Preventative Effects of Milk Fat Globule Membrane Ingredients on DSS-Induced Mucosal Injury in Intestinal Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Digestion of MFGM Ingredients
2.2. Caco-2/HT29-MTX Co-Culture
2.3. Transepithelial Electrical Resistance (TEER) and FITC-Dextran Permeability
2.4. Slot Blot for Mucin Detection
2.5. Scanning Electron Microscopy (SEM)
2.6. Glycoprotein Staining
2.7. Bioinformatic Analysis
2.8. Statistical Analysis
3. Results
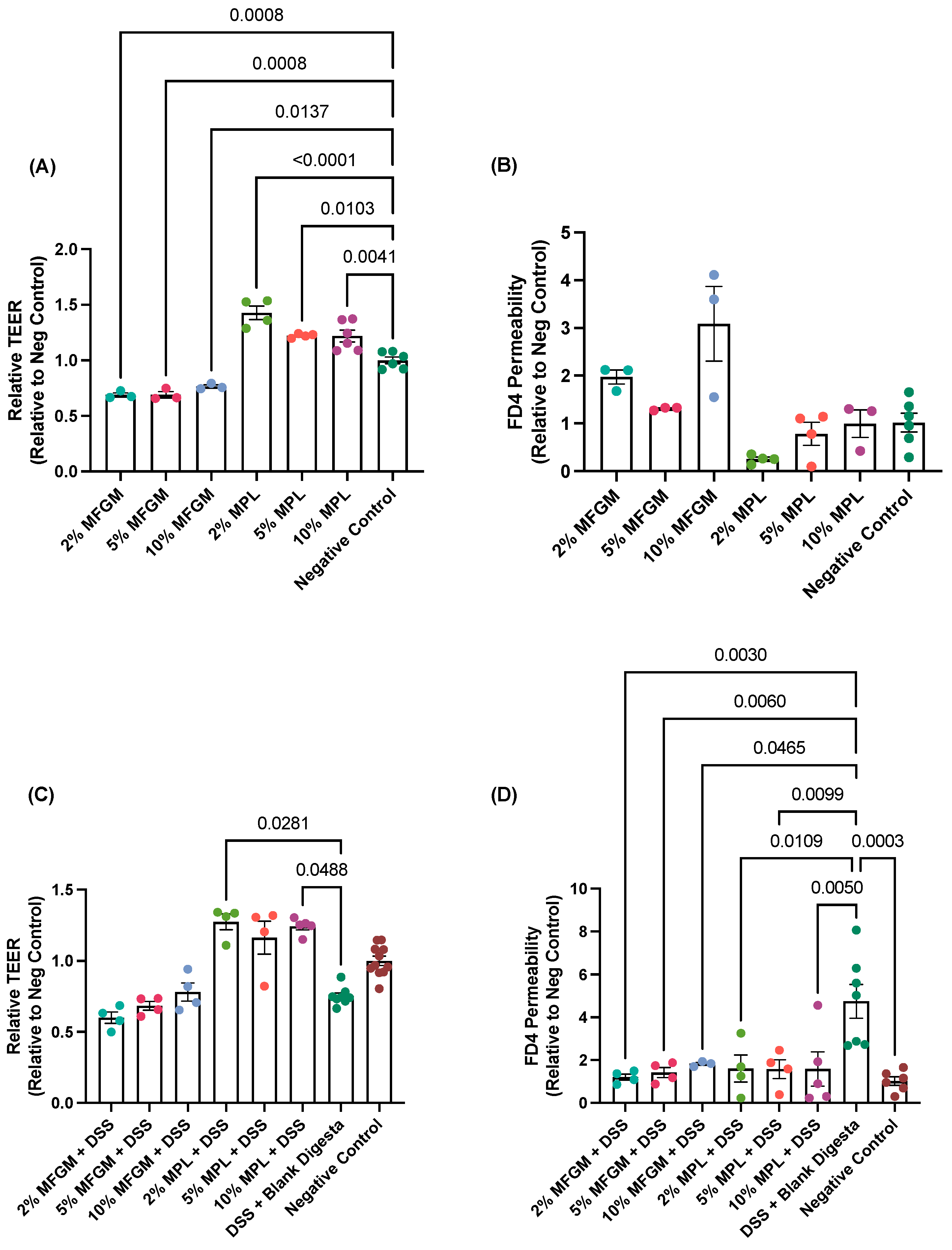
3.1. Protective Effects of MFGM and MPL on Caco-2/HT29-MTX Barrier Integrity and Permeability
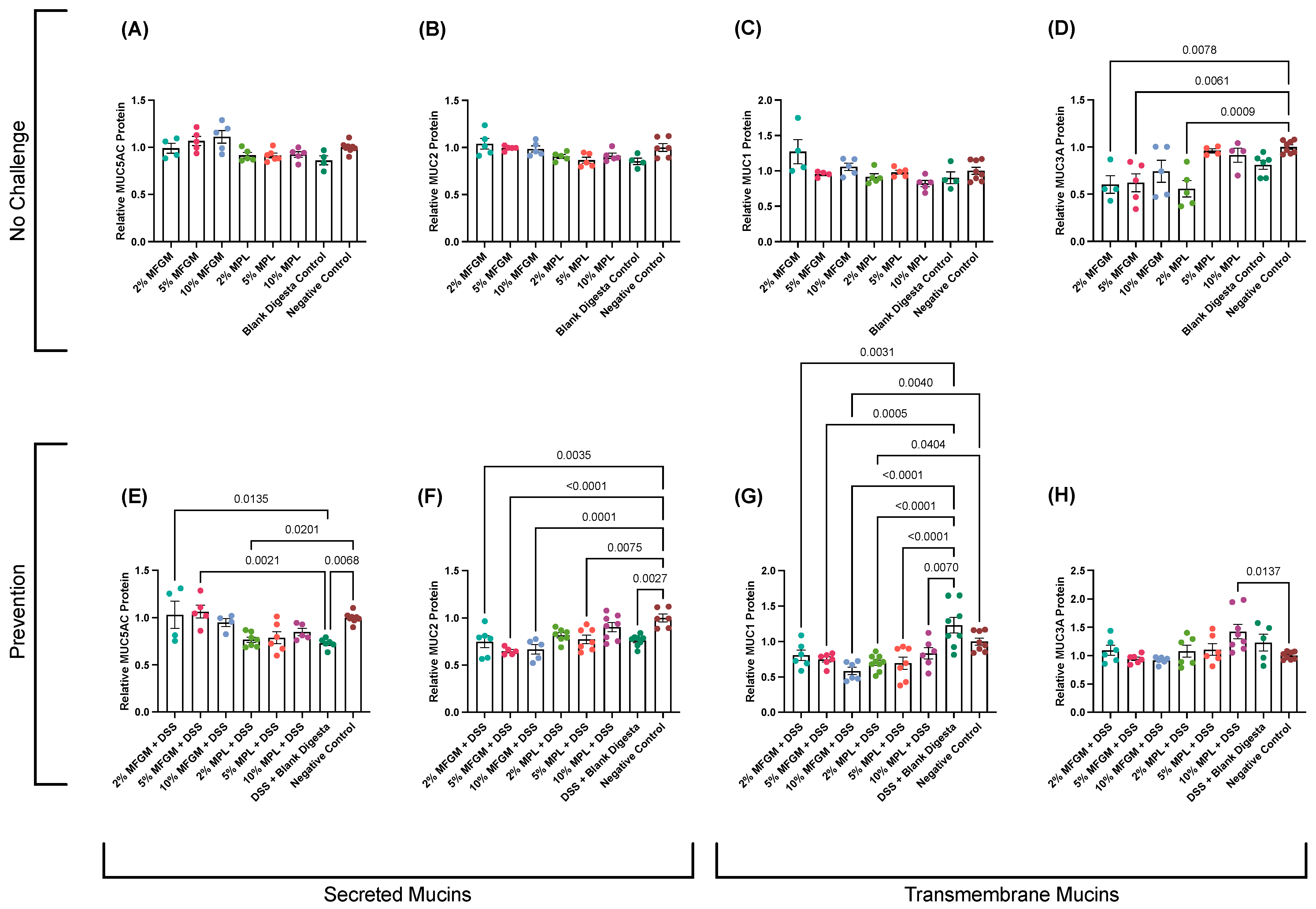
3.2. Effects of MFGM or MPL on DSS-Treated Cells Demonstrated Protective Effects on Mucin Protein Abundance
3.3. DSS-Induced Impaired Brush Border Is Prevented through Digested MFGM and MPL Treatments
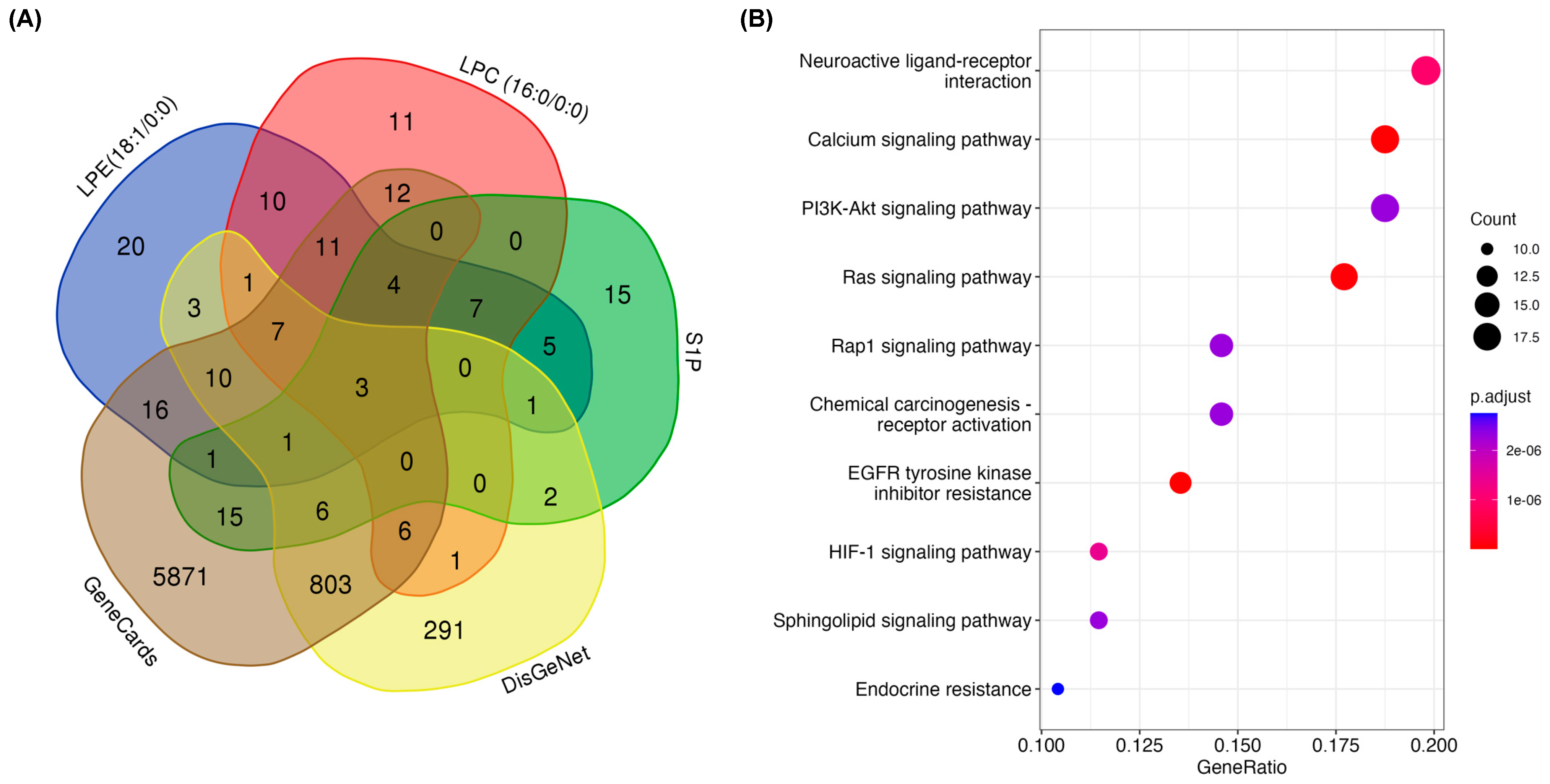
3.4. In Silico Prediction of Pathways Involved in Prevention of DSS-Induced Mucosal Damage by MFGM Lipids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbara, G.; Barbaro, M.R.; Fuschi, D.; Palombo, M.; Falangone, F.; Cremon, C.; Marasco, G.; Stanghellini, V. Inflammatory and Microbiota-Related Regulation of the Intestinal Epithelial Barrier. Front. Nutr. 2021, 8, 718356. [Google Scholar] [CrossRef] [PubMed]
- Carraway, K.L.; Ramsauer, V.P.; Haq, B.; Carothers Carraway, C.A. Cell Signaling through Membrane Mucins. Bioessays 2003, 25, 66–71. [Google Scholar] [CrossRef] [PubMed]
- van Putten, J.P.M.; Strijbis, K. Transmembrane Mucins: Signaling Receptors at the Intersection of Inflammation and Cancer. J. Innate Immun. 2017, 9, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Vancamelbeke, M.; Vermeire, S. The Intestinal Barrier: A Fundamental Role in Health and Disease. Expert. Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Haddad, M.J.; Sztupecki, W.; Delayre-Orthez, C.; Rhazi, L.; Barbezier, N.; Depeint, F.; Anton, P.M. Complexification of In Vitro Models of Intestinal Barriers, A True Challenge for a More Accurate Alternative Approach. Int. J. Mol. Sci. 2023, 24, 3595. [Google Scholar] [CrossRef] [PubMed]
- Dijk, W.; Villa, C.; Benedé, S.; Vassilopoulou, E.; Mafra, I.; Garrido-Arandia, M.; Martínez Blanco, M.; Bouchaud, G.; Hoppenbrouwers, T.; Bavaro, S.L.; et al. Critical Features of an in Vitro Intestinal Absorption Model to Study the First Key Aspects Underlying Food Allergen Sensitization. Compr. Rev. Food Sci. Food Saf. 2023, 22, 971–1005. [Google Scholar] [CrossRef]
- Turner, J.R. Intestinal Mucosal Barrier Function in Health and Disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Toutounji, M.; Wanes, D.; El-Harakeh, M.; El-Sabban, M.; Rizk, S.; Naim, H.Y. Dextran Sodium Sulfate-Induced Impairment of Protein Trafficking and Alterations in Membrane Composition in Intestinal Caco-2 Cell Line. Int. J. Mol. Sci. 2020, 21, 2726. [Google Scholar] [CrossRef] [PubMed]
- Eichele, D.D.; Kharbanda, K.K. Dextran Sodium Sulfate Colitis Murine Model: An Indispensable Tool for Advancing Our Understanding of Inflammatory Bowel Diseases Pathogenesis. World J. Gastroenterol. 2017, 23, 6016–6029. [Google Scholar] [CrossRef]
- Kiesler, P.; Fuss, I.J.; Strober, W. Experimental Models of Inflammatory Bowel Diseases. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 154–170. [Google Scholar] [CrossRef]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary Emulsifiers Impact the Mouse Gut Microbiota Promoting Colitis and Metabolic Syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L.; Ocansey, D.K.W.; Wang, B.; Wang, L.; Xu, Z. Mucin-Type O-Glycans: Barrier, Microbiota, and Immune Anchors in Inflammatory Bowel Disease. J. Inflamm. Res. 2021, 14, 5939–5953. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, K.; Xia, L. The Barrier and beyond: Roles of Intestinal Mucus and Mucin-Type O-Glycosylation in Resistance and Tolerance Defense Strategies Guiding Host-Microbe Symbiosis. Gut Microbes 2022, 14, 2052699. [Google Scholar] [CrossRef] [PubMed]
- De Santis, S.; Cavalcanti, E.; Mastronardi, M.; Jirillo, E.; Chieppa, M. Nutritional Keys for Intestinal Barrier Modulation. Front. Immunol. 2015, 6, 612. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, L.; Wu, Y.; Zhou, P. Changes in Milk Fat Globule Membrane Proteome after Pasteurization in Human, Bovine and Caprine Species. Food Chem. 2019, 279, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Manoni, M.; Di Lorenzo, C.; Ottoboni, M.; Tretola, M.; Pinotti, L. Comparative Proteomics of Milk Fat Globule Membrane (MFGM) Proteome across Species and Lactation Stages and the Potentials of MFGM Fractions in Infant Formula Preparation. Foods 2020, 9, 1251. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Anaya, J.; Jiménez-Flores, R. Symposium Review: The Relevance of Bovine Milk Phospholipids in Human Nutrition—Evidence of the Effect on Infant Gut and Brain Development. J. Dairy Sci. 2019, 102, 11. [Google Scholar] [CrossRef] [PubMed]
- Bhinder, G.; Allaire, J.M.; Garcia, C.; Lau, J.T.; Chan, J.M.; Ryz, N.R.; Bosman, E.S.; Graef, F.A.; Crowley, S.M.; Celiberto, L.S.; et al. Milk Fat Globule Membrane Supplementation in Formula Modulates the Neonatal Gut Microbiome and Normalizes Intestinal Development. Sci. Rep. 2017, 7, 45274. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wu, S.S.; Berseth, C.L.; Harris, C.L.; Richards, J.D.; Wampler, J.L.; Zhuang, W.; Cleghorn, G.; Rudolph, C.D.; Liu, B.; et al. Improved Neurodevelopmental Outcomes Associated with Bovine Milk Fat Globule Membrane and Lactoferrin in Infant Formula: A Randomized, Controlled Trial. J. Pediatr. 2019, 215, 24–31. [Google Scholar] [CrossRef]
- Moukarzel, S.; Dyer, R.A.; Garcia, C.; Wiedeman, A.; Boyce, G.; Weinberg, J.; Keller, B.O.; Elango, R.; Innis, S.M. Milk Fat Globule Membrane Supplementation in Formula-Fed Rat Pups Improves Reflex Development and May Alter Brain Lipid Composition. Sci. Rep. 2018, 8, 9. [Google Scholar] [CrossRef]
- Han, L.; Du, M.; Ren, F.; Mao, X. Milk Polar Lipids Supplementation to Obese Rats During Pregnancy and Lactation Benefited Skeletal Outcomes of Male Offspring. Mol. Nutr. Food Res. 2021, 65, 2001208. [Google Scholar] [CrossRef] [PubMed]
- Rogers, T.S.; Demmer, E.; Rivera, N.; Gertz, E.R.; German, J.B.; Smilowitz, J.T.; Zivkovic, A.M.; Van Loan, M.D. The Role of a Dairy Fraction Rich in Milk Fat Globule Membrane in the Suppression of Postprandial Inflammatory Markers and Bone Turnover in Obese and Overweight Adults: An Exploratory Study. Nutr. Metab. 2017, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Parenti, M.; Grip, T.; Domellöf, M.; Lönnerdal, B.; Hernell, O.; Timby, N.; Slupsky, C.M. Metabolic Phenotype of Breast-Fed Infants, and Infants Fed Standard Formula or Bovine MFGM Supplemented Formula: A Randomized Controlled Trial. Sci. Rep. 2019, 9, 339. [Google Scholar] [CrossRef]
- Li, T.; Gao, J.; Du, M.; Song, J.; Mao, X. Milk Fat Globule Membrane Attenuates High-Fat Diet-Induced Obesity by Inhibiting Adipogenesis and Increasing Uncoupling Protein 1 Expression in White Adipose Tissue of Mice. Nutrients 2018, 10, 331. [Google Scholar] [CrossRef]
- Rosqvist, F.; Smedman, A.; Lindmark-Månsson, H.; Paulsson, M.; Petrus, P.; Straniero, S.; Rudling, M.; Dahlman, I.; Risérus, U. Potential Role of Milk Fat Globule Membrane in Modulating Plasma Lipoproteins, Gene Expression, and Cholesterol Metabolism in Humans: A Randomized Study1. Am. J. Clin. Nutr. 2015, 102, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Anto, L.; Warykas, S.W.; Torres-Gonzalez, M.; Blesso, C.N. Milk Polar Lipids: Underappreciated Lipids with Emerging Health Benefits. Nutrients 2020, 12, 1001. [Google Scholar] [CrossRef] [PubMed]
- Millar, C.L.; Jiang, C.; Norris, G.H.; Garcia, C.; Seibel, S.; Anto, L.; Lee, J.-Y.; Blesso, C.N. Cow’s Milk Polar Lipids Reduce Atherogenic Lipoprotein Cholesterol, Modulate Gut Microbiota and Attenuate Atherosclerosis Development in LDL-Receptor Knockout Mice Fed a Western-Type Diet. J. Nutr. Biochem. 2020, 79, 108351. [Google Scholar] [CrossRef]
- Yu, Z.; Li, Y.; Niu, Y.; Tang, Q.; Wu, J. Milk Fat Globule Membrane Enhances Colonic–Mucus-Barrier Function in a Rat Model of Short-Bowel Syndrome. J. Parenter. Enter. Nutr. 2021, 45, 916–925. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, X.; Huang, S.; Li, T.; Zhang, X.; Pang, J.; Zhao, J.; Chen, L.; Zhang, B.; Wang, J.; et al. Milk Fat Globule Membrane Attenuates Acute Colitis and Secondary Liver Injury by Improving the Mucus Barrier and Regulating the Gut Microbiota. Front. Immunol. 2022, 13, 865273. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Kleiveland, C.R. Co-Cultivation of Caco-2 and HT-29MTX. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 135–140. ISBN 978-3-319-16104-4. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Sánchez-Juanes, F.; Alonso, J.M.; Zancada, L.; Hueso, P. Distribution and Fatty Acid Content of Phospholipids from Bovine Milk and Bovine Milk Fat Globule Membranes. Int. Dairy J. 2009, 19, 273–278. [Google Scholar] [CrossRef]
- Fong, B.Y.; Norris, C.S.; MacGibbon, A.K.H. Protein and Lipid Composition of Bovine Milk-Fat-Globule Membrane. Int. Dairy J. 2007, 17, 275–288. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinform. 2016, 54, 1.30.1–1.30.33. [Google Scholar] [CrossRef] [PubMed]
- Piñero, J.; Ramírez-Anguita, J.M.; Saüch-Pitarch, J.; Ronzano, F.; Centeno, E.; Sanz, F.; Furlong, L.I. The DisGeNET Knowledge Platform for Disease Genomics: 2019 Update. Nucleic Acids Res. 2020, 48, D845–D855. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; van der Mei, H.C.; Busscher, H.J.; Peterson, B.W. Two-Stage Interpretation of Changes in TEER of Intestinal Epithelial Layers Protected by Adhering Bifidobacteria During E. Coli Challenges. Front. Microbiol. 2020, 11, 599555. [Google Scholar] [CrossRef]
- Jiang, R.; Du, X.; Brink, L.; Lönnerdal, B. The Role of Orally Ingested Milk Fat Globule Membrane on Intestinal Barrier Functions Evaluated with a Suckling Rat Pup Supplementation Model and a Human Enterocyte Model. J. Nutr. Biochem. 2022, 108, 109084. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER Measurement Techniques for in Vitro Barrier Model Systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef]
- Wang, L.; Llorente, C.; Hartmann, P.; Yang, A.-M.; Chen, P.; Schnabl, B. Methods to Determine Intestinal Permeability and Bacterial Translocation during Liver Disease. J. Immunol. Methods 2015, 421, 44–53. [Google Scholar] [CrossRef]
- Zarepour, M.; Bhullar, K.; Montero, M.; Ma, C.; Huang, T.; Velcich, A.; Xia, L.; Vallance, B.A. The Mucin Muc2 Limits Pathogen Burdens and Epithelial Barrier Dysfunction during Salmonella Enterica Serovar Typhimurium Colitis. Infect. Immun. 2013, 81, 3672–3683. [Google Scholar] [CrossRef] [PubMed]
- Sommer, K.M.; Lee, Y.; Donovan, S.M.; Dilger, R.N. Purification Methods to Reduce Interference by Dextran Sodium Sulfate with Quantification of Gene Expression in Intestinal Tissue Samples from a Piglet Model of Colitis. J. Anim. Sci. 2023, 101, skad202. [Google Scholar] [CrossRef] [PubMed]
- Lesuffleur, T.; Porchet, N.; Aubert, J.-P.; Swallow, D.; Gum, J.R.; Real, F.X.; Zweibaum, A. Differential Expression of the Human Mucin Genes MUC1 to MUC5 in Relation to Growth and Differentiation of Different Mucus-Secreting HT-29 Cell Subpopulations. J. Cell Sci. 1993, 106, 771–783. [Google Scholar] [CrossRef]
- Dhanisha, S.S.; Guruvayoorappan, C.; Drishya, S.; Abeesh, P. Mucins: Structural Diversity, Biosynthesis, Its Role in Pathogenesis and as Possible Therapeutic Targets. Crit. Rev. Oncol./Hematol. 2018, 122, 98–122. [Google Scholar] [CrossRef]
- Breugelmans, T.; Van Spaendonk, H.; De Man, J.G.; De Schepper, H.U.; Jauregui-Amezaga, A.; Macken, E.; Lindén, S.K.; Pintelon, I.; Timmermans, J.-P.; De Winter, B.Y.; et al. In-Depth Study of Transmembrane Mucins in Association with Intestinal Barrier Dysfunction During the Course of T Cell Transfer and DSS-Induced Colitis. J. Crohn’s Colitis 2020, 14, 974–994. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, N.; Jin, C.; Long, M.D.; Rajabi, H.; Yasumizu, Y.; Fushimi, A.; Yamashita, N.; Hagiwara, M.; Zheng, R.; et al. MUC1-C Drives Stemness in Progression of Colitis to Colorectal Cancer. JCI Insight 2020, 5, e137112. [Google Scholar] [CrossRef] [PubMed]
- Patergnani, S.; Danese, A.; Bouhamida, E.; Aguiari, G.; Previati, M.; Pinton, P.; Giorgi, C. Various Aspects of Calcium Signaling in the Regulation of Apoptosis, Autophagy, Cell Proliferation, and Cancer. Int. J. Mol. Sci. 2020, 21, 8323. [Google Scholar] [CrossRef] [PubMed]
- Birchenough, G.M.H.; Johansson, M.E.; Gustafsson, J.K.; Bergström, J.H.; Hansson, G.C. New Developments in Goblet Cell Mucus Secretion and Function. Mucosal Immunol. 2015, 8, 712–719. [Google Scholar] [CrossRef]
- Huang, X.; Gao, Y.; Li, S.; Wu, C.; Wang, J.; Zheng, N. Modulation of Mucin (MUC2, MUC5AC and MUC5B) mRNA Expression and Protein Production and Secretion in Caco-2/HT29-MTX Co-Cultures Following Exposure to Individual and Combined Aflatoxin M1 and Ochratoxin A. Toxins 2019, 11, 132. [Google Scholar] [CrossRef]
- Malmberg, E.K.; Noaksson, K.A.; Phillipson, M.; Johansson, M.E.V.; Hinojosa-Kurtzberg, M.; Holm, L.; Gendler, S.J.; Hansson, G.C. Increased Levels of Mucins in the Cystic Fibrosis Mouse Small Intestine, and Modulator Effects of the Muc1 Mucin Expression. Am. J. Physiol.-Gastrointest. Liver Physiol. 2006, 291, G203–G210. [Google Scholar] [CrossRef]
- Petersson, J.; Schreiber, O.; Hansson, G.C.; Gendler, S.J.; Velcich, A.; Lundberg, J.O.; Roos, S.; Holm, L.; Phillipson, M. Importance and Regulation of the Colonic Mucus Barrier in a Mouse Model of Colitis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2011, 300, G327–G333. [Google Scholar] [CrossRef] [PubMed]
- Olli, K.E.; Rapp, C.; O’Connell, L.; Collins, C.B.; McNamee, E.N.; Jensen, O.; Jedlicka, P.; Allison, K.C.; Goldberg, M.S.; Gerich, M.E.; et al. Muc5ac Expression Protects the Colonic Barrier in Experimental Colitis. Inflamm. Bowel Dis. 2020, 26, 1353–1367. [Google Scholar] [CrossRef] [PubMed]
- Van der Sluis, M.; De Koning, B.A.E.; De Bruijn, A.C.J.M.; Velcich, A.; Meijerink, J.P.P.; Van Goudoever, J.B.; Büller, H.A.; Dekker, J.; Van Seuningen, I.; Renes, I.B.; et al. Muc2-Deficient Mice Spontaneously Develop Colitis, Indicating That MUC2 Is Critical for Colonic Protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, Å.; Duan, R.-D.; Ohlsson, L. Digestion and Absorption of Milk Phospholipids in Newborns and Adults. Front. Nutr. 2021, 8, 724006. [Google Scholar] [CrossRef]
- Nilsson, Å.; Duan, R.-D. Pancreatic and Mucosal Enzymes in Choline Phospholipid Digestion. Am. J. Physiol.-Gastrointest. Liver Physiol. 2019, 316, G425–G445. [Google Scholar] [CrossRef] [PubMed]
- Kosmerl, E.; Martínez-Sánchez, V.; Calvo, M.V.; Jiménez-Flores, R.; Fontecha, J.; Pérez-Gálvez, A. Food Matrix Impacts Bioaccessibility and Assimilation of Acid Whey-Derived Milk Fat Globule Membrane Lipids in Caco-2 Cells. Front. Nutr. 2023, 10, 1177152. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.a.T.; Gomes, F.; Vaz, W.L.C.; Moreno, M.J. Binding of Phospholipids to Beta-Lactoglobulin and Their Transfer to Lipid Bilayers. Biochim. Biophys. Acta 2008, 1778, 1308–1315. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mandalari, G.; Mackie, A.M.; Rigby, N.M.; Wickham, M.S.J.; Mills, E.N.C. Physiological Phosphatidylcholine Protects Bovine Beta-Lactoglobulin from Simulated Gastrointestinal Proteolysis. Mol. Nutr. Food Res. 2009, 53 (Suppl. S1), S131–S139. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, X.; Han, D.; Pi, Y.; Tao, S.; Zhang, S.; Wang, S.; Zhao, J.; Chen, L.; Wang, J. Early Life Administration of Milk Fat Globule Membrane Promoted SCFA-Producing Bacteria Colonization, Intestinal Barriers and Growth Performance of Neonatal Piglets. Anim. Nutr. 2021, 7, 346–355. [Google Scholar] [CrossRef]
- Gong, H.; Yuan, Q.; Pang, J.; Li, T.; Li, J.; Zhan, B.; Chang, R.; Mao, X. Dietary Milk Fat Globule Membrane Restores Decreased Intestinal Mucosal Barrier Development and Alterations of Intestinal Flora in Infant-Formula-Fed Rat Pups. Mol. Nutr. Food Res. 2020, 64, 2000232. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Ye, H.; Feng, C.; Han, D.; Tao, S.; Pi, Y.; Zhao, J.; Chen, L.; Wang, J. Dietary Milk Fat Globule Membrane Supplementation during Late Gestation Increased the Growth of Neonatal Piglets by Improving Their Plasma Parameters, Intestinal Barriers, and Fecal Microbiota. RSC Adv. 2020, 10, 16987–16998. [Google Scholar] [CrossRef] [PubMed]
- Stremmel, W.; Ehehalt, R.; Staffer, S.; Stoffels, S.; Mohr, A.; Karner, M.; Braun, A. Mucosal Protection by Phosphatidylcholine. Dig. Dis. 2012, 30 (Suppl. S3), 85–91. [Google Scholar] [CrossRef] [PubMed]
- Bootman, M.D.; Bultynck, G. Fundamentals of Cellular Calcium Signaling: A Primer. Cold Spring Harb. Perspect. Biol. 2020, 12, a038802. [Google Scholar] [CrossRef] [PubMed]

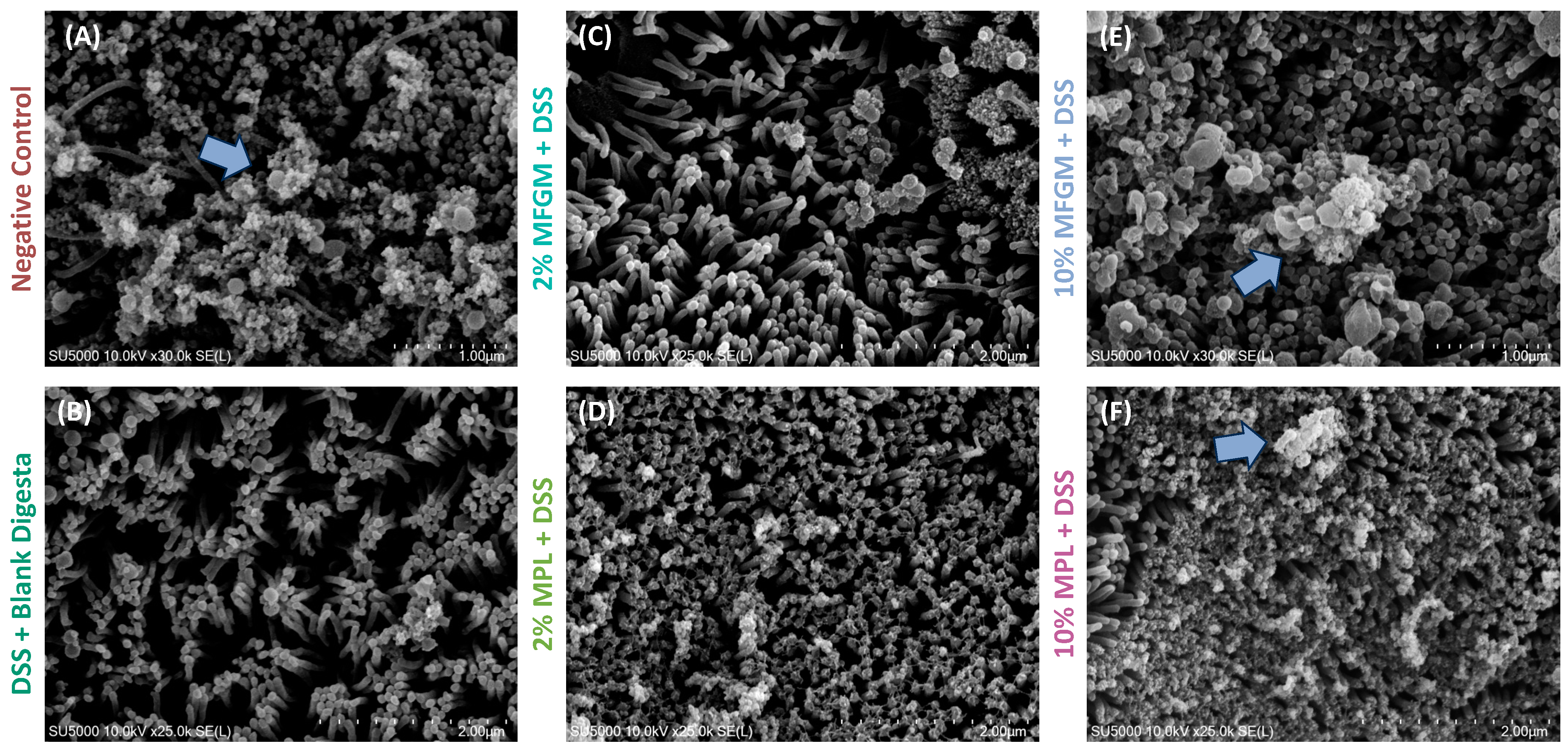

| Negative Control | DSS + Blank Digesta | 2% MFGM + DSS | 2% MPL + DSS | 10% MFGM + DSS | 10% MPL + DSS | |
|---|---|---|---|---|---|---|
| % Coverage | 86.6 ± 2.04 | 71.2 ± 2.03 | 78.2 ± 6.74 | 88.9 ± 0.714 | 99.3 ± 0.501 | 99.8 ± 0.087 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosmerl, E.; Miller, C.; Jiménez-Flores, R. Preventative Effects of Milk Fat Globule Membrane Ingredients on DSS-Induced Mucosal Injury in Intestinal Epithelial Cells. Nutrients 2024, 16, 954. https://doi.org/10.3390/nu16070954
Kosmerl E, Miller C, Jiménez-Flores R. Preventative Effects of Milk Fat Globule Membrane Ingredients on DSS-Induced Mucosal Injury in Intestinal Epithelial Cells. Nutrients. 2024; 16(7):954. https://doi.org/10.3390/nu16070954
Chicago/Turabian StyleKosmerl, Erica, Celeste Miller, and Rafael Jiménez-Flores. 2024. "Preventative Effects of Milk Fat Globule Membrane Ingredients on DSS-Induced Mucosal Injury in Intestinal Epithelial Cells" Nutrients 16, no. 7: 954. https://doi.org/10.3390/nu16070954
APA StyleKosmerl, E., Miller, C., & Jiménez-Flores, R. (2024). Preventative Effects of Milk Fat Globule Membrane Ingredients on DSS-Induced Mucosal Injury in Intestinal Epithelial Cells. Nutrients, 16(7), 954. https://doi.org/10.3390/nu16070954








