Is There Evidence of Crohn’s Disease Exclusion Diet (CDED) in Remission of Active Disease in Children and Adults? A Systematic Review
Highlights
- Certain dietary components are hostile to gut microbiota and increase intestinal permeability. This directly influences the inflammatory process in Crohn's disease.
- The Crohn’s Disease Exclusion Diet (CDED) is a new nutritional approach recently tested in clinical trials, showing promising results.
- The CDED has shown better tolerance than the currently used nutritional approach and similar beneficial results in achieving remission.
Abstract
1. Introduction
- ✓
- Phase 1 (week 0 to week 6): During this phase, there are mandatory, allowed, and disallowed foods. Enteral formula provides 50% of total energy requirements.
- ✓
- Phase 2 (week 7 to week 12): The list of mandatory and disallowed foods remains unchanged, while the list of allowed foods becomes less restrictive. Enteral formula provides 25% of total energy requirements.
- ✓
- Maintenance phase (after week 13): No mandatory foods are specified in this phase. The list of disallowed foods remains the same, and the list of allowed foods becomes more flexible. Enteral formula continues to provide 25% of total energy requirements. Additionally, this phase includes instructions for incorporating free meals on weekends, which may include some initially excluded dietary components [31,33].
2. Materials and Methods
- ((Gastrointestinal diseases[MeSH Terms]) OR (inflammatory bowel diseases[MeSH Terms]) OR (IBD[Title/Abstract]) OR (crohn disease[MeSH Terms]) OR (crohn[Title/Abstract])) AND ((crohn* disease exclusion diet[Title/Abstract]) OR (CDED[Title/Abstract]) OR (partial enteral nutrition[Title/Abstract]) OR (PEN[Title/Abstract])).
2.1. Inclusion and Exclusion Criteria
2.1.1. Inclusion Criteria
- Date of publication: Studies published between 30 June 2014 and 2 August 2022.
- Population: Human participants, including children and adults with active CD.
- Outcome: CDED as a nutritional intervention for achieving remission in CD.
- Study Design: Case–control studies, cohort studies, clinical trials, and randomized clinical trials.
- Language: Studies published in Portuguese, English, or Spanish.
2.1.2. Exclusion Criteria
- Studies that addressed nutritional therapy for CD in children and/or adults with active disease but did not involve the use of CDED.
- Studies that did not provide results or prevalence data.
2.1.3. Dietary Intervention
3. Results
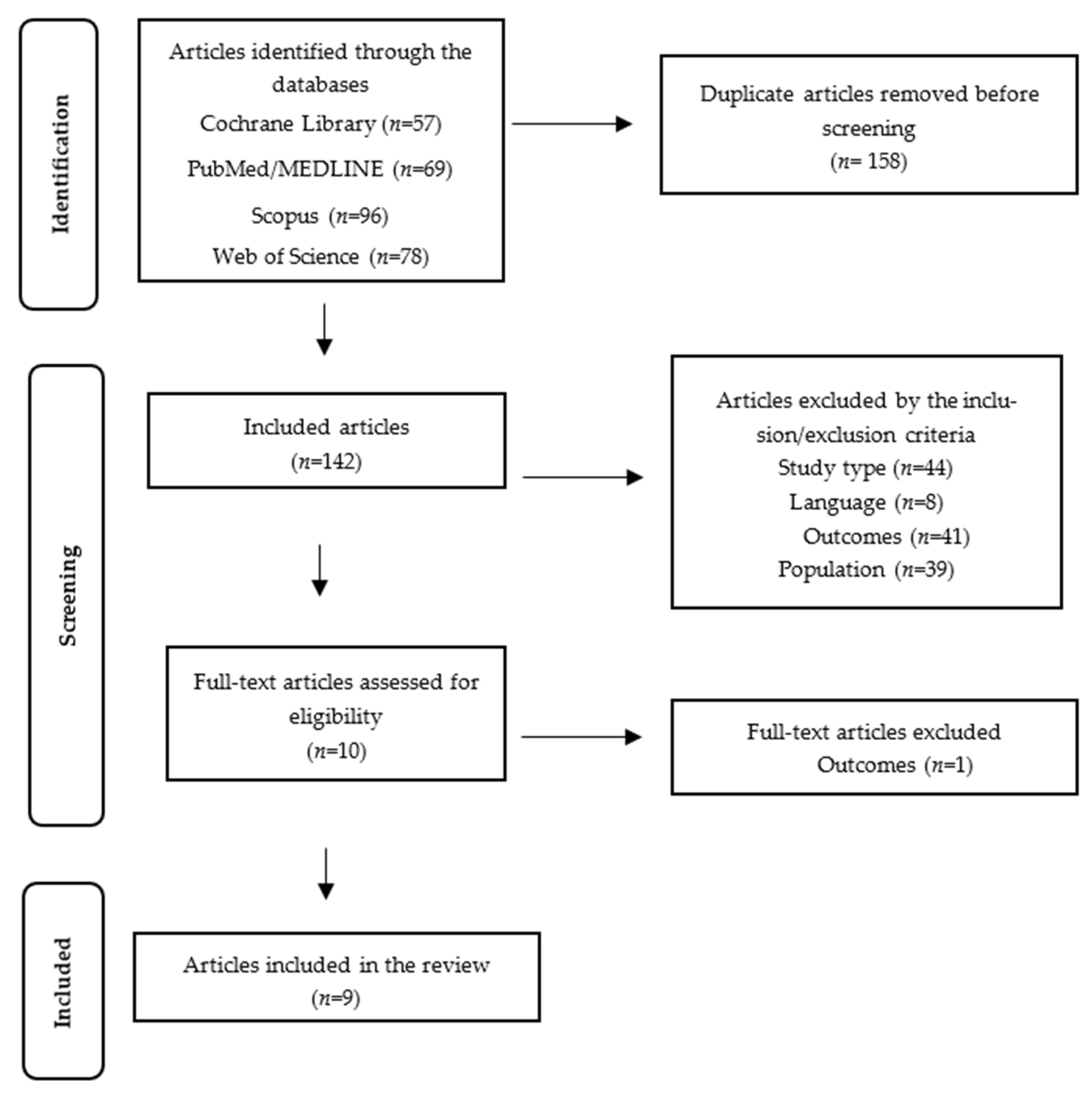
3.1. Included Studies
3.2. General Characteristics of the Studies
3.3. Diet
3.4. Outcomes
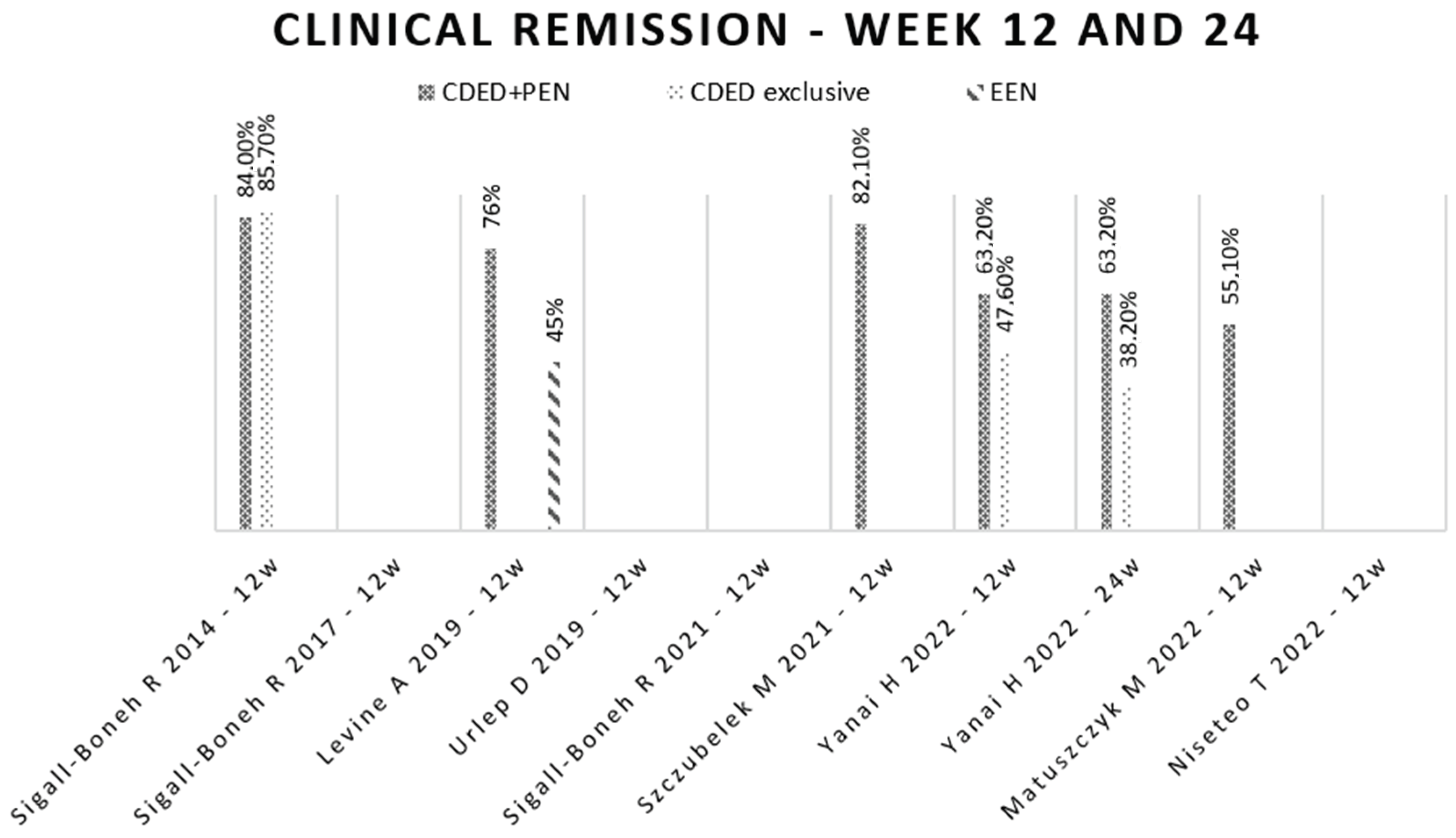
3.5. Disease’s Remission
3.6. Clinical Response
3.7. Tolerance
3.8. Fecal Calprotectin
3.9. Biochemical Parameters
3.9.1. C-Reactive Protein
3.9.2. Erythrocyte Sedimentation Rate
3.9.3. Albumin
| Study | Dietary Intervention | Fecal Calprotectin | CRP | ESR | Albumin |
|---|---|---|---|---|---|
| Sigall-Boneh, 2014 [31] | CDED + PEN CDED exclusive | NA | Significant decrease. No results available by intervention group. | Significant decrease. No results available by intervention group. | Significant increase. No results available by intervention group. |
| Sigall-Boneh, 2017 [32] | CDED + PEN CDED exclusive EEN + CDED + PEN | NA | Significant decrease in the total sample. No results available by intervention group. | NA | Non-significant increase. No results available by intervention group. |
| Levine, 2019 [33] | CDED + PEN EEN + free diet | Significant decrease. Non-significant differences between groups. | Significant decrease. Non-significant differences between groups. | NA | NA |
| Urlep, 2019 [40] | CDED adapted + PEN EEN | Significant decrease. Non-significant differences between groups. | Significant decrease. Non-significant differences between groups. | Significant decrease. Non-significant differences between groups. | Significant increase. Non-significant differences between groups. |
| Sigall-Boneh, 2021 [39] | CDED + PEN EEN | NA | Significant decrease. Non-significant differences between groups. | NA | |
| Szczubelek, 2021 [42] | CDED + PEN | Significant decrease. | Significant decrease. | Non-significant decrease. | No alterations were observed. |
| Yanai, 2022 [45] | CDED + PEN CDED exclusive | Significant decrease. Non-significant differences between groups. | Significant decrease. Non-significant differences between groups. | NA | No alterations were observed. |
| Matuszcz, 2022 [46] | CDED + PEN | Significant decrease. | Significant decrease. | Significant decrease. | NA |
| Niseteo, 2022 [41] | CDED + PEN EEN + CDED + PEN EEN | NA | Non-significant decrease in any group. | NA | NA |
3.10. Anthropometric Measurements
3.11. Endoscopic Remission
3.12. Intestinal Permeability and Microbiota
3.13. Quality of Life
3.14. Bias Risk Analysis
4. Discussion
4.1. Disease’s Remission
4.2. Biochemical Parameters, Intestinal Permeability, and Microbiota
4.3. Duration of Intervention
4.4. Enteral Formula
4.5. Nutritional Status
4.6. Importance of Registered Dietitians
4.7. Highlights of Our Review
4.8. What Is New?
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernstein, C.N.; Eliakim, A.; Fedail, S.; Fried, M.; Gearry, R.; Goh, K.-L.; Hamid, S.; Khan, A.G.; Khalif, I.; Ng, S.C.; et al. World Gastroenterology Organisation Global Guidelines Inflammatory Bowel Disease. J. Clin. Gastroenterol. 2016, 50, 803–818. [Google Scholar] [CrossRef]
- Van Assche, G.; Dignass, A.; Panes, J.; Beaugerie, L.; Karagiannis, J.; Allez, M.; Ochsenkühn, T.; Orchard, T.; Rogler, G.; Louis, E.; et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Definitions and diagnosis. J. Crohn’s Colitis 2010, 4, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN practical guideline: Clinical Nutrition in inflammatory bowel disease. Clin. Nutr. 2020, 39, 632–653. [Google Scholar] [CrossRef] [PubMed]
- Wark, G.; Samocha-Bonet, D.; Ghaly, S.; Danta, M. The role of diet in the pathogenesis and management of inflammatory bowel disease: A review. Nutrients 2021, 13, 135. [Google Scholar] [CrossRef]
- Torres, J.; Mehandru, S.; Colombel, J.-F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef] [PubMed]
- Satsangi, J.; Silverberg, M.S.; Vermeire, S.; Colombel, J.F. The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut 2006, 55, 749–753. [Google Scholar] [CrossRef]
- Levine, A.; Griffiths, A.; Markowitz, J.; Wilson, D.C.; Turner, D.; Russell, R.K.; Fell, J.; Ruemmele, F.M.; Walters, T.; Sherlock, M.; et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: The Paris classification. Inflamm. Bowel Dis. 2011, 17, 1314–1321. [Google Scholar] [CrossRef]
- Best, W.R.; Becktel, J.M.; Singleton, J.W.; Kern, F. Development of a Crohn’s Disease Activity Index. Gastroenterology 1976, 70, 439–444. [Google Scholar] [CrossRef]
- Best, W.R. Predicting the Crohn’s disease activity index from the harvey-bradshaw index. Inflamm. Bowel Dis. 2006, 12, 304–310. [Google Scholar] [CrossRef]
- Harvey, R.; Bradshaw, J. A SIMPLE INDEX OF CROHN’S-DISEASE ACTIVITY. Lancet 1980, 315, 514. [Google Scholar] [CrossRef]
- Hyams, J.S.; Ferry, G.D.; Mandel, F.S.; Gryboski, J.D.; Kibort, P.M.; Kirschner, B.S.; Griffiths, A.M.; Katz, A.J.; Grand, R.J.; Boyle, J.T. Development and validation of a pediatric Crohn’s disease activity index. J. Pediatr. Gastroenterol. Nutr. 1991, 12, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Mak, W.Y.; Zhao, M.; Ng, S.C.; Burisch, J. The epidemiology of inflammatory bowel disease: East meets west. J. Gastroenterol. Hepatol. 2020, 35, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Wine, E. Effects of enteral nutrition on Crohn’s Disease: Clues to the impact of diet on disease pathogenesis. Inflamm. Bowel Dis. 2013, 19, 1322–1329. [Google Scholar] [CrossRef]
- Petagna, L.; Antonelli, A.; Ganini, C.; Bellato, V.; Campanelli, M.; Divizia, A.; Efrati, C.; Franceschilli, M.; Guida, A.M.; Ingallinella, S.; et al. Pathophysiology of Crohn’s disease inflammation and recurrence. Biol. Direct 2020, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Boneh, R.S.; Wine, E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut 2018, 67, 1726–1738. [Google Scholar] [CrossRef] [PubMed]
- Van Rheenen, P.F.; Aloi, M.; Assa, A.; Bronsky, J.; Escher, J.C.; Fagerberg, U.L.; Gasparetto, M.; Gerasimidis, K.; Griffiths, A.; Henderson, P.; et al. The Medical Management of Paediatric Crohn’s Disease: An ECCO-ESPGHAN Guideline Update. J. Crohn’s Colitis 2021, 15, 171–194. [Google Scholar] [CrossRef]
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; et al. ECCO guidelines on therapeutics in Crohn’s disease: Medical treatment. J. Crohn’s Colitis 2020, 14, 4–22. [Google Scholar] [CrossRef]
- Borrelli, O.; Cordischi, L.; Cirulli, M.; Paganelli, M.; Labalestra, V.; Uccini, S.; Russo, P.M.; Cucchiara, S. Polymeric Diet Alone Versus Corticosteroids in the Treatment of Active Pediatric Crohn’s Disease: A Randomized Controlled Open-Label Trial. Clin. Gastroenterol. Hepatol. 2006, 4, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Pigneur, B.; Lepage, P.; Mondot, S.; Schmitz, J.; Goulet, O.; Doré, J.; Ruemmele, F.M. Mucosal Healing and Bacterial Composition in Response to Enteral Nutrition Vs Steroid-based Induction Therapy—A Randomised Prospective Clinical Trial in Children With Crohn’s Disease. J. Crohn’s Colitis 2019, 13, 846–855. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, K.-C.; Chen, J. Exclusive enteral nutrition versus corticosteroids for treatment of pediatric Crohn’s disease: A meta-analysis. World J. Pediatr. 2019, 15, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Albenberg, L.; Compher, C.; Baldassano, R.; Piccoli, D.; Lewis, J.D.; Wu, G.D. Diet in the pathogenesis and treatment of inflammatory bowel diseases. Gastroenterology 2015, 148, 1087–1106. [Google Scholar] [CrossRef]
- Ashton, J.J.; Gavin, J.; Beattie, R.M. Exclusive enteral nutrition in Crohn’s disease: Evidence and practicalities. Clin. Nutr. 2019, 38, 80–89. [Google Scholar] [CrossRef]
- Hansen, T.; Duerksen, D.R. Enteral Nutrition in the Management of Pediatric and Adult Crohn’s Disease. Nutrients 2018, 10, 537. [Google Scholar] [CrossRef] [PubMed]
- Whitten, K.E.; Rogers, P.; Ooi, C.K.Y.; Day, A.S. International survey of enteral nutrition protocols used in children with Crohn’s disease. J. Dig. Dis. 2012, 13, 107–112. [Google Scholar] [CrossRef]
- Logan, M.; Gkikas, K.; Svolos, V.; Nichols, B.; Milling, S.; Gaya, D.R.; Seenan, J.P.; Macdonald, J.; Hansen, R.; Ijaz, U.Z.; et al. Analysis of 61 exclusive enteral nutrition formulas used in the management of active Crohn’s disease—New insights into dietary disease triggers. Aliment. Pharmacol. Ther. 2020, 51, 935–947. [Google Scholar] [CrossRef]
- Wall, C.L.; Day, A.S.; Gearry, R.B. Use of exclusive enteral nutrition in adults with Crohn’s disease: A review. World J. Gastroenterol. 2013, 19, 7652–7660. [Google Scholar] [CrossRef]
- Matuszczyk, M.; Kierkus, J. Nutritional therapy in pediatric crohn’s disease—are we going to change the guidelines? J. Clin. Med. 2021, 10, 3027. [Google Scholar] [CrossRef]
- Sigall-Boneh, R.; Pfeffer-Gik, T.; Segal, I.; Zangen, T.; Boaz, M.; Levine, A. Partial enteral nutrition with a Crohn’s disease exclusion diet is effective for induction of remission in children and young adults with Crohn’s disease. Inflamm. Bowel Dis. 2014, 20, 1353–1360. [Google Scholar] [CrossRef]
- Boneh, R.S.; Shabat, C.S.; Yanai, H.; Chermesh, I.; Ben Avraham, S.; Boaz, M.; Levine, A. Dietary therapy with the Crohn’s disease exclusion diet is a successful strategy for induction of Remission in children and adults failing biological therapy. J. Crohn’s Colitis 2017, 11, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Wine, E.; Assa, A.; Boneh, R.S.; Shaoul, R.; Kori, M.; Cohen, S.; Peleg, S.; Shamaly, H.; On, A.; et al. Crohn’s Disease Exclusion Diet Plus Partial Enteral Nutrition Induces Sustained Remission in a Randomized Controlled Trial. Gastroenterology 2019, 157, 440–450.e8. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- National Institute for Health and Care Research. International Prospective Register of Systematic Reviews: PROSPERO n.d. Available online: https://www.crd.york.ac.uk/prospero/ (accessed on 21 June 2022).
- Rayyan. Rayyan-AI Powered Tool for Systematic Literature Reviews n.d. Available online: https://www.rayyan.ai/ (accessed on 1 July 2022).
- Cochrane. RevMan: Systematic Review and Meta-Analysis Tool for Researchers Worldwide. Available online: https://revman.cochrane.org/info (accessed on 30 July 2022).
- Boneh, R.S.; Van Limbergen, J.; Wine, E.; Assa, A.; Shaoul, R.; Milman, P.; Cohen, S.; Kori, M.; Peleg, S.; On, A.; et al. Dietary Therapies Induce Rapid Response and Remission in Pediatric Patients With Active Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2021, 19, 752–759. [Google Scholar] [CrossRef]
- Urlep, D.; Benedik, E.; Brecelj, J.; Orel, R. Partial enteral nutrition induces clinical and endoscopic remission in active pediatric Crohn’s disease: Results of a prospective cohort study. Eur. J. Pediatr. 2020, 179, 431–438. [Google Scholar] [CrossRef]
- Niseteo, T.; Sila, S.; Trivić, I.; Mišak, Z.; Kolaček, S.; Hojsak, I. Modified Crohn’s disease exclusion diet is equally effective as exclusive enteral nutrition: Real-world data. Nutr. Clin. Pr. 2022, 37, 435–441. [Google Scholar] [CrossRef]
- Szczubełek, M.; Pomorska, K.; Korólczyk-Kowalczyk, M.; Lewandowski, K.; Kaniewska, M.; Rydzewska, G. Effectiveness of crohn’s disease exclusion diet for induction of remission in crohn’s disease adult patients. Nutrients 2021, 13, 4112. [Google Scholar] [CrossRef]
- Turner, D.; Griffiths, A.M.; Walters, T.D.; Seah, T.; Markowitz, J.; Pfefferkorn, M.; Keljo, D.; Waxman, J.; Otley, A.; LeLeiko, N.S.; et al. Mathematical weighting of the pediatric Crohn’s disease activity index (PCDAI) and comparison with its other short versions. Inflamm. Bowel Dis. 2012, 18, 55–62. [Google Scholar] [CrossRef]
- Koutroumpakis, E.; Katsanos, K.H. Implementation of the simple endoscopic activity score in crohn’s disease. Saudi J. Gastroenterol. 2016, 22, 183–191. [Google Scholar] [CrossRef]
- Yanai, H.; Levine, A.; Hirsch, A.; Boneh, R.S.; Kopylov, U.; Eran, H.B.; A Cohen, N.; Ron, Y.; Goren, I.; Leibovitzh, H.; et al. The Crohn’s disease exclusion diet for induction and maintenance of remission in adults with mild-to-moderate Crohn’s disease (CDED-AD): An open-label, pilot, randomised trial. Lancet Gastroenterol. Hepatol. 2022, 7, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Matuszczyk, M.; Meglicka, M.; Wiernicka, A.; Jarzębicka, D.; Osiecki, M.; Kotkowicz-Szczur, M.; Kierkuś, J. Effect of the Crohn’s Disease Exclusion Diet (CDED) on the Fecal Calprotectin Level in Children with Active Crohn’s Disease. J. Clin. Med. 2022, 11, 4146. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.; Guyatt, G.; Singer, J.; Irvine, E.J.; Goodacre, R.; Tompkins, C.; Williams, N.; Wagner, F. Quality of Life in Patients with Inflammatory Bowel Disease. J. Clin. Gastroenterol. 1988, 10, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.; Langner, C.; Driessen, A.; Ensari, A.; Geboes, K.; Mantzaris, G.; Villanacci, V.; Becheanu, G.; Nunes, P.B.; Cathomas, G.; et al. European consensus on the histopathology of inflammatory bowel disease. J. Crohn’s Colitis 2013, 7, 827–851. [Google Scholar] [CrossRef] [PubMed]
- Ihara, S.; Hirata, Y.; Koike, K. TGF-β in inflammatory bowel disease: A key regulator of immune cells, epithelium, and the intestinal microbiota. J. Gastroenterol. 2017, 52, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Bager, P.; Escher, J.; Forbes, A.; Hébuterne, X.; Hvas, C.L.; Joly, F.; Klek, S.; Krznaric, Z.; Ockenga, J.; et al. ESPEN guideline on Clinical Nutrition in inflammatory bowel disease. Clin. Nutr. 2023, 42, 352–379. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Lei, Y.; Lin, Z. Effects of Crohn’s disease exclusion diet on remission: A systematic review. Ther. Adv. Gastroenterol. 2023, 16. [Google Scholar] [CrossRef] [PubMed]
- Urlep, D.; Orel, R.; Kunstek, P.; Benedik, E. Treatment of Active Crohn’s Disease in Children Using Partial Enteral Nutrition Combined with a Modified Crohn’s Disease Exclusion Diet: A Pilot Prospective Cohort Trial on Clinical and Endoscopic Outcomes. Nutrients 2023, 15, 4676. [Google Scholar] [CrossRef]
- Fliss-Isakov, N.; Cohen, N.A.; Bromberg, A.; Elbert, G.; Anbar, R.; Ron, Y.; Hirsch, A.; Thurm, T.; Maharshak, N. Crohn’s Disease Exclusion Diet for the Treatment of Crohn’s Disease: Real-World Experience from a Tertiary Center. J. Clin. Med. 2023, 12, 5428. [Google Scholar] [CrossRef]
- Stein, R.; Daniel, S.G.; Baldassano, R.N.; Feigenbaum, K.R.; Kachelries, K.R.; Sigall-Boneh, R.R.; Weston, S.R.; Levine, A.; Bittinger, K. Outcomes and Predictors of Sustained Remission After Drug Withdrawal in Pediatric Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.C.J.; Muncunill, G.P.; Ruf, A.L.; Carnero, L.; Miravet, V.V.; Arenas, D.G.; Castillo, N.E.; de Carpi, J.M. Efficacy of Crohn’s disease exclusion diet in treatment -naïve children and children progressed on biological therapy: A retrospective chart review. BMC Gastroenterol. 2023, 23, 225. [Google Scholar] [CrossRef]
- Martín-Masot, R.; Herrador-López, M.; Navas-López, V.M. Dietary Habit Modifications in Paediatric Patients after One Year of Treatment with the Crohn’s Disease Exclusion Diet. Nutrients 2023, 15, 554. [Google Scholar] [CrossRef]


| Study | Criteria for Clinical Remission | Criteria for Clinical Response |
|---|---|---|
| Sigall-Boneh, 2014 [31] | PCDAI ≤ 7.5 (without height criteria at the age of diagnosis) or HBI ≤ 3 + CRP (0.5 mg/dL) | Decrease ≥ 12.5 points in PCDAI or decrease ≥2 points in HBI or improvement in biochemical parameters (Hg, ESR, albumin, and CRP) |
| Sigall-Boneh, 2017 [32] | PGA score + HBI < 5 | Decrease ≥3 HBI points or remission |
| Levine, 2019 [33] | PCDAI ≤ 10 or PCDAI ≤ 7.5 (without height criteria at the age of diagnosis) | Decrease ≥12.5 points in PCDAI or remission |
| Urlep, 2019 [40] | PCDAI ≤ 10 | Decrease ≥15 points in PCDAI or endoscopic response (50% reduction in SES-CD score) or changes in PCDAI and SES-CD or improvement in biochemical levels (ESR, CRP, Hg, albumin, thrombocytes, and calprotectin) or improvement in anthropometric measurements (weight and BMI) |
| Sigall-Boneh, 2021 [39] | PCDAI ≤ 10 | Decrease ≥12.5 points in PCDAI or remission |
| Szczubelek, 2021 [42] | CDAI <150 | Decrease ≥100 CDAI points or statistically significant decrease in CRP and/or leukocyte levels or Improvement in QoL or improvement in biochemical levels (total protein, albumin, Vit D, Vit B12, folic acid, Na, K, Ca, Fe, and ferritin) or improvement in BMI |
| Yanai, 2022 [45] | HBI < 5 at week 6 | Decrease ≥3 HBI points |
| Matuszczyk, 2022 [46] | PCDAI ≤ 10 | Decrease ≥12.5 points in PCDAI or remission |
| Niseteo, 2022 [41] | wPCDAI ≤ 12.5 | NA |
| Study | Time of Intervention | Diet Therapy | Nutritional Formula | Main Outcomes | Secondary Outcomes |
|---|---|---|---|---|---|
| Sigall-Boneh, 2014 [31] | 12 weeks | Group 1 (n = 40) 0–6 weeks: CDED + PEN 50% 7–12 weeks: CDED + PEN 25% Group 2 (n = 7) 0–12 weeks: CDED exclusive | Modulen, Nestlé, Switzerland Pediasure, Abbott, The Netherlands | Clinical response Clinical remission | Weight Biochemical parameters |
| Sigall-Boneh, 2017 [32] | 6 weeks | Group 1 (n = 12) 0–6 weeks: CDED + PEN 50% Group 2 (n = 4) 0–6 weeks: CDED exclusive Group 3 (n = 5) 0–2 weeks: EEN 3–6 weeks: CDED + PEN 50% | Modulen, Nestlé, Switzerland Pediasure, Abbott, The Netherlands | Clinical response Clinical remission | - |
| Levine, 2019 [33] | 12 weeks | Group 1 (n = 40) 0–6 weeks: CDED + PEN 50% 7–12 weeks: CDED + PEN 25% Group 2 (n = 38) 0–6 weeks: EEN 7–12 weeks: PEN 25% + free diet | Modulen, Nestlé, Switzerland | Tolerance | Clinical response Clinical remission Intestinal permeability Poor adherence Microbiota composition Weight Biochemical parameters Fecal calprotectin |
| Urlep, 2019 [40] | 6 weeks | Group 1 (n = 13) 0–6 weeks: EEN Group 2 (n = 12) 0–12 weeks: Diet adapted from CDED + PEN 75% | Alicalm, Nutricia, The Netherlands | Clinical remission | Clinical response Endoscopic remission Anthropometric parameters Biochemical parameters Fecal calprotectin |
| Sigall-Boneh, 2021 [39] | 6 weeks | Group 1 (n = 39) 0–6 weeks: CDED + PEN 50% Group 2 (n = 34) 0–6 weeks: EEN | Modulen, Nestlé, Switzerland | Rapid clinical response | Clinical remission |
| Szczubelek, 2021 [42] | 12 weeks | Total sample (n = 32) 0–6 weeks: CDED + PEN 50% 7–12 weeks: CDED + PEN 25% | Modulen, Nestlé, Switzerland | Clinical remission | Clinical response Anthropometric parameters Biochemical parameters Quality of life Fecal calprotectin |
| Yanai, 2022 [45] | 24 weeks | Group 1 (n = 19) 0–6 weeks: CDED + PEN (1000 kcal) 7–12 weeks: CDED + PEN (600 kcal) 13–24 weeks: CDED + PEN (600 kcal) or CDED exclusive Group 2 (n = 21) CDED exclusive | Modulen, Nestlé, Switzerland | Clinical remission | Corticosteroid-free remission Clinical response Endoscopic remission Biochemical parameters Fecal calprotectin Anthropometric parameters Adherence |
| Matuszczyk, 2022 [46] | 12 weeks | Total sample (n = 48) 0–6 weeks: CDED + PEN 50% 7–12 weeks: CDED + PEN 25% | Modulen, Nestlé, Switzerland | Normal fecal calprotectin after 12 weeks | Clinical response Clinical remission Biochemical parameters Anthropometric parameters |
| Niseteo, 2022 [41] | 6–9 weeks | Group 1 (n = 16) 0–1/2 weeks: EEN 2/3–8/9 weeks: CDED + PEN 50% Group 2 (n = 4) 0–6 weeks: CDED + PEN 50% Group 3 (n = 41) 0–6/8 weeks: EEN | Modulen, Nestlé, Switzerland Pediasure, Abbott, The Netherlands NutriniDrink, Nutricia, The Netherlands Resource Jr, Nestlé, Switzerland Resource 2.0, Nestlé, Switzerland | Clinical remission | Anthropometric parameters |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correia, I.; Oliveira, P.A.; Antunes, M.L.; Raimundo, M.d.G.; Moreira, A.C. Is There Evidence of Crohn’s Disease Exclusion Diet (CDED) in Remission of Active Disease in Children and Adults? A Systematic Review. Nutrients 2024, 16, 987. https://doi.org/10.3390/nu16070987
Correia I, Oliveira PA, Antunes ML, Raimundo MdG, Moreira AC. Is There Evidence of Crohn’s Disease Exclusion Diet (CDED) in Remission of Active Disease in Children and Adults? A Systematic Review. Nutrients. 2024; 16(7):987. https://doi.org/10.3390/nu16070987
Chicago/Turabian StyleCorreia, Inês, Patrícia Almeida Oliveira, Maria Luz Antunes, Maria da Graça Raimundo, and Ana Catarina Moreira. 2024. "Is There Evidence of Crohn’s Disease Exclusion Diet (CDED) in Remission of Active Disease in Children and Adults? A Systematic Review" Nutrients 16, no. 7: 987. https://doi.org/10.3390/nu16070987
APA StyleCorreia, I., Oliveira, P. A., Antunes, M. L., Raimundo, M. d. G., & Moreira, A. C. (2024). Is There Evidence of Crohn’s Disease Exclusion Diet (CDED) in Remission of Active Disease in Children and Adults? A Systematic Review. Nutrients, 16(7), 987. https://doi.org/10.3390/nu16070987








