Ketone Monoester Followed by Carbohydrate Ingestion after Glycogen-Lowering Exercise Does Not Improve Subsequent Endurance Cycle Time Trial Performance
Highlights
- Ketone ester + carbohydrate (KME+CHO) ingestion increased circulating blood ketones (β-hydroxybutyrate) and insulin concentrations during recovery, but it did not enhance performance in the subsequent time trials.
- KME+CHO ingestion resulted only in a modest increase in blood glucose concentrations relative to the same amount of CHO alone.
- KME+CHO ingestion may induce a greater insulinotropic response, which could enhance glycogen resynthesis, although no performance differences were seen in this study when compared to CHO alone.
- KME may be used as a potential therapeutic agent in the treatment of type 2 diabetics given the effect it may have on blood glucose.
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Preliminary Sessions
2.4. Experimental Sessions
2.5. Time Trials (TT20km and TT5km)
2.6. Statistical Analysis
3. Results
3.1. Blood Ketones (Beta-Hydroxybutyrate—βHB)
3.2. Blood Glucose
3.3. Blood Insulin
3.4. Measures of Exercise Performance
Time Trials (TT20km and TT5km)
4. Discussion
5. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeukendrup, A.E. Carbohydrate intake during exercise and performance. Nutrition 2004, 20, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Cermak, N.M.; Van Loon, L.J.C. The use of carbohydrates during exercise as an ergogenic aid. Sports Med. 2013, 43, 1139–1155. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Leckey, J.J. Carbohydrate dependence during prolonged, intense endurance exercise. Sports Med. 2015, 45, S5–S12. [Google Scholar] [CrossRef] [PubMed]
- Saltin, B. Metabolic fundamentals in exercise. Med. Sci. Sports 1973, 5, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Krustrup, P.; Mohr, M.; Steensberg, A.; Bencke, J.; Kjaer, M.; Bangsbo, J. Muscle and blood metabolites during a soccer game: Implications for sprint performance. Med. Sci. Sports Exerc. 2006, 38, 1165–1174. [Google Scholar] [CrossRef]
- Coyle, E.; Coggan, A.; Hemmert, M.K.; Ivy, J.L. Muscle glycogen utilization during prolonged strenuous exercise when fed carbohydrate. J. Appl. Physiol. 1986, 61, 165–172. [Google Scholar] [CrossRef]
- Berardi, J.M.; Noreen, E.E.; Lemon, P.W.R. Recovery from a cycling time trial is enhanced with carbohydrate- protein supplementation vs. isoenergetic carbohydrate supplementation. Sports Nutr. Rev. J. 2008, 5, 24. [Google Scholar] [CrossRef]
- Upshaw, A.U.; Wong, T.S.; Bandegan, A.; Lemon, P.W.R. Cycling time trial performance 4 hours after glycogen-lowering exercise is similarly enhanced by recovery nondairy chocolate beverages versus chocolate milk. Int. J. Sport. Nutr. Exerc. Metab. 2016, 26, 65–70. [Google Scholar] [CrossRef]
- Van Loon, L.J.C.; Saris, W.H.M.; Kruijshoop, M.; Wagenmakers, A.J.M. Maximizing postexercise muscle glycogen synthesis: Carbohydrate supplementation and the application of amino acid or protein hydrolysate mixtures. Am. J. Clin. Nutr. 2000, 72, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Laffel, L. Ketone bodies: A review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab. Res. Rev. 1999, 15, 412–426. [Google Scholar] [CrossRef]
- Puchalska, P.; Crawford, P.A. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef]
- Robinson, A.M.; Williamson, D.H. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol. Rev. 1980, 60, 143–187. [Google Scholar] [CrossRef]
- Cox, P.J.; Kirk, T.; Ashmore, T.; Willerton, K.; Evans, R.; Smith, A.; Murray, A.J.; Stubbs, B.; West, J.; McLure, S.W.; et al. Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab. 2016, 24, 256–268. [Google Scholar] [CrossRef]
- Dearlove, D.J.; Harrison, O.K.; Hodson, L.; Jefferson, A.; Clarke, K.; Cox, P.J. The effect of blood ketone concentration and exercise intensity on exogenous ketone oxidation rates in athletes. Med. Sci. Sports Exerc. 2021, 53, 505–516. [Google Scholar] [CrossRef]
- Newman, J.C.; Verdin, E. Ketone bodies as signaling metabolites. Trends Endocrinol. Metab. 2014, 25, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Vandoorne, T.; De Smet, S.; Ramaekers, M.; Van Thienen, R.; De Bock, K.; Clarke, K.; Hespel, P. Intake of a ketone ester drink during recovery from exercise promotes mtorc1 signaling but not glycogen resynthesis in human muscle. Front. Physiol. 2017, 8, 310. [Google Scholar] [CrossRef] [PubMed]
- Rittig, N.; Svart, M.; Thomsen, H.H.; Vestergaard, E.T.; Rehfeld, J.F.; Hartmann, B.; Holst, J.J.; Johannsen, M.; Møller, N.; Jessen, N. Oral D/L-3-Hydroxybutyrate stimulates cholecystokinin and insulin secretion and slows gastric emptying in healthy males. J. Clin. Endocrinol. Metab. 2020, 10, e3597–e3605. [Google Scholar] [CrossRef] [PubMed]
- Maizels, E.Z.; Ruderman, N.B.; Goodman, N.M.; Lau, D. Effect of acetoacetate on glucose metabolism in the soleus and extensor digitorum longus muscles of the rat. Biochem. J. 1977, 162, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, M.R.; Taylor, J.; Chesnick, A.S.; Balaban, R.S. Nonglucose substrates increase glycogen synthesis in vivo in dog heart. Am. J. Physiol. 1994, 267, H217–H223. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Terada, S.; Banjo, M.; Seike, K.; Nakano, S.; Hatta, H. Effects of B-hydroxybutyrate treatment on glycogen repletion and its related signaling cascades in epitrochlearis muscle during 120 min of postexercise recovery. Appl. Physiol. Nutr. Metab. 2019, 44, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Holdsworth, D.A.; Cox, P.J.; Kirk, T.; Stradling, H.; Impey, S.G.; Clarke, K. A ketone ester drink increases postexercise muscle glycogen synthesis in humans. Med. Sci. Sports Exerc. 2017, 49, 1789–1795. [Google Scholar] [CrossRef]
- Thomas, S.; Reading, J.; Shephard, R.J. Revision of the physical activity readiness questionnaire (PAR-Q). Can. J. Sport. Sci. 1992, 17, 338–345. [Google Scholar] [PubMed]
- Noreen, E.E.; Lemon, P.W.R. Reliability of air displacement plethysmography in a large, heterogeneous sample. Med. Sci. Sports Exerc. 2006, 38, 1505–1509. [Google Scholar] [CrossRef] [PubMed]
- Keizer, H.A.; Kulpers, G.; Van Kranenburg, G. Influence of liquid and solid meals on muscle glycogen resynthesis, plasma fuel hormone response, and maximal physical working capacity. Int. J. Sports Med. 1987, 8, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Biden, T.J.; Taylor, K.W. Effects of ketone bodies on insulin release and islet-cell metabolism in the rat. Biochem. J. 1983, 212, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Duchman, S.M.; Ryan, A.J.; Schedl, H.P.; Summers, R.W.; Bleiler, T.L.; Gisolfi, C.V. Upper limit for intestinal absorption of a dilute glucose solution in men at rest. Med. Sci. Sports Exerc. 1997, 29, 482–488. [Google Scholar] [CrossRef]
- Cox, G.R.; Clark, S.A.; Cox, A.J.; Halson, S.L.; Hargreaves, M.; Hawley, J.A.; Jeacocke, N.; Snow, R.J.; Yeo, W.K.; Burke, L.M. Daily training with high carbohydrate availability increases exogenous carbohydrate oxidation during endurance cycling. J. Appl. Physiol. 2010, 109, 126–134. [Google Scholar] [CrossRef]
- Jeukendrup, A.E. Training the gut for athletes. Sports Med. 2017, 47, 101–110. [Google Scholar] [CrossRef]
- Evans, M.; Egan, B. Intermittent running and cognitive performance after ketone ester ingestion. Med. Sci. Sports Exerc. 2018, 50, 2330–2338. [Google Scholar] [CrossRef]
- Dearlove, D.J.; Faull, O.K.; Rolls, E.; Clarke, K.; Cox, P.J. Nutritional ketoacidosis during incremental exercise in healthy athletes. Front. Physiol. 2019, 10, 290. [Google Scholar] [CrossRef]
- Miles, J.M.; Haymond, M.W.; Gerich, J.E. Suppression of glucose production and stimulation of insulin secretion by physiological concentrations of ketone bodies in man. J. Clin. Endocrinol. Metab. 1981, 52, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, K.H.; Seifert, T.; Secher, N.H.; Grondal, T.; Van Hall, G. Systemic, cerebral and skeletal muscle ketone body and energy metabolism during acute hyper-D-beta-hydroxybutyratemia in post-absorptive healthy males. J. Clin. Endocrinol. Metab. 2015, 100, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Soto-Mota, A.; Norwitz, N.G.; Evans, R.; Clarke, K.; Barber, T.M. Exogenous ketosis in patients with type 2 diabetes: Safety, tolerability and effect on glycaemic control. Endocrinol. Diabetes Metab. J. 2021, 4, e00264. [Google Scholar] [CrossRef] [PubMed]

 = treatment ingestion (KME or PLAC).
= treatment ingestion (KME or PLAC).  = CHO drink. βHB = beta-hydroxybutyrate.
= CHO drink. βHB = beta-hydroxybutyrate.
 = treatment ingestion (KME or PLAC).
= treatment ingestion (KME or PLAC).  = CHO drink. βHB = beta-hydroxybutyrate.
= CHO drink. βHB = beta-hydroxybutyrate.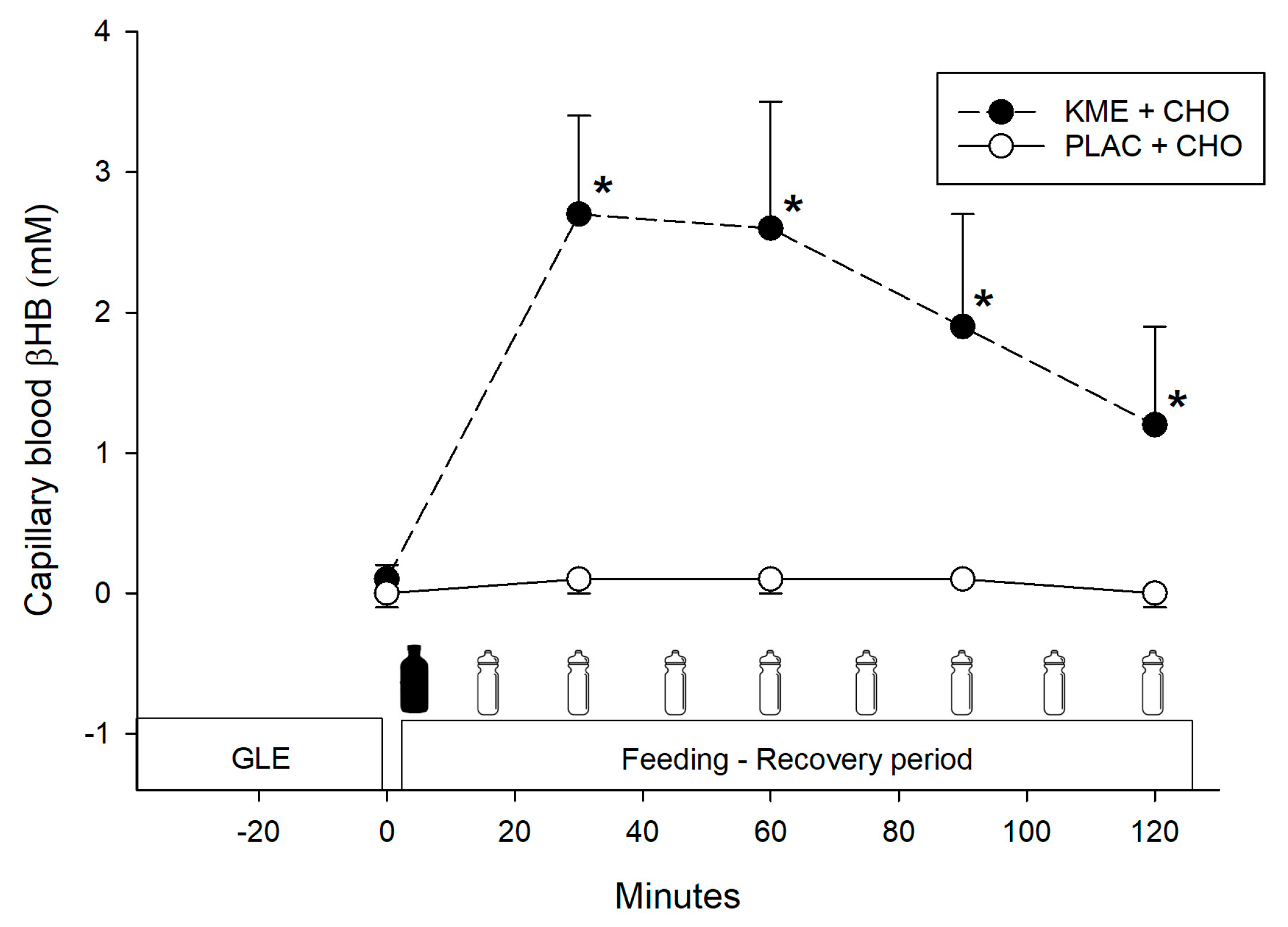
 = treatment ingestion (KME or PLAC).
= treatment ingestion (KME or PLAC).  = CHO drink.
= CHO drink.
 = treatment ingestion (KME or PLAC).
= treatment ingestion (KME or PLAC).  = CHO drink.
= CHO drink.
 = treatment ingestion (KME or PLAC).
= treatment ingestion (KME or PLAC).  = CHO drink.
= CHO drink.
 = treatment ingestion (KME or PLAC).
= treatment ingestion (KME or PLAC).  = CHO drink.
= CHO drink.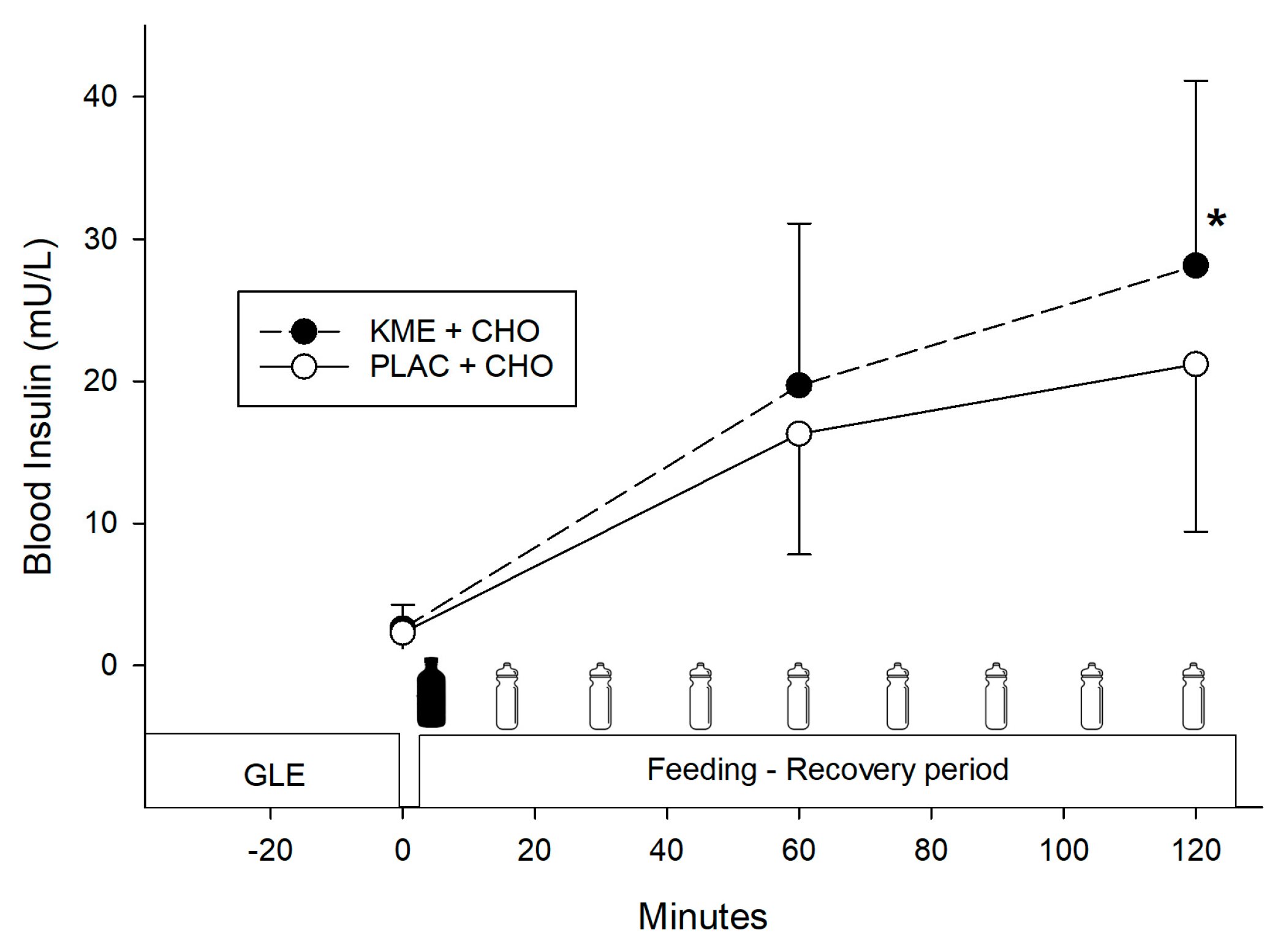
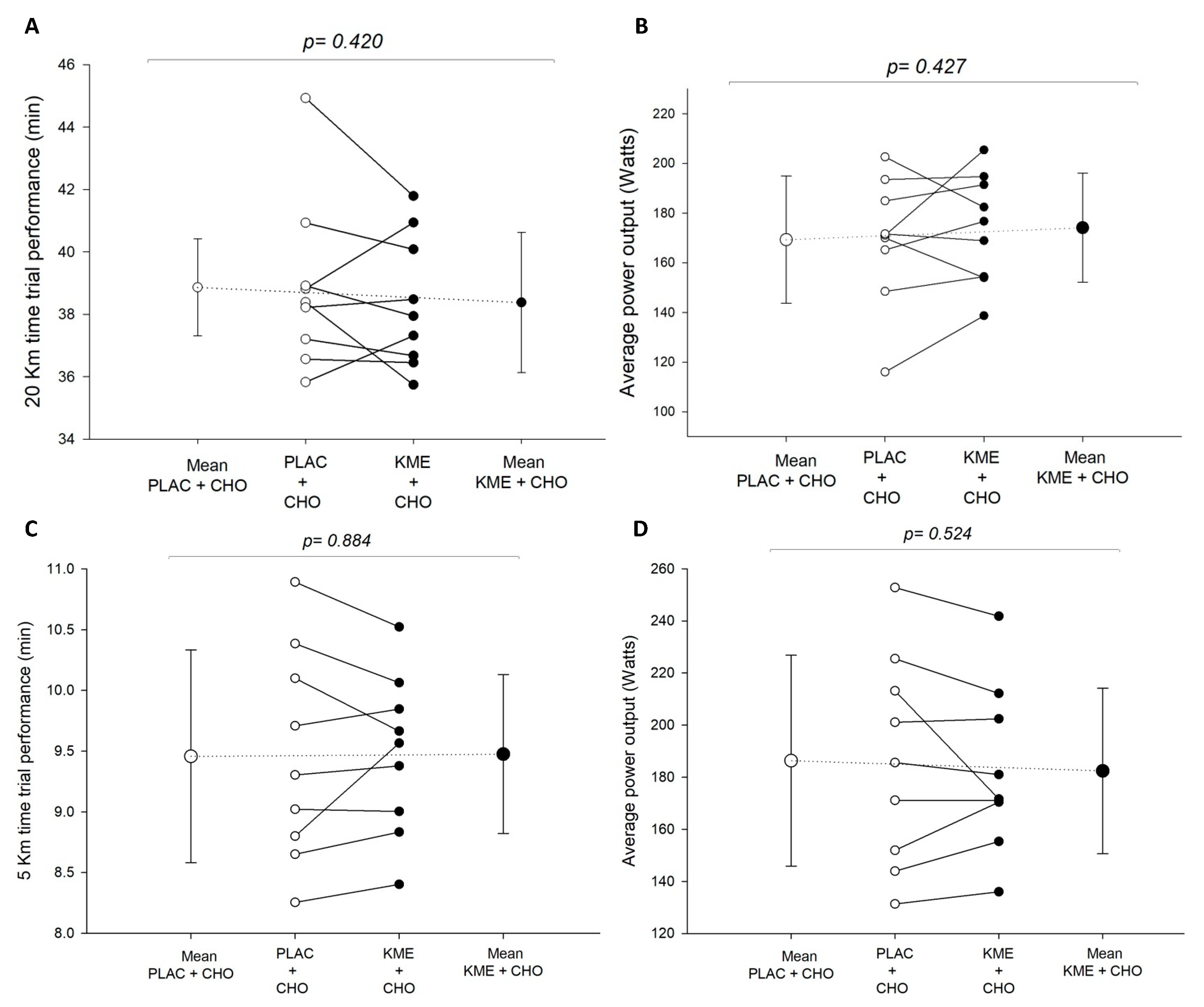
| Characteristics | Value |
|---|---|
| Height, cm | 175.6 ± 5.3 |
| Body mass, kg | 72.9 ± 7.7 |
| Age, y | 28.0 ± 5.0 |
| Body fat a, % | 12.2 ± 3.2 |
| Wmax b | 314.6 ± 42.3 |
| VO2max c, mL·kg−1·min−1 | 56.2 ± 5.8 |
| Intakes d | |
| Energy, kcal·kg−1·d−1 | 32.2 ± 3.5 |
| Carbohydrate, g·kg−1·d−1 | 3.5 ± 0.4 |
| Protein, g·kg−1·d−1 | 1.3 ± 0.3 |
| Fat, g·kg−1·d−1 | 1.6 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quinones, M.D.; Weiman, K.; Lemon, P.W.R. Ketone Monoester Followed by Carbohydrate Ingestion after Glycogen-Lowering Exercise Does Not Improve Subsequent Endurance Cycle Time Trial Performance. Nutrients 2024, 16, 932. https://doi.org/10.3390/nu16070932
Quinones MD, Weiman K, Lemon PWR. Ketone Monoester Followed by Carbohydrate Ingestion after Glycogen-Lowering Exercise Does Not Improve Subsequent Endurance Cycle Time Trial Performance. Nutrients. 2024; 16(7):932. https://doi.org/10.3390/nu16070932
Chicago/Turabian StyleQuinones, Manuel D., Kyle Weiman, and Peter W. R. Lemon. 2024. "Ketone Monoester Followed by Carbohydrate Ingestion after Glycogen-Lowering Exercise Does Not Improve Subsequent Endurance Cycle Time Trial Performance" Nutrients 16, no. 7: 932. https://doi.org/10.3390/nu16070932
APA StyleQuinones, M. D., Weiman, K., & Lemon, P. W. R. (2024). Ketone Monoester Followed by Carbohydrate Ingestion after Glycogen-Lowering Exercise Does Not Improve Subsequent Endurance Cycle Time Trial Performance. Nutrients, 16(7), 932. https://doi.org/10.3390/nu16070932





