Abstract
The lipid accumulation product (LAP) is a reliable marker of metabolic syndrome, which includes conditions like obesity. However, the correlation between the circulating selenium (CSe) concentration and the LAP is currently unclear. This study aimed to ascertain this correlation. Overall, 12,815 adults aged ≥20 years were enrolled in this study. After adjusting for all the confounding variables, CSe was positively correlated to the LAP (β = 0.41; 95% confidence interval [CI]: 0.28, 0.54; p < 0.001). Compared with the lowest quartile of CSe, the highest quartile of CSe was positively related to the LAP (β = 0.16; 95% CI: 0.12, 0.21; p < 0.001). Moreover, the correlation between CSe and the LAP revealed a positive non-linear trend. In the subgroup analysis, interaction effects were observed for age, sex, smoking, and stroke (p for interaction < 0.05). The effects were stronger for males (β = 0.64, 95% CI: 0.47, 0.80; p < 0.001) and individuals who smoke at the time of the trial (β = 0.64, 95% CI: 0.37, 0.91; p < 0.001). In conclusion, our results indicated that CSe was positively correlated with the LAP in a non-linear manner. Future research is warranted to explore their relationship and better understand the mechanisms underlying this association.
1. Introduction
Obesity is a global problem [1,2], contributing to the incidence and mortality of a range of diseases, including hypertension, coronary heart disease, type 2 diabetes mellitus (T2DM), dyslipidemia, cerebrovascular accidents, and cancer [3,4,5,6]. It also results in a substantial increase in healthcare costs. However, to date, no country has implemented a successful public health model to reduce the prevalence of obesity, despite continued efforts to do so [7]. In light of this, tackling obesity has become a global health priority.
Obesity is characterized by the growth of adipose cells and the enlargement of adipose tissue [8]. Body mass index (BMI) is a conventional, simple measurement that is applied to assess relative weight. However, BMI is neither specific for adipose or lean tissues, nor is it a reliable predictor of cardiovascular events or mortality [9,10]. The lipid accumulation product (LAP) is calculated from the waist circumference (WC) and triglyceride (TG), and is a reliable marker of metabolic syndrome, which encompasses obesity [11]. Furthermore, the LAP is superior to BMI in distinguishing cardiovascular risk and T2DM [11,12].
Selenium (Se), an essential trace element and constituent of selenoproteins, plays many vital physiological roles, including antioxidation, anti-inflammation, anti-aging, energy metabolism, immune regulation, redox signaling, cellular differentiation, protein folding, and gene expression [13,14,15,16,17,18,19,20,21,22]. Oxidative stress (OS), inflammation, endocrine function dysfunction, and energy metabolism disorders are key in the pathogenesis of obesity [8,23,24,25]. Thus, it is hypothesized that Se may influence obesity levels. Notably, a previous meta-analysis, including 65 articles, highlighted that the relationship between Se and being overweight or obese was controversial [26]. Furthermore, to date, the association between the circulating selenium (CSe) concentration and LAP has not been explored. Hence, based on the National Health and Nutrition Examination Survey (NHANES), this research aimed to assess the correlation between CSe and the LAP.
2. Materials and Methods
2.1. Study Population
The NHANES was conducted in order to make a health evaluation for all Americans [27]. The NHANES project was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of the National Center for Health Statistics (NCHS) (protocol code: 2011-17, date of approval 2011; protocol code: 2018-01, date of approval 2018). A written notice was submitted to all adult individuals. The survey utilized open-access data from the NHANES. A secondary analysis in our study was based on the NHANES. The data analyzed in our study combined four survey cycles from the NHANES (2011–2012, 2013–2014, 2015–2016, and 2017–2018). After screening the data of 39,156 participants, 26,341 participants were eliminated due to the following reasons: Age < 20 years (n = 16,539), cancer (n = 2184), pregnancy (n = 245), missing CSe (n = 6423), missing WC (n = 684), and missing TG (n = 266). A total of 12,815 participants were eligible for inclusion and therefore incorporated into the analyses (Figure 1).
Figure 1.
Flow diagram of screening for individuals. NHANES, National Health and Nutrition Examination Survey; CSe, circulating selenium; WC, waist circumference; TG, triglyceride.
2.2. Acquisition of Variables
Age, sex, race, education, family poverty ratio of income (FPRI), marital status, and pregnancy status were acquired from the demographic data. The BMI, WC, and blood pressure were retrieved from the examination data. A history of various self-reported diseases, medication use status, alcohol consumption, and smoking status were acquired from the questionnaire data. Serum lipid, fasting plasma glucose, hemoglobin A1c (HbA1c), and whole blood Se levels were retrieved from laboratory data. More detailed information is available from the NHANES.
2.3. Case Definition
Hypertension was defined using the following criteria: Self-reported hypertension; diagnosed by a physician; patients taking antihypertensive medication; an average systolic blood pressure (SBP) of ≥130 mmHg; and/or diastolic blood pressure (DBP) of ≥80 mmHg [28]. T2DM was defined as follows: a diagnosis of diabetes mellitus; taking hypoglycemic medications or using insulin; HbA1c ≥ 6.5%; fasting plasma glucose ≥ 7.0 mmol/L; and/or a 2 h plasma glucose ≥ 11.1 mmol/L [29]. Stroke or coronary heart disease was defined as a self-reported stroke, or a coronary heart disease as diagnosed by a physician.
2.4. Lipid Accumulation Product Index Calculation
The LAP index was calculated from the WC and TG using the following formula [11]:
LAP (males) = (WC [cm] − 65) × TG (mmol/L)
LAP (females) = (WC [cm] − 58) × TG (mmol/L)
2.5. Covariates Assessment
Covariates included age (≤44 [young], 45–59 [middle-aged], ≥60 years [old]) [30], sex (males, females), race (Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, others), FPRI (<1, 1–3, >3, unavailable [Un]), education (<high school, high school, >high school, Un), BMI (<25, 25–30, ≥30 kg/m2, Un) [31], alcohol consumption (≤3, >3 drinks/day, Un), total cholesterol (TC) (<6.22, ≥6.22 mmol/L, Un) [32], TG (<2.26, ≥2.26 mmol/L, Un) [32], glucose (<6.1, 6.1–7.0, ≥7.0 mmol/L, Un) [29], diastolic blood pressure (DBP) (<80, ≥80 mmHg, Un) [28], systolic blood pressure (SBP) (<130, ≥130 mmHg, Un) [28], marital status (married, never married, others, Un), hypertension, T2DM (no, yes), stroke, current smoking status, coronary heart disease, current taking of hypotensive drugs, and current injection of insulin (no, yes, Un).
2.6. Statistical Analysis
Regarding the NHANES guidelines, the statistical analysis adopted suitable sampling weights. Categorical variables are presented as numbers (%), whereas continuous variables are presented as medians (interquartile range) for skewed distributions. Differences were calculated via applying the chi-square test for categorical variables and the rank sum test for continuous variables. The potential confounders were explored using univariate linear regression analysis. After adjusting for multiple factors in different models, multivariate linear regression analyses were used to analyze the independent correlation between CSe and the LAP. Covariates with p < 0.05 in univariate analysis were included in the multivariate analysis as adjusting confounders. Three different models were adopted to verify independent correlations according to the guidelines of the STROBE statement. Model A was not adjusted for any variables. Model B was adjusted for age, sex, and race. Model C was adjusted for age, sex, race, education, marital status, alcohol consumption, BMI, hypertension, T2DM, stroke, coronary heart disease, TC, glucose, SBP, DBP, current injection of insulin, and current taking of hypotensive drugs. Dose–response analysis was adopted to test linear or non-linear relationships after adjusting for the same variables in model C. Subgroup analyses were applied to explore the variable interactions. The natural logarithmic (Ln) transformation of CSe and the LAP was carried out due to its non-normal distribution.
All statistical analyses were performed using R (version 4.2.0) and EmpowerStats (version 5.0). p < 0.05 (two-sided) was deemed statistically significant.
3. Results
3.1. Baseline Characteristics
The participants were divided into three groups based on the LAP tertiles (Table 1). When compared with individuals in the low LAP, subjects in the high LAP were significantly more likely to be elderly, male, Mexican American, less educated, poor, married, and obese. They were also more likely to be currently injecting insulin or taking hypotensive drugs, or display symptoms of hypertension, T2DM, stroke, coronary heart disease, and higher levels of SBP, DBP, TC, glucose, and CSe (all p < 0.05).

Table 1.
Baseline characteristics of individuals based on the LAP tertiles.
3.2. Univariate Analysis
As shown in Table 2, the results of the univariate linear regression analysis demonstrated that age, alcohol consumption, hypertension, coronary heart disease, T2DM, stroke, glucose, TC, SBP, DBP, BMI, the taking of hypotensive drugs, and the injecting of insulin and Ln CSe were positively related to Ln LAP. In contrast, sex, race, education, and marital status were negatively correlated with Ln LAP (all p < 0.05). The FPRI and current smoking status were not associated with Ln LAP (p > 0.05).

Table 2.
Results of univariate linear regression analysis of each variable.
3.3. Multivariate Analysis
A multivariate linear regression analysis was carried out to detect the correlation between CSe and the LAP. In model A, which had no adjustments for variables, the correlation between CSe and the LAP was positive (β = 0.76, 95% confidence interval [CI]: 0.56, 0.95; p < 0.001). In model B, which was adjusted for age, sex, and race, the correlation between CSe and the LAP was also positive (β = 0.66; 95% CI: 0.47, 0.84; p < 0.001). In model C, which was adjusted for all significant variables in the univariate analysis, the correlation between CSe and the LAP was also positive (β = 0.41; 95% CI: 0.28, 0.54; p < 0.001). For sensitivity analysis, we also processed CSe as a categorical variable (quartiles), and a similar trend was observed (p for trend < 0.001), as shown in Table 3.

Table 3.
The detection of the independent relationship between Cse and the LAP using multivariate linear regression analysis.
3.4. Dose–Response Analysis
The dose–response analysis of the multivariate-adjusted linear regression was also performed (Figure 2). We found that the correlation between CSe and the LAP showed a positive non-linear manner in the log-likelihood ratio test (p = 0.003). In threshold effect analysis, when Ln CSe < 1.10, CSe was significantly positively related to the LAP (β = 0.45; 95% CI: 0.35, 0.55; p < 0.001). In contrast, when Ln CSe ≥ 1.10, CSe was not significantly positively related to the LAP (β = −0.13; 95% CI: −0.48, 0.21; p = 0.454) (Table 4).
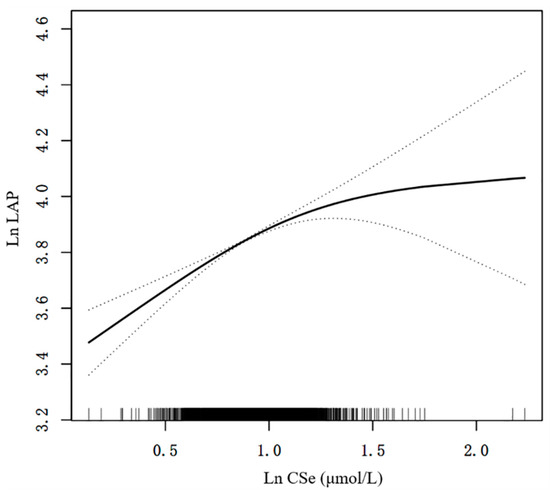
Figure 2.
A positive non-linear manner between CSe and the LAP. A positive non-linear manner was identified after adjusting for multiple confounders in model C. The solid and dashed lines describe the β value and 95% CI, respectively. CI, confidence interval; Ln, natural logarithmic; LAP, lipid accumulation product; CSe, circulating selenium.

Table 4.
The threshold effect analysis.
3.5. Subgroup Analysis
A subgroup analysis was adopted to explore variable interactions (Table 5). Interaction effects were observed for age, sex, current smoking status, and stroke (all p < 0.05 for interactions). Conversely, no significant interactions were observed for race, education, FPRI, BMI, T2DM, glucose, coronary heart disease, hypertension, SBP, DBP, marital status, alcohol consumption, TC, the current taking of hypotensive drugs, and current injections of insulin (all p > 0.05 for interactions). Stronger effects were found for males (β = 0.64, 95% CI: 0.47, 0.80; p < 0.001) and individuals who smoked at the time of the analysis (β = 0.64, 95% CI: 0.37, 0.91; p < 0.001).

Table 5.
Interaction effects in the subgroup analysis.
4. Discussion
As an indispensable trace element in the human body, Se plays an important role in antioxidation, anti-inflammation, anti-aging, energy metabolism regulation, etc. [13,14,15,16,17,18,19,20,21,22]. In nature, Se exists in two forms: inorganic Se and organic Se. Se is absorbed by the small intestine, and then distributed into various tissues of the body. After being absorbed by the small intestine, it is divided into various tissues of the body, which are then used to synthesize various selenoproteins that play important biological roles [14]. There are twenty-five types of human selenoproteins, all of which are very small, including five glutathione peroxidases (GPx), three thioredoxin reductases, three iodothyronine deiodinases, and others [20]. The synthesis of these selenoproteins requires the insertion of a Se-containing homolog of cysteine and 25 coding genes [13,14]. GPx1 is the most abundant selenoprotein in mammals, and it is an enzyme that is universally expressed in various cell types. Along with the consumption of reduced glutathione, GPx1 consumes reduced glutathione in order to convert lipid peroxides to their respective alcohols, and to convert H2O2 to water [17]. This physiological process is beneficial, as it alleviates oxidative damage to biomolecules such as lipids, lipoproteins, and DNA [17], in addition to maintaining membrane integrity, and reducing the related risks of various diseases [17]. Se can also intervene in energy metabolism by activating adipose tissue and regulating thyroid hormones [22].
To date, our analysis is the first to explore the correlation between CSe and the LAP. A positive non-linear correlation between CSe and the LAP was observed in our study. Moreover, the positive relationship between CSe and the LAP was more substantial in participants who were male and currently smoking. Previous literature has shown that the connection between Se levels and obesity is complex and contradictory. Previous observational research has demonstrated that the plasma Se content was negatively related to obesity among children [33]. In contrast, a case-control study of 847 adults reported that a high serum Se concentration was related to a high BMI [34]. A separate study in women revealed that hair Se levels increased in obese individuals [35]. However, a study on French adults reported that the serum Se content was not correlated with the BMI, but rather with serum cholesterol levels [36]. Furthermore, a previous NHANES study also reported that the Se dietary intake was unrelated to the BMI and WC [37]. Nevertheless, another study revealed a positive correlation between Se dietary intake and obesity in adults [38].
Not only are the results of observational studies inconsistent, but those of interventional studies are also. In animal models, the BMI of obese mice was reduced following the dietary selenomethionine intake, which facilitated the browning reaction [39]. However, a randomized prospective survey observed that the BMI was not changed, but there was a significant increase in lean muscle mass and a decrease in leptin levels after 3 months in participants taking oral 240 μg L of selenomethionine per day [40].
In the subgroup analysis, we observed that the connection between CSe and the LAP was influenced by age, sex, current smoking status, and stroke. Serum Se levels were higher in the older group [34]. It is known that differences in adipose distribution and proportions between males and females directly affect the evaluation of the LAP. In addition, lifestyle, behavior, and sex hormones differ between males and females [41,42]. The gene expression of selenoproteins differs between the sexes [43,44]. A Japanese study found a strong connection between the LAP and diabetes mellitus in both sexes [45]. The clinical features of diabetes differ between the sexes [46]. An American study reported that the whole blood Se concentration was higher in male non-smokers [47]. Our previous study found that CSe levels were negatively correlated with stroke [48], meaning that CSe levels were decreased in stroke patients. However, CSe was positively related to the LAP. This contrast amplified the relationship between CSe and the LAP, and made this relationship more significant.
This study has several strengths. First, this is the first analysis of the relationship between CSe and the LAP. Second, we found that CSe and the LAP were positively correlated in a non-linear manner. Third, the sample size of our study was relatively large.
Nevertheless, several additional limitations existed in this research. First, because our analysis was based on an observational survey, we can only draw a correlation, not a causal conclusion. Second, recall biases existed in our study due to some diseases identified based on self-reported diagnostic histories. This is despite the fact that self-reported diagnostic histories were consistent in medical records, particularly for stroke, hypertension, and diabetes mellitus [49]. Nonetheless, the individuals enrolled into our analysis were American adults. Thus, there may be inherent population bias, and further investigation is required to generalize our results to other populations.
5. Conclusions
In conclusion, our results indicated that CSe was positively correlated with the LAP in a non-linear manner. Future investigations are warranted to explore their relationship and better understand the mechanisms underlying this association.
Author Contributions
Conceptualization, Y.Z.; methodology, K.Z. and W.S.; analysis, K.Z.; writing—original draft preparation, K.Z.; writing—review and editing, Y.Z. and W.S.; funding acquisition, Y.Z. and W.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82000411, 82030051), the National Key R & D Program of China (2021YFF0501403), and the Key R & D Program of Shandong Province (ZR2020QH023, 2021SFGC0503, 2021ZDSYS05, 2020ZLYS05).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of the NCHS (protocol code: 2011-17, date of approval 2011; protocol code: 2018-01, date of approval 2018).
Informed Consent Statement
A written notice was submitted to all adult individuals before enrollment.
Data Availability Statement
The data are openly accessible via the NHANES and can be found here: https://www.cdc.gov/ (accessed on 12 March 2023).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Friedrich, M.J. Global obesity epidemic worsening. JAMA 2017, 318, 603. [Google Scholar] [CrossRef]
- Visscher, T.L.; Heitmann, B.L.; Rissanen, A.; Lahti-Koski, M.; Lissner, L. A break in the obesity epidemic? Explained by biases or misinterpretation of the data? Int. J. Obes. 2015, 39, 189–198. [Google Scholar] [CrossRef]
- Willett, W.C.; Dietz, W.H.; Colditz, G.A. Guidelines for healthy weight. N. Engl. J. Med. 1999, 341, 427–434. [Google Scholar] [CrossRef]
- Dong, Q.; Sidra, S.; Gieger, C.; Wang-Sattler, R.; Rathmann, W.; Prehn, C.; Adamski, J.; Koenig, W.; Peters, A.; Grallert, H.; et al. Metabolic signatures elucidate the effect of body mass index on type 2 diabetes. Metabolites 2023, 13, 227. [Google Scholar] [CrossRef] [PubMed]
- Field, A.E.; Coakley, E.H.; Must, A.; Spadano, J.L.; Laird, N.; Dietz, W.H.; Rimm, E.; Colditz, G.A. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch. Intern. Med. 2001, 161, 1581–1586. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, R.; Snarski, P.; King, A.N.; Ghimire, J.; Ruiz, E.; Lau, F.; Savkovic, S.D. Epiploic adipose tissue (EPAT) in obese individuals promotes colonic tumorigenesis: A novel model for EPAT-dependent colorectal cancer progression. Cancers 2023, 15, 977. [Google Scholar] [CrossRef] [PubMed]
- Swinburn, B.A.; Sacks, G.; Hall, K.D.; McPherson, K.; Finegood, D.T.; Moodie, M.L.; Gortmaker, S.L. The global obesity pandemic: Shaped by global drivers and local environments. Lancet 2011, 378, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Cruciani, S.; Delitala, A.P.; Cossu, M.L.; Ventura, C.; Maioli, M. Management of obesity and obesity-related disorders: From stem cells and epigenetics to its treatment. Int. J. Mol. Sci. 2023, 24, 2310. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Bautista, L.; Franzosi, M.G.; Commerford, P.; Lang, C.C.; Rumboldt, Z.; Onen, C.L.; Lisheng, L. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: A case-control study. Lancet 2005, 366, 1640–1649. [Google Scholar] [CrossRef] [PubMed]
- Romero-Corral, A.; Montori, V.M.; Somers, V.K.; Korinek, J.; Thomas, R.J.; Allison, T.G.; Mookadam, F.; Lopez-Jimenez, F. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: A systematic review of cohort studies. Lancet 2006, 368, 666–678. [Google Scholar] [CrossRef]
- Kahn, H.S. The “lipid accumulation product” performs better than the body mass index for recognizing cardiovascular risk: A population-based comparison. BMC Cardiovasc. Disord. 2005, 5, 26. [Google Scholar] [CrossRef]
- Kahn, H.S. The lipid accumulation product is better than BMI for identifying diabetes: A population-based comparison. Diabetes Care 2006, 29, 151–153. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Speckmann, B.; Klotz, L.O. Selenoproteins: Antioxidant selenoenzymes and beyond. Arch. Biochem. Biophys. 2016, 595, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Tan, H.Y.; Li, S.; Xu, Y.; Guo, W.; Feng, Y. Supplementation of micronutrient selenium in metabolic diseases: Its role as an antioxidant. Oxid. Med. Cell Longev. 2017, 2017, 7478523. [Google Scholar] [CrossRef] [PubMed]
- Handy, D.E.; Joseph, J.; Loscalzo, J. Selenium, a micronutrient that modulates cardiovascular health via redox enzymology. Nutrients 2021, 13, 3238. [Google Scholar] [CrossRef]
- Chen, J.; Feng, T.; Wang, B.; He, R.; Xu, Y.; Gao, P.; Zhang, Z.H.; Zhang, L.; Fu, J.; Liu, Z.; et al. Enhancing organic selenium content and antioxidant activities of soy sauce using nano-selenium during soybean soaking. Front. Nutr. 2022, 9, 970206. [Google Scholar] [CrossRef]
- Liu, H.; Xu, H.; Huang, K. Selenium in the prevention of atherosclerosis and its underlying mechanisms. Metallomics 2017, 9, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, A.; Casas, C.; Herrero, E. Selenite-induced cell death in saccharomyces cerevisiae: Protective role of glutaredoxins. Microbiology 2010, 156, 2608–2620. [Google Scholar] [CrossRef]
- Mangiapane, E.; Pessione, A.; Pessione, E. Selenium and selenoproteins: An overview on different biological systems. Curr. Protein Pept. Sci. 2014, 15, 598–607. [Google Scholar] [CrossRef]
- Gao, X.; Shan, P.; Feng, T.; He, P.; Ran, J.; Fu, J.; Zhou, C. Enhancing selenium and key flavor compounds contents in soy sauce using selenium-enriched soybean. J. Food Compos. Anal. 2022, 106, 104299. [Google Scholar] [CrossRef]
- Shimada, B.K.; Watanabe, L.M.; Swanson, S.; Toh, P.; Seale, L.A. Selenium and selenoproteins in thermogenic adipocytes. Arch. Biochem. Biophys. 2022, 731, 109445. [Google Scholar] [CrossRef] [PubMed]
- Davanzo, G.G.; Castro, G.; Monteiro, L.B.; Castelucci, B.G.; Jaccomo, V.H.; da Silva, F.C.; Marques, A.M.; Francelin, C.; de Campos, B.B.; de Aguiar, C.F.; et al. Obesity increases blood-brain barrier permeability and aggravates the mouse model of multiple sclerosis. Mult. Scler. Relat. Disord. 2023, 72, 104605. [Google Scholar] [CrossRef] [PubMed]
- Sonnefeld, L.; Rohmann, N.; Geisler, C.; Laudes, M. Is human obesity an inflammatory disease of the hypothalamus? Eur. J. Endocrinol. 2023, 188, R37–R45. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Xu, H.; Li, C.; Yang, H.; Mao, Y. Intermittent fasting and immunomodulatory effects: A systematic review. Front. Nutr. 2023, 10, 1048230. [Google Scholar] [CrossRef] [PubMed]
- Fontenelle, L.C.; Cardoso de Araújo, D.S.; da Cunha Soares, T.; Clímaco Cruz, K.J.; Henriques, G.S.; Marreiro, D.D.N. Nutritional status of selenium in overweight and obesity: A systematic review and meta-analysis. Clin. Nutr. 2022, 41, 862–884. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.L.; Paulose-Ram, R.; Ogden, C.L. National health and nutrition examination survey: Analytic guidelines, 1999–2010. Vital Health Stat. 2 2013, 161, 1–24. [Google Scholar]
- American Heart Association. Blood Pressure Categories 2018. Available online: https://www.health.harvard.edu/heart-health/reading-the-new-blood-pressure-guidelines (accessed on 10 May 2023).
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef]
- Ding, N.; He, L.; Li, C.; Su, Y. Uric acid and blood pressure in NHANES dated from 2009 to 2018: A cross-sectional research. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2568–2578. [Google Scholar] [CrossRef]
- Bao, W.; Liu, B.; Simonsen, D.W.; Lehmler, H.J. Association between exposure to pyrethroid insecticides and risk of all-cause and cause-specific mortality in the general U.S. adult population. JAMA Intern. Med. 2020, 180, 367–374. [Google Scholar] [CrossRef]
- Sun, H.; Wang, N.; Chen, C.; Nie, X.; Han, B.; Li, Q.; Zhu, C.; Chen, Y.; Xia, F.; Chen, Y.; et al. Cadmium exposure and its association with serum uric acid and hyperuricemia. Sci. Rep. 2017, 7, 550. [Google Scholar] [CrossRef]
- González-Domínguez, Á.; Millán-Martínez, M.; Domínguez-Riscart, J.; Mateos, R.M.; Lechuga-Sancho, A.M.; González-Domínguez, R. Altered metal homeostasis associates with inflammation, oxidative stress, impaired glucose metabolism, and dyslipidemia in the crosstalk between childhood obesity and insulin resistance. Antioxidants 2022, 11, 2439. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.W.; Chang, H.H.; Yang, K.C.; Kuo, C.S.; Lee, L.T.; Huang, K.C. High serum selenium levels are associated with increased risk for diabetes mellitus independent of central obesity and insulin resistance. BMJ Open Diabetes Res. Care 2016, 4, e000253. [Google Scholar] [CrossRef] [PubMed]
- Fatani, S.H.; Saleh, S.A.; Adly, H.M.; Abdulkhaliq, A.A. Trace element alterations in the hair of diabetic and obese women. Biol. Trace Elem. Res. 2016, 174, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Galan, P.; Viteri, F.E.; Bertrais, S.; Czernichow, S.; Faure, H.; Arnaud, J.; Ruffieux, D.; Chenal, S.; Arnault, N.; Favier, A.; et al. Serum concentrations of beta-carotene, vitamins C and E, zinc and selenium are influenced by sex, age, diet, smoking status, alcohol consumption and corpulence in a general French adult population. Eur. J. Clin. Nutr. 2005, 59, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Ma, X.; Li, M.; Yan, S.; Zhao, H.; Pan, Y.; Wang, C.; Yao, Y.; Jin, L.; Li, B. Association between dietary mineral nutrient intake, body mass index, and waist circumference in U.S. adults using quantile regression analysis NHANES 2007–2014. PeerJ 2020, 8, e9127. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, H.; Zhang, Y.; Chen, L.; Tian, C.; Huang, B.; Chen, Y.; Ma, L. Associations of dietary antioxidant micronutrients with the prevalence of obesity in adults. Front. Nutr. 2023, 10, 1098761. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, B.; Sun, G.; Gao, J.; Huang, T.; Liu, J.; Zhou, Q.; He, X.; Zhang, S.; Wang, C.Y.; et al. Dietary selenomethionine attenuates obesity by enhancing beiging process in white adipose tissue. J. Nutr. Biochem. 2023, 113, 109230. [Google Scholar] [CrossRef] [PubMed]
- Cavedon, E.; Manso, J.; Negro, I.; Censi, S.; Serra, R.; Busetto, L.; Vettor, R.; Plebani, M.; Pezzani, R.; Nacamulli, D.; et al. Selenium supplementation, body mass composition, and leptin levels in patients with obesity on a balanced mildly hypocaloric diet: A pilot study. Int. J. Endocrinol. 2020, 2020, 4802739. [Google Scholar] [CrossRef]
- Viegas-Crespo, A.M.; Pavão, M.L.; Paulo, O.; Santos, V.; Santos, M.C.; Nève, J. Trace element status (Se, Cu, Zn) and serum lipid profile in Portuguese subjects of San Miguel Island from Azores’archipelago. J. Trace Elem. Med. Biol. 2000, 14, 1–5. [Google Scholar] [CrossRef]
- Kafai, M.R.; Ganji, V. Sex, age, geographical location, smoking, and alcohol consumption influence serum selenium concentrations in the USA: Third National Health and Nutrition Examination Survey, 1988–1994. J. Trace Elem. Med. Biol. 2003, 17, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, L. Selene, the goddess of the moon: Does she shine on men only? Eur. Heart J. 2007, 28, 2043–2044. [Google Scholar] [CrossRef] [PubMed]
- Alanne, M.; Kristiansson, K.; Auro, K.; Silander, K.; Kuulasmaa, K.; Peltonen, L.; Salomaa, V.; Perola, M. Variation in the selenoprotein S gene locus is associated with coronary heart disease and ischemic stroke in two independent Finnish cohorts. Hum. Genet 2007, 122, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, I.; Daimon, T. A strong association between lipid accumulation product and diabetes mellitus in japanese women and men. J. Atheroscler. Thromb. 2014, 21, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.; Haider, S.M.S.; Ali, S.M.; Haider, T.; Anwar, A.; Hashmi, A.A. Overall clinical features of type 2 diabetes mellitus with respect to gender. Cureus 2023, 15, e35771. [Google Scholar] [CrossRef] [PubMed]
- Swanson, C.A.; Longnecker, M.P.; Veillon, C.; Howe, M.; Levander, O.A.; Taylor, P.R.; McAdam, P.A.; Brown, C.C.; Stampfer, M.J.; Willett, W.C. Selenium intake, age, gender, and smoking in relation to indices of selenium status of adults residing in a seleniferous area. Am. J. Clin. Nutr. 1990, 52, 858–862. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, Y.; Sui, W. Association between blood selenium levels and stroke: A study based on the NHANES (2011–2018). Biol. Trace Elem. Res. 2023, 202, 25–33. [Google Scholar] [CrossRef]
- Okura, Y.; Urban, L.H.; Mahoney, D.W.; Jacobsen, S.J.; Rodeheffer, R.J. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J. Clin. Epidemiol. 2004, 57, 1096–1103. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).