Waly, M.I.; Ali, A.; Guizani, N.; Al-Rawahi, A.S.; Farooq, S.A.; Rahman, M.S. Pomegranate (Punica granatum) peel extract efficacy as a dietary antioxidant against azoxymethane-induced colon cancer in rat. Asian Pac. J. Cancer Prev. 2012, 13, 4051–4055. [Google Scholar] [CrossRef]
- Bardelčíková, A.; Šoltys, J.; Mojžiš, J. Oxidative Stress, Inflammation and Colorectal Cancer: An Overview. Antioxidants 2023, 12, 901. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, X.; Zhang, C.; Zhu, H.; Xu, Q.; Bu, Y.; Lei, Y. Redox Imbalance in the Development of Colorectal Cancer. J. Cancer 2017, 8, 1586–1597. [Google Scholar] [CrossRef]
- Moradi-Marjaneh, R.; Hassanian, S.M.; Mehramiz, M.; Rezayi, M.; Ferns, G.A.; Khazaei, M.; Avan, A. Reactive oxygen species in colorectal cancer: The therapeutic impact and its potential roles in tumor progression via perturbation of cellular and physiological dysregulated pathways. J. Cell. Physiol. 2019, 234, 10072–10079. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Li, Y.; Zamyatnin, A.A.; Werner, J.; Bazhin, A.V. Reactive oxygen species and colorectal cancer. J. Cell. Physiol. 2018, 233, 5119–5132. [Google Scholar] [CrossRef]
- Basak, D.; Uddin, M.N.; Hancock, J. The Role of Oxidative Stress and Its Counteractive Utility in Colorectal Cancer (CRC). Cancers 2020, 12, 3336. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, K.; Mik, M.; Dziki, Ł.; Dziki, A.; Majsterek, I. Evaluation of antioxidant defense in patients with colorectal carcinoma. Pol. Prz. Chir. Pol. J. Surg. 2015, 87, 357–361. [Google Scholar] [CrossRef][Green Version]
- Kumar, V.L.; Verma, S.; Das, P. Artesunate suppresses inflammation and oxidative stress in a rat model of colorectal cancer. Drug Dev. Res. 2019, 80, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Liu, Y. Anti-inflammatory and Antioxidant Effect of Lycoperoside H against the 1,2-Dimethyl Hydrazine (DMH) Induced Colorectal Cancer in Rats. J. Oleo Sci. 2022, 71, 1021–1029. [Google Scholar]
- Kopiasz, Ł.; Dziendzikowska, K.; Gajewska, M.; Wilczak, J.; Harasym, J.; Żyła, E.; Kamola, D.; Oczkowski, M.; Królikowski, T.; Gromadzka-Ostrowska, J. Time-dependent indirect antioxidative effects of oat beta-glucans on peripheral blood parameters in the animal model of colon inflammation. Antioxidants 2020, 9, 375. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, M.; Shi, S.; Wang, H.; Li, N.; Su, J.; Liu, R.; Huang, Z.; Jin, H.; Ji, X.; et al. Hypoglycemic effect and mechanism of a pectic polysaccharide with hexenuronic acid from the fruits of Ficus pumila L. in C57BL/KsJ db/db mice. Carbohydr. Polym. 2017, 178, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Sala, R.J.; Ery, J.; Cuesta-Peredo, D.; Muedra, V.; Rodilla, V. Complete Blood Count Alterations Prior to the Diagnosis of Colorectal Cancer May Help in the Detection of Synchronous Liver Metastases. J. Clin. Med. 2023, 12, 6540. [Google Scholar] [CrossRef]
- Virdee, P.S.; Marian, I.R.; Mansouri, A.; Elhussein, L.; Kirtley, S.; Holt, T.; Birks, J. The Full Blood Count Blood Test for Colorectal Cancer Detection: A Systematic Review, Meta-Analysis, and Critical Appraisal. Cancers 2020, 12, 2348. [Google Scholar] [CrossRef]
- May, J.E.; Marques, M.B.; Reddy, V.V.B.; Gangaraju, R. Three neglected numbers in the CBC: The RDW, MPV, and NRBC count. Cleve Clin. J. Med. 2019, 86, 167–172. [Google Scholar] [CrossRef]
- Goyal, H.; Lippi, G.; Gjymishka, A.; John, B.; Chhabra, R.; May, E. Prognostic significance of red blood cell distribution width in gastrointestinal disorders. World J. Gastroenterol. 2017, 23, 4879–4891. [Google Scholar] [CrossRef]
- Wen, Z.L.; Zhou, X.; Xiao, D.C. Is red blood cell distribution width a prognostic factor for colorectal cancer? A meta-analysis. Front. Surg. 2022, 9, 945126. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, T.J.; Biederstädt, A.; Rezvani, K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat. Rev. Cancer 2022, 22, 557–575. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Xu, X.; Chen, D.; Zhao, F.; Wang, W. Therapeutic potential of targeting the Wnt/β-catenin signaling pathway in colorectal cancer. Biomed. Pharmacother. 2019, 110, 473–481. [Google Scholar] [CrossRef]
- Bian, J.; Dannappel, M.; Wan, C.; Firestein, R. Transcriptional Regulation of Wnt/β-Catenin Pathway in Colorectal Cancer. Cells 2020, 9, 2125. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, E.; Razmi, M.; Tajik, F.; Asadi-Lari, M.; Ghods, R.; Madjd, Z. Oncogenic functions and clinical significances of DCLK1 isoforms in colorectal cancer: A systematic review and meta-analysis. Cancer Cell Int. 2022, 22, 217. [Google Scholar] [CrossRef] [PubMed]


| Control Groups (n = 21) | CRC Groups (n = 24) | |||||
|---|---|---|---|---|---|---|
| OBG_0 | OBG_1 | OBG_3 | OBG_0 | OBG_1 | OBG_3 | |
| Feed | Without OBG | +1% OBG | +3% OBG | Without OBG | +1% OBG | +3% OBG |
| OBG | Molar Mass [g/mol] | Purity [%] | TPC [GAE/ 1 g s.m.] | DPPH [μmol TE/1 g s.m.] | Protein (%/db) | Nitrogen × 5.83 (%/s.m.) |
|---|---|---|---|---|---|---|
| Low molar mass | 5.2 × 104 ± 0.6 × 104 | 99.3 ± 0.23 | 0.17 ± 0.040 | 5.94 ± 0.21 | 0.12 ± 0.04 | 1.52 ± 0.18 |
| Control Groups | CRC Groups | |||||
|---|---|---|---|---|---|---|
| OBG_0 | OBG_1 | OBG_3 | OBG_0 | OBG_1 | OBG_3 | |
| Feed intake [g/day] | 25.84 ± 0.05 | 25.29 ± 0.44 | 25.48 ± 0.15 | 25.24 ± 0.33 | 24.98 ± 0.17 | 25.23 ± 0.33 |
| Total body weight gain [g] | 207.6 ± 3.57 | 196.1 ± 7.5 | 207.9 ± 10.58 | 205.1 ± 8.42 | 212.0 ± 5.82 | 199.1 ± 6.65 |
| Feed growth efficiency [g/MJ] (mg/kcal) | 9.006 ± 0.16 (38.65 ± 0.7) | 8.842 ± 0.23 (37.68 ± 1.0) | 9.644 ± 0.47 (40.53 ± 2.0) | 9.107 ± 0.34 (39.09 ± 1.5) | 9.691 ± 0.24 (41.29 ± 1.0) | 9.342 ± 0.33 (39.26 ± 1.4) |
| Control Groups | CRC Groups | χ2 | p-Value/ V | |||||
|---|---|---|---|---|---|---|---|---|
| OBG_0 (n = 7) | OBG_1 (n = 7) | OBG_3 (n = 7) | OBG_0 (n = 8) | OBG_1 (n = 8) | OBG_3 (n = 8) | |||
| colon epithelium changes | NC = 7 (100%) | NC = 7 (100%) | NC = 6 (86%) HP = 1 (14%) | NC = 3 (37.5%) HP = 5 (62.5%) | NC = 6 (75%) HP = 2 (25%) | NC = 7 (87.5%) HP = 1 (12.5%) | 13.08 | 0.023/ 0.54 |
| Intestinal Crypts Parameters | Control Groups | CRC Groups | ||||
|---|---|---|---|---|---|---|
| OBG_0 | OBG_1 | OBG_3 | OBG_0 | OBG_1 | OBG_3 | |
| Crypt length [μm] | 196.8 ± 10.23 | 196.4 ± 9.96 | 214.2 ± 9.10 | 220.5 ± 6.28 | 207.5 ± 8.20 | 223.5 ± 9.45 |
| Crypt width bottom [μm] | 33.41 ± 0.33 | 32.63 ± 0.52 | 32.88 ± 0.62 | 36.17 ± 1.33 | 34.21 ± 1.39 | 35.82 ± 0.69 |
| Crypt width middle part [μm] | 34.76 ± 1.28 | 34.37 ± 0.68 | 35.58 ± 0.87 | 34.02 ± 1.36 | 33.15 ± 0.97 | 34.88 ± 1.36 |
| Crypt width upper part [μm] | 37.39 ± 1.05 | 38.96 ± 1.32 | 39.96 ± 0.73 | 40.60 ± 1.69 | 38.51 ± 0.90 | 40.65 ± 1.09 |
| Feature | Control Groups | CRC Groups | ||||
|---|---|---|---|---|---|---|
| OBG_0 | OBG_1 | OBG_3 | OBG_0 | OBG_1 | OBG_3 | |
| RBC [1012/L] | 8.18 ± 0.29 | 8.01 ± 0.19 | 7.85 ± 0.28 | 8.07 ± 0.15 | 7.99 ± 0.17 | 8.09 ± 0.17 |
| HBG [g/dL] | 14.42 ± 0.26 | 14.23 ± 0.23 | 13.94 ± 0.42 | 14.45 ± 0.17 | 14.24 ± 0.32 | 14.50 ± 0.15 |
| HCT [%] | 42.52 ± 0.6 | 41.24 ± 0.56 | 41.03 ± 0.92 | 40.92 ± 0.84 | 39.92 ± 0.8 | 41.42 ± 0.59 |
| MCV [fl] | 53.00 ± 0.68 | 51.71 ± 0.87 | 51.83 ± 0.95 | 51.25 ± 0.67 | 50.00 ± 0.46 | 51.25 ± 0.80 |
| MCH [pg] | 17.64 ± 0.3 | 17.80 ± 0.25 | 17.77 ± 0.18 | 17.93 ± 0.21 | 17.80 ± 0.19 | 17.98 ± 0.28 |
| MCHC [g/dL] | 33.91 ± 0.2 | 34.49 ± 0.29 | 33.97 ± 0.43 A | 34.96 ± 0.24 * | 35.31 ± 0.19 ** | 35.30 ± 0.22 **, A |
| RDWc [%] | 17.59 ± 0.23 | 18.29 ± 0.24 | 17.98 ± 0.14 | 17.70 ± 0.21 | 18.04 ± 0.18 | 18.43 ± 0.19 * |
| PLT [109/L] | 718.7 ± 38.3 | 747.3 ± 32.2 | 788.9 ± 25.2 | 710.6 ± 22.7 | 781.4 ± 45.5 | 759.0 ± 36.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harasym, J.; Dziendzikowska, K.; Kopiasz, Ł.; Wilczak, J.; Sapierzyński, R.; Gromadzka-Ostrowska, J. Consumption of Feed Supplemented with Oat Beta-Glucan as a Chemopreventive Agent against Colon Cancerogenesis in Rats. Nutrients 2024, 16, 1125. https://doi.org/10.3390/nu16081125
Harasym J, Dziendzikowska K, Kopiasz Ł, Wilczak J, Sapierzyński R, Gromadzka-Ostrowska J. Consumption of Feed Supplemented with Oat Beta-Glucan as a Chemopreventive Agent against Colon Cancerogenesis in Rats. Nutrients. 2024; 16(8):1125. https://doi.org/10.3390/nu16081125
Chicago/Turabian StyleHarasym, Joanna, Katarzyna Dziendzikowska, Łukasz Kopiasz, Jacek Wilczak, Rafał Sapierzyński, and Joanna Gromadzka-Ostrowska. 2024. "Consumption of Feed Supplemented with Oat Beta-Glucan as a Chemopreventive Agent against Colon Cancerogenesis in Rats" Nutrients 16, no. 8: 1125. https://doi.org/10.3390/nu16081125
APA StyleHarasym, J., Dziendzikowska, K., Kopiasz, Ł., Wilczak, J., Sapierzyński, R., & Gromadzka-Ostrowska, J. (2024). Consumption of Feed Supplemented with Oat Beta-Glucan as a Chemopreventive Agent against Colon Cancerogenesis in Rats. Nutrients, 16(8), 1125. https://doi.org/10.3390/nu16081125







