Measures for Identifying Malnutrition in Geriatric Rehabilitation: A Scoping Review
Abstract
1. Introduction
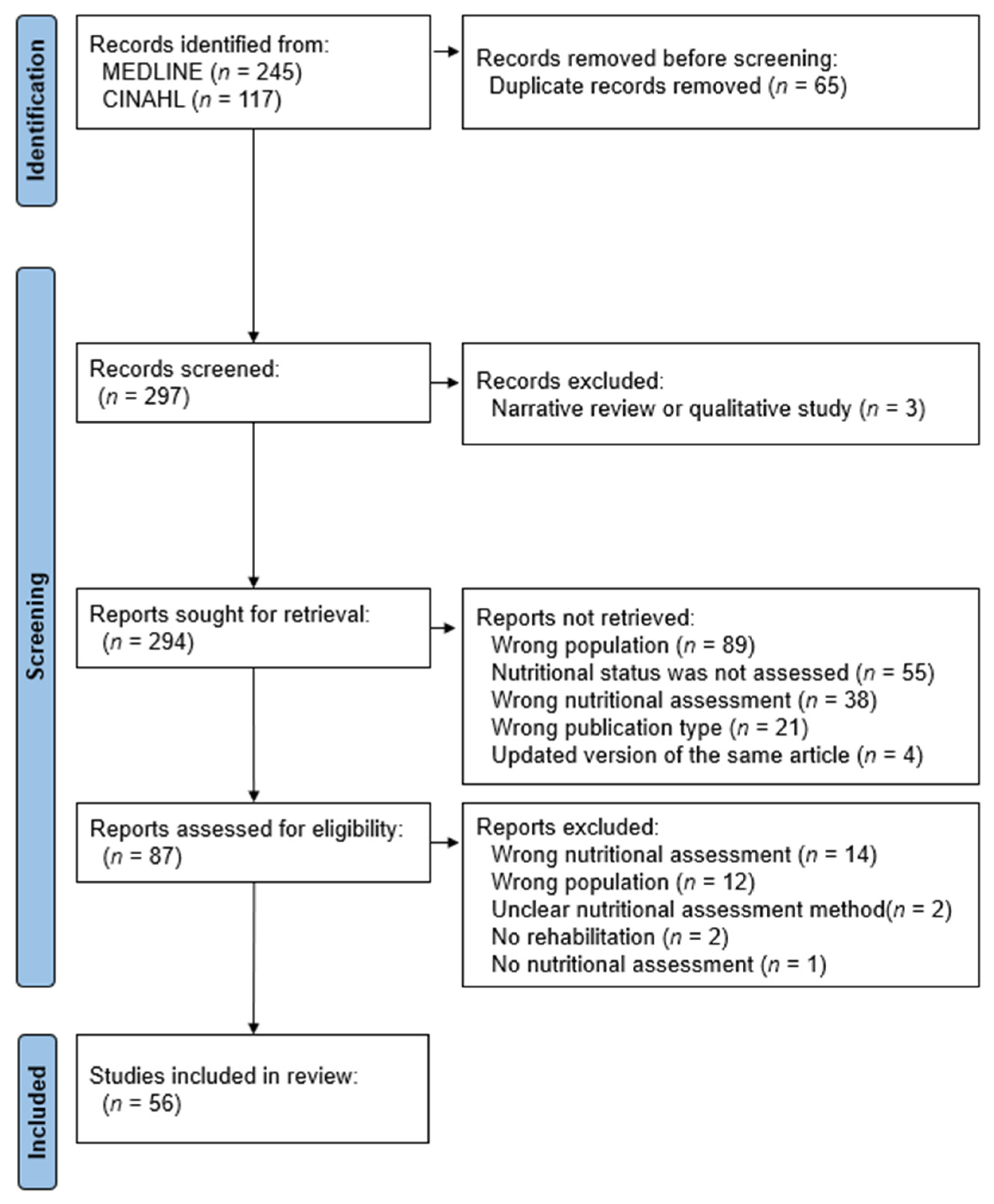
2. Materials and Methods
- What are the characteristics of existing measures for identifying malnutrition in geriatric rehabilitation patients?
- What are the accuracy and predictive validities of these methods?
3. Results
3.1. Measures for Identifying Malnutrition Used in the Literature
3.2. Prevalence of Malnutrition
3.3. Accuracy
3.4. Predictive Validity
4. Discussion
4.1. Components of the Measures for Identifying Malnutrition
4.2. Accuracy and Predictive Validity
4.3. Usability and Considerations of Nutritional Assessment Tools in Geriatric Rehabilitation
4.4. Application of the Global Leadership Initiative on Malnutrition Criteria in Geriatric Rehabilitation
4.5. Potential Biases in the Review Process
4.6. Agreements and Disagreements with Other Studies or Reviews
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Cederholm, T.; Jensen, G.L.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.; Wright, O.R.L.; Woo, J.; Hoogendijk, E.O. Malnutrition in older adults. Lancet 2023, 10380, 951–966. [Google Scholar] [CrossRef] [PubMed]
- Wolters, M.; Volkert, D.; Streicher, M.; Kiesswetter, E.; Torbahn, G.; O’Connor, E.M.; O’Keeffe, M.; Kelly, M.; O’Herlihy, E.; O’Toole, P.W.; et al. Prevalence of malnutrition using harmonised definitions in older adults from different settings—A MaNuEL study. Clin. Nutr. 2019, 38, 2389–2398. [Google Scholar] [CrossRef] [PubMed]
- Cereda, E.; Pedrolli, C.; Klersy, C.; Klersy, C.; Bonardi, C.; Quarleri, L.; Cappello, S.; Turri, A.; Rondanelli, M.; Caccialanza, R. Nutritional status in older persons according to healthcare setting: A systematic review and meta-analysis of prevalence data using MNA®. Clin. Nutr. 2016, 35, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Wojzischke, J.; van Wijngaarden, J.; van den Berg, C.; Diekmann, R.; Luiking, Y.; Bauer, J. Nutritional status and functionality in geriatric rehabilitation patients: A systematic review and meta-analysis. Eur. Geriatr. Med. 2020, 11, 195–207. [Google Scholar] [CrossRef]
- Hettiarachchi, J.; Reijnierse, E.M.; Soh, C.H.; Agius, B.; Fetterplace, K.; Lim, W.K.; Maier, A.B. Malnutrition is associated with poor trajectories of activities of daily living in geriatric rehabilitation inpatients: RESORT. Mech. Ageing Dev. 2021, 197, 111500. [Google Scholar] [CrossRef]
- Nishioka, S.; Omagari, K.; Nishioka, E.; Mori, N.; Taketani, Y.; Kayashita, J. Concurrent and predictive validity of the Mini Nutritional Assessment Short-Form and the Geriatric Nutritional Risk Index in older stroke rehabilitation patients. J. Hum. Nutr. Diet. 2020, 33, 12–22. [Google Scholar] [CrossRef]
- Nishioka, S.; Wakabayashi, H.; Kayashita, J.; Taketani, Y.; Momosaki, R. Predictive validity of the Mini Nutritional Assessment Short-Form for rehabilitation patients: A retrospective analysis of the Japan Rehabilitation Nutrition Database. J. Hum. Nutr. Diet. 2021, 34, 881–889. [Google Scholar] [CrossRef]
- Nishioka, S.; Wakabayashi, H. Interaction between malnutrition and physical disability in older adults: Is there a malnutrition-disability cycle? Nutr. Rev. 2023, 81, 191–205. [Google Scholar] [CrossRef]
- Guigoz, Y. The Mini Nutritional Assessment (MNA) review of the literature—What does it tell us? J. Nutr. Health Aging 2006, 10, 466–485; discussion 85–87. [Google Scholar]
- Detsky, A.S.; McLaughlin, J.R.; Baker, J.P.; Johnston, N.; Whittaker, S.; Mendelson, R.A.; Jeejeebhoy, K.N. What is subjective global assessment of nutritional status? JPEN J. Parenter. Enteral Nutr. 1987, 11, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Ottery, F.D. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition 1996, 12, S15–S19. [Google Scholar] [CrossRef]
- Marshall, S.; Young, A.; Bauer, J.; Isenring, E. Malnutrition in geriatric rehabilitation: Prevalence, patient outcomes, and criterion validity of the scored Patient-Generated Subjective Global Assessment and the Mini Nutritional Assessment. J. Acad. Nutr. Diet 2016, 116, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- JBI Manual for Evidence Synthesis. Available online: https://synthesismanual.jbi.global (accessed on 25 February 2023).
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Moloney, L.; Jarrett, B. Nutrition assessment and interventions for the prevention and treatment of malnutrition in older adults: An evidence analysis center scoping review. J. Acad. Nutr. Diet. 2021, 121, 2108–2140.e6. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Power, L.; de van der Schueren, M.A.E.; Leij-Halfwerk, S.; Bauer, J.; Clarke, M.; Visser, M.; Volkert, D.; Bardon, L.; Gibney, E.; Corish, C.A.; et al. Development and application of a scoring system to rate malnutrition screening tools used in older adults in community and healthcare settings—A MaNuEL study. Clin. Nutr. 2019, 38, 1807–1819. [Google Scholar] [CrossRef]
- Andersson, P.; Westergren, A.; Karlsson, S.; Hallberg, I.R.; Renver, S. Oral health and nutritional status in a group of geriatric rehabilitation patients. Scand. J. Caring Sci. 2002, 16, 311–318. [Google Scholar] [CrossRef]
- Avenell, A.; Smith, T.O.; Curtain, J.P.; Mak, J.C.; Myint, P.K. Nutritional supplementation for hip fracture aftercare in older people. Cochrane Database Syst Rev. 2016, 11, CD001880. [Google Scholar] [CrossRef] [PubMed]
- Babineau, J.; Villalon, L.; Laporte, M.; Payette, H. Outcomes of screening and nutritional intervention among older adults in healthcare facilities. Can. J. Diet. Pract. Res. 2008, 69, 89–94. [Google Scholar] [CrossRef]
- Bellelli, G.; Bernardini, B.; Pievani, M.; Frisoni, G.B.; Guaita, A.; Trabucchi, M. A score to predict the development of adverse clinical events after transition from acute hospital wards to post-acute care settings. Rejuvenation Res. 2012, 15, 553–563. [Google Scholar] [CrossRef]
- Bouillanne, O.; Curis, E.; Hamon-Vilcot, B.; Nicolis, I.; Chrétien, P.; Schauer, N.; Vincent, J.-P.; Cynober, L.; Ausselet, C. Impact of protein pulse feeding on lean mass in malnourished and at-risk hospitalized elderly patients: A randomized controlled trial. Clin. Nutr. 2013, 32, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Bouillanne, O.; Neveux, N.; Nicolis, I.; Curis, E.; Cynober, L.; Aussel, C. Long-lasting improved amino acid bioavailability associated with protein pulse feeding in hospitalized elderly patients: A randomized controlled trial. Nutrition 2014, 30, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Charlton, K.E.; Nichols, C.; Bowden, S.; Lambert, K.; Barone, L.; Mason, M.; Milosavljevic, M. Older rehabilitation patients are at high risk of malnutrition: Evidence from a large Australian database. J. Nutr. Health Aging 2010, 14, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.B.; Reijnierse, E.M.; Lim, W.K.; Maier, A.B. Prevalence of malnutrition comparing the GLIM criteria, ESPEN definition and MST malnutrition risk in geriatric rehabilitation patients: RESORT. Clin. Nutr. 2020, 39, 3504–3511. [Google Scholar] [CrossRef]
- Duerksen, D.R.; Yeo, T.A.; Siemens, J.L.; O’Connor, M.P. The validity and reproducibility of clinical assessment of nutritional status in the elderly. Nutrition 2000, 16, 740–744. [Google Scholar] [CrossRef]
- Frangos, E.; Trombetti, A.; Graf, C.; Lachat, V.; Samaras, N.; Vischer, U.M.; Zekry, D.; Rizzoli, R.; Herrmann, F.R. Malnutrition in very old hospitalized patients: A new etiologic factor of anemia? J. Nutr. Health Aging 2016, 20, 705–713. [Google Scholar] [CrossRef]
- Groenendijk, I.; Kramer, C.S.; den Boeft, L.M.; Hobbelen, H.S.M.; van der Putten, G.J.; de Groot, L. Hip fracture patients in geriatric rehabilitation show poor nutritional status, dietary intake and muscle health. Nutrients 2020, 12, 2528. [Google Scholar] [CrossRef]
- Lelli, D.; Calle, A.; Perez, L.M.; Onder, G.; Morandi, A.; Ortolani, E.; Colominas, M.; Pedone, C.; Inzitari, M. Nutritional status and functional outcomes in older adults admitted to geriatric rehabilitations: The SAFARI Study. J. Am. Coll. Nutr. 2019, 38, 441–446. [Google Scholar] [CrossRef]
- Maeda, K.; Koga, T.; Akagi, J. Nutritional variables predict chances of returning home and activities of daily living in post-acute geriatric care. Clin. Interv. Aging 2018, 13, 151–157. [Google Scholar] [CrossRef]
- Malafarina, V.; Malafarina, C.; Ugarte, A.B.; Martinez, J.A.; Goni, I.A.; Zulet, M.A. Factors associated with sarcopenia and 7-year mortality in very old patients with hip fracture admitted to rehabilitation units: A pragmatic study. Nutrients 2019, 11, 2243. [Google Scholar] [CrossRef]
- Marshall, S.; Young, A.; Bauer, J.; Isenring, E. Nutrition screening in geriatric rehabilitation: Criterion (concurrent and predictive) validity of the malnutrition screening tool and the Mini Nutritional Assessment-Short Form. J. Acad. Nutr. Diet. 2016, 116, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, S.; Wakabayashi, H.; Nishioka, E.; Yoshida, T.; Mori, N.; Watanabe, R. Nutritional improvement correlates with recovery of activities of daily living among malnourished elderly stroke patients in the convalescent stage: A cross-sectional study. J. Acad. Nutr. Diet. 2016, 116, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, S.; Wakabayashi, H.; Yoshida, T.; Mori, N.; Watanabe, R.; Nishioka, E. Obese Japanese patients with stroke have higher functional recovery in convalescent rehabilitation wards: A retrospective cohort study. J. Stroke Cerebrovasc. Dis. 2016, 25, 26–33. [Google Scholar] [CrossRef]
- Nishioka, S.; Wakabayashi, H.; Momosaki, R. Nutritional status changes and activities of daily living after hip fracture in convalescent rehabilitation units: A retrospective observational cohort study from the Japan Rehabilitation Nutrition Database. J. Acad. Nutr. Diet. 2018, 118, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, S.; Yamasaki, K.; Ogawa, K.; Oishi, K.; Yano, Y.; Okazaki, Y.; Nakashima, R.; Kurihara, M. Impact of nutritional status, muscle mass and oral status on recovery of full oral intake among stroke patients receiving enteral nutrition: A retrospective cohort study. Nutr. Diet. 2020, 77, 456–466. [Google Scholar] [CrossRef]
- Nishioka, S.; Matsushita, T.; Yamanouchi, A.; Okazaki, Y.; Oishi, K.; Nishioka, E.; Mori, N.; Tokunaga, S.; Onizuka, S. Prevalence and associated factors of coexistence of malnutrition and sarcopenia in geriatric rehabilitation. Nutrients 2021, 13, 3745. [Google Scholar] [CrossRef]
- Nomoto, A.; Shimizu, A.; Ohno, T.; Tohara, H.; Hashidume, M.; Hatano, M.; Fujishima, I. Poor oral health and anorexia in older rehabilitation patients. Gerodontology 2022, 39, 59–66. [Google Scholar] [CrossRef]
- O’Leary, F.; Flood, V.M.; Petocz, P.; Allman-Farinel, M.; Samman, S. B vitamin status, dietary intake and length of stay in a sample of elderly rehabilitation patients. J. Nutr. Health Aging 2011, 15, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Onaga, A.; Oshiro, N.; Oshiro, A.; Kitagawa, Y.; Taira, Y.; Nakahodo, S.; Oshiro, K. Older patients with less skeletal muscle mass gain more skeletal muscle in rehabilitation wards after fractures. Eur. Geriatr. Med. 2022, 13, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Rojer, A.G.M.; Denneman, B.C.M.; Brouwer, P.; Ramsey, K.A.; Trappenburg, M.C.; Meskers, C.G.M.; Pijnappels, M.; Goonan, R.; Marston, C.; Kay, J.E.; et al. Determinants of instrumented sedentary and physical activity behavior in geriatric rehabilitation inpatients: RESORT. Exp. Gerontol. 2021, 154, 111524. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Nakayama, E.; Tohara, H.; Maeda, T.; Sugimoto, M.; Takehisa, T.; Takehisa, Y.; Ueda, K. Tongue strength is associated with grip strength and nutritional status in older adult inpatients of a rehabilitation hospital. Dysphagia 2017, 32, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rodriguez, D.; Marco, E.; Ronquillo-Moreno, N.; Miralles, R.; Vázquez-Ibar, O.; Escalada, F.; Muniesa, J.M. Prevalence of malnutrition and sarcopenia in a post-acute care geriatric unit: Applying the new ESPEN definition and EWGSOP criteria. Clin. Nutr. 2017, 36, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Savina, C.; Donini, L.M.; Anzivino, R.; De Felice, M.R.; De Bernardini, L.; Cannella, C. Administering the “AHSP Questionnaire” (appetite, hunger, sensory perception) in a geriatric rehabilitation care. J. Nutr. Health Aging 2003, 7, 385–389. [Google Scholar]
- Shimazu, S.; Yoshimura, Y.; Kudo, M.; Nagano, F.; Bise, T.; Shiraishi, A.; Sunahara, T. Frequent and personalised nutritional support leads to improved nutritional status, activities of daily living, and dysphagia after stroke. Nutrition 2021, 83, 111091. [Google Scholar] [CrossRef]
- Shimizu, A.; Maeda, K.; Tanaka, K.; Ogawa, M.; Kayashita, J. Texture-modified diets are associated with decreased muscle mass in older adults admitted to a rehabilitation ward. Geriatr. Gerontol. Int. 2018, 18, 698–704. [Google Scholar] [CrossRef]
- Shimizu, A.; Maeda, K.; Koyanagi, Y.; Kayashita, J.; Fujishima, I.; Mori, N. The Global Leadership Initiative on Malnutrition-defined malnutrition predicts prognosis in persons with stroke-related dysphagia. J. Am. Med. Dir. Assoc. 2019, 20, 1628–1633. [Google Scholar] [CrossRef]
- Shimizu, A.; Maeda, K.; Honda, T.; Ishida, Y.; Ueshima, J.; Nagami, S.; Nagano, A.; Inoue, T.; Murotani, K.; Kayashita, J.; et al. Comparison between the Global Leadership Initiative on Malnutrition and the European Society for Clinical Nutrition and Metabolism definitions for the prevalence of malnutrition in geriatric rehabilitation care. Geriatr. Gerontol. Int. 2020, 20, 1221–1227. [Google Scholar] [CrossRef]
- Shimizu, A.; Maeda, K.; Wakabayashi, H.; Nishioka, S.; Ohno, T.; Nomoto, A.; Kayashita, J.; Fujishima, I. Sarcopenic dysphagia with low tongue pressure is associated with worsening of swallowing, nutritional status, and activities of daily living. J. Nutr. Health Aging 2021, 25, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A.; Fujishima, I.; Maeda, K.; Murotani, K.; Kayashita, J.; Ohno, T.; Nomoto, A.; Ueshima, J.; Ishida, Y.; Inoue, T. Texture-modified diets are associated with poor appetite in older adults who are admitted to a post-acute rehabilitation hospital. J. Am. Med. Dir. Assoc. 2021, 22, 1960–1965. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A.; Fujishima, I.; Maeda, K.; Wakabayashi, H.; Nishioka, S.; Ohno, T.; Nomoto, A.; Shigematsu, T.; Kayashita, J.; Japanese Working Group on Sarcopenic Dysphagia. Effect of low tongue pressure on nutritional status and improvement of swallowing function in sarcopenic dysphagia. Nutrition 2021, 90, 111295. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A.; Fujishima, I.; Maeda, K.; Murotani, K.; Inoue, T.; Ohno, T.; Nomoto, A.; Ueshima, J.; Ishida, Y.; Nagano, A.; et al. Accuracy of the Simplified Nutritional Appetite Questionnaire for malnutrition and sarcopenia screening among older patients requiring rehabilitation. Nutrients 2021, 13, 2738. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, A.; Yoshimura, Y.; Wakabayashi, H.; Tsuji, Y. Poor oral status is associated with rehabilitation outcome in older people. Geriatr. Gerontol. Int. 2017, 17, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Soderhamn, U.; Soderhamn, O. Reliability and validity of the nutritional form for the elderly (NUFFE). J. Adv. Nurs. 2002, 37, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Tamamura, Y.; Matsuura, M.; Shiba, S.; Nishikimi, T. Effect of heart failure and malnutrition, alone and in combination, on rehabilitation effectiveness in patients with hip fracture. Clin. Nutr. ESPEN 2021, 44, 356–366. [Google Scholar] [CrossRef]
- Tanaka, M.; Momosaki, R.; Wakabayashi, H.; Kikura, T.; Maeda, K. Relationship between nutritional status and improved ADL in individuals with cervical spinal cord injury in a convalescent rehabilitation ward. Spinal Cord 2019, 57, 501–508. [Google Scholar] [CrossRef]
- Than, S.; Crabtree, A.; Moran, C. Examination of risk scores to better predict hospital-related harms. Intern. Med. J. 2019, 49, 1125–1131. [Google Scholar] [CrossRef]
- Thiam, C.N.; Mathavan, S.; Abdullah, A.; Chong, E.G.M. Malnutrition among patients admitted to the subacute geriatric ward during the COVID-19 pandemic era: A cross-sectional study in a tertiary hospital in Malaysia. Med. J. Malays. 2022, 77, 313–319. [Google Scholar]
- Urquiza, M.; Fernandez, N.; Arrinda, I.; Sierra, I.; Irazusta, J.; Larrad, A.R. Nutritional status is associated with function, physical performance and falls in older adults admitted to geriatric rehabilitation: A retrospective cohort study. Nutrients 2020, 12, 2855. [Google Scholar] [CrossRef]
- van Zwienen-Pot, J.I.; Visser, M.; Kuijpers, M.; Grimmerink, M.F.A.; Kruizenga, H.M. Undernutrition in nursing home rehabilitation patients. Clin. Nutr. 2017, 36, 755–759. [Google Scholar] [CrossRef] [PubMed]
- van Zwienen-Pot, J.I.; Visser, M.; Kruizenga, H.M. Predictors for achieving adequate protein and energy intake in nursing home rehabilitation patients. Aging Clin. Exp. Res. 2018, 30, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Verstraeten, L.M.G.; van Wijngaarden, J.P.; Pacifico, J.; Reijnierse, E.M.; Meskers, C.G.M.; Maier, A.B. Association between malnutrition and stages of sarcopenia in geriatric rehabilitation inpatients: RESORT. Clin. Nutr. 2021, 40, 4090–4096. [Google Scholar] [CrossRef]
- Vivanti, A.; Ward, N.; Haines, T. Nutritional status and associations with falls, balance, mobility and functionality during hospital admission. J. Nutr. Health Aging 2011, 15, 388–391. [Google Scholar] [CrossRef]
- Wester, P.; Angus, R.; Easlea, D.; Lin, M.; Chen, B.; Bisset, L. Use of the malnutrition screening tool by non-dietitians to identify at-risk patients in a rehabilitation setting: A validation study. Nutr. Diet. 2018, 75, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Nijmeijer, W.S.; van Dartel, D.; de Groot, R.; Woudsma, S.; Folbert, E.C.; den Braber, N.; Vermeer, M.; Hegeman, J.H.; Vollenbroek-Hutten, M.M.; Up&Go after a Hip Fracture Group. Transparency in hip fracture recovery over institutional boundaries: The transmural monitoring pathway. Clin. Rehabil. 2023, 37, 1406–1419. [Google Scholar] [CrossRef]
- Blanquet, M.; Guiguet-Auclair, C.; Berland, P.; Ducher, G.; Sauvage, A.; Dadet, S.; Guiyedi, V.; Farigon, N.; Bohatier, J.; Gerbaud, L.; et al. Are energy and protein intakes lower than requirements in older adults? An urgent issue in hospitals and nursing homes. Nutrients 2023, 15, 3307. [Google Scholar] [CrossRef]
- Kayhan Kocak, F.O.; Sahin, S.; Taşkıran, E.; Simsek, H.; Daylan, A.; Arman, P.; Dikmeer, A.; Kılıc, F.; Balci, C.; Tosun Tasaret, P.; et al. Frequency and risk factors of re-hospitalization in geriatric inpatient wards: A multicenter retrospective analysis. Exp. Aging Res. 2023, 49, 70–82. [Google Scholar] [CrossRef]
- Kurmann, S.; Reber, E.; Schönenberger, K.A.; Schuetz, P.; Uhlmann, K.; Vasiloglou, M.F.; Schoenenberger, A.W.; Bertschi, D.; Sterchi, A.B.; Stanga, Z. MEDPass versus conventional administration of oral nutritional supplements—A randomised controlled trial comparing coverage of energy and protein requirements. Clin. Nutr. 2023, 42, 108–115. [Google Scholar] [CrossRef]
- Laporte, M.; Villalon, L.; Payette, H. Simple nutrition screening tools for healthcare facilities: Development and validity assessment. Can. J. Diet. Pract. Res. 2001, 62, 26–34. [Google Scholar] [PubMed]
- How to Use the SGA Form. Available online: https://nutritioncareincanada.ca/sites/default/uploads/files/SGA%20Guidance%20Document%202017_EN_BW.PDF (accessed on 3 December 2023).
- Power, L.; Mullally, D.; Gibney, E.R.; Clarke, M.; Visser, M.; Volkert, D.; Bardon, L.; de van der Schueren, M.A.E.; Corish, C.A.; MaNuEL Consortium. A review of the validity of malnutrition screening tools used in older adults in community and healthcare settings—A MaNuEL study. Clin. Nutr. ESPEN 2018, 24, 1–13. [Google Scholar] [CrossRef]
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Van Gossum, A.; Klek, S.; Muscaritoli, M.; Nyulasi, I.; Ockenga, J.; Schneideret, S.M.; et al. Diagnostic criteria for malnutrition—An ESPEN Consensus Statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef]
- White, J.V.; Guenter, P.; Jensen, G.; Malone, A.; Schofield, M.; Academy Malnutrition Work Group; A.S.P.E.N. Malnutrition Task Force; A.S.P.E.N. Board of Directors. Consensus statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: Characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). JPEN J. Parenter. Enteral Nutr. 2012, 36, 275–283. [Google Scholar] [CrossRef] [PubMed]
- de van der Schueren, M.A.E.; Keller, H.; GLIM Consortium; Cederholm, T.; Barazzoni, R.; Compher, C.; Correia, M.I.T.D.; Gonzalez, M.C.; Jager-Wittenaar, H.; Pirlich, M.; et al. Global Leadership Initiative on Malnutrition (GLIM): Guidance on validation of the operational criteria for the diagnosis of protein-energy malnutrition in adults. Clin. Nutr. 2020, 39, 2872–2880. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, D.; Marco, E.; Meza-Valderrama, D.; Davalos-Yerovi, V.; Duarte, E. Taking a step toward implementation of Global Leadership Initiative on Malnutrition (GLIM) criteria in geriatric rehabilitation. Eur. Geriatr. Med. 2020, 11, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Peters, L.; O’Connor, C.; Giroux, I.; Teasell, R.; Foley, N. Screening and assessment of nutritional status following stroke: Results from a national survey of registered dietitians in Canada. Disabil. Rehabil. 2015, 37, 2413–2417. [Google Scholar] [CrossRef]
- Miyai, I.; Sonoda, S.; Nagai, S.; Takayama, Y.; Inoue, Y.; Kakehi, A.; Kurihara, M.; Ishikawa, M. Results of new policies for inpatient rehabilitation coverage in Japan. Neurorehabil. Neural Repair 2011, 25, 540–547. [Google Scholar] [CrossRef]
- Grund, S.; Gordon, A.L.; van Balen, R.; Bachmann, S.; Cherubini, A.; Landi, F.; Stuck, A.E.; Becker, C.; Achterberg, W.P.; Bauer, A.M.; et al. European consensus on core principles and future priorities for geriatric rehabilitation: Consensus statement. Eur. Geriatr. Med. 2020, 11, 233–238. [Google Scholar] [CrossRef]
- Muscat, F.; Camilleri, L.; Attard, C.; Lungaro Mifsud, S. Inpatient geriatric rehabilitation: Definitions and appropriate admission criteria, as established by Maltese national experts. J. Clin. Med. 2022, 11, 7230. [Google Scholar] [CrossRef] [PubMed]
- Academy of Nutrition and Dietetics Evidence Analysis Library. Malnutrition in Older Adults Evidence-Based Nutrition Practice Guideline (2020–2023). Available online: https://www.andeal.org/topic.cfm?menu=6064 (accessed on 5 January 2024).

| Items | Contents |
|---|---|
| Patient | Older patients in geriatric rehabilitation settings with a mean age of 65 or older |
| Concept | Types and characteristics of nutritional assessment methods, accuracy, and outcome measures related to malnutrition, identified by these tools or to the single parameter used in these tools |
| Context | Any study that utilized nutritional assessment methods for identifying malnutrition in older adults admitted to rehabilitation hospital/ward/facility or equivalent setting. Descriptive studies or studies that used nutritional assessment but focused on other variables were not excluded. |
| Design | Cross-sectional, cohort, case–control, systematic reviews, meta-analyses, and intervention studies. |
| Category | Methods | Body Weight/BMI | Weight Loss | Appetite Loss/Reduced Nutritional Intake | Anthropo-Metric Indices | Physical Functions/Activities | Cognitive Functions | Stress/Inflammatory Response from Disease | Others |
|---|---|---|---|---|---|---|---|---|---|
| Nutritional screening tool | MNA-SF | ✔ | ✔ | ✔ | (✔) a | ✔ | ✔ | ✔ | |
| MUST | ✔ | ✔ | ✔ | ✔ | |||||
| NRS2002 | ✔ | ✔ | ✔ | ✔ | ✔ b | ||||
| SNAQRC | ✔ | ✔ | ✔ | ✔ c | |||||
| Laporte’s method [23,68] | ✔ | ✔ | ✔ d | ||||||
| van Zwienen-Pot’s method [63,64] | ✔ | ✔ | |||||||
| Nutritional assessment tool | MNA | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ e |
| SGA | ✔ | ✔ | ✔ | ✔ | ✔ | ||||
| PG-SGA | ✔ | ✔ | ✔ | ✔ | ✔ f | ||||
| Diagnostic criteria for malnutrition | GLIM | ✔ | ✔ | ✔ | ✔ | ✔ g | |||
| ESPEN | ✔ | ✔ | ✔ h | ||||||
| ICD-10-AM | ✔ | ✔ | ✔ | ✔ i |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishioka, S.; Kokura, Y.; Momosaki, R.; Taketani, Y. Measures for Identifying Malnutrition in Geriatric Rehabilitation: A Scoping Review. Nutrients 2024, 16, 223. https://doi.org/10.3390/nu16020223
Nishioka S, Kokura Y, Momosaki R, Taketani Y. Measures for Identifying Malnutrition in Geriatric Rehabilitation: A Scoping Review. Nutrients. 2024; 16(2):223. https://doi.org/10.3390/nu16020223
Chicago/Turabian StyleNishioka, Shinta, Yoji Kokura, Ryo Momosaki, and Yutaka Taketani. 2024. "Measures for Identifying Malnutrition in Geriatric Rehabilitation: A Scoping Review" Nutrients 16, no. 2: 223. https://doi.org/10.3390/nu16020223
APA StyleNishioka, S., Kokura, Y., Momosaki, R., & Taketani, Y. (2024). Measures for Identifying Malnutrition in Geriatric Rehabilitation: A Scoping Review. Nutrients, 16(2), 223. https://doi.org/10.3390/nu16020223








