The Vitamin D Receptor as a Prognostic Marker in Breast Cancer—A Cohort Study
Abstract
1. Introduction
2. Material and Methods
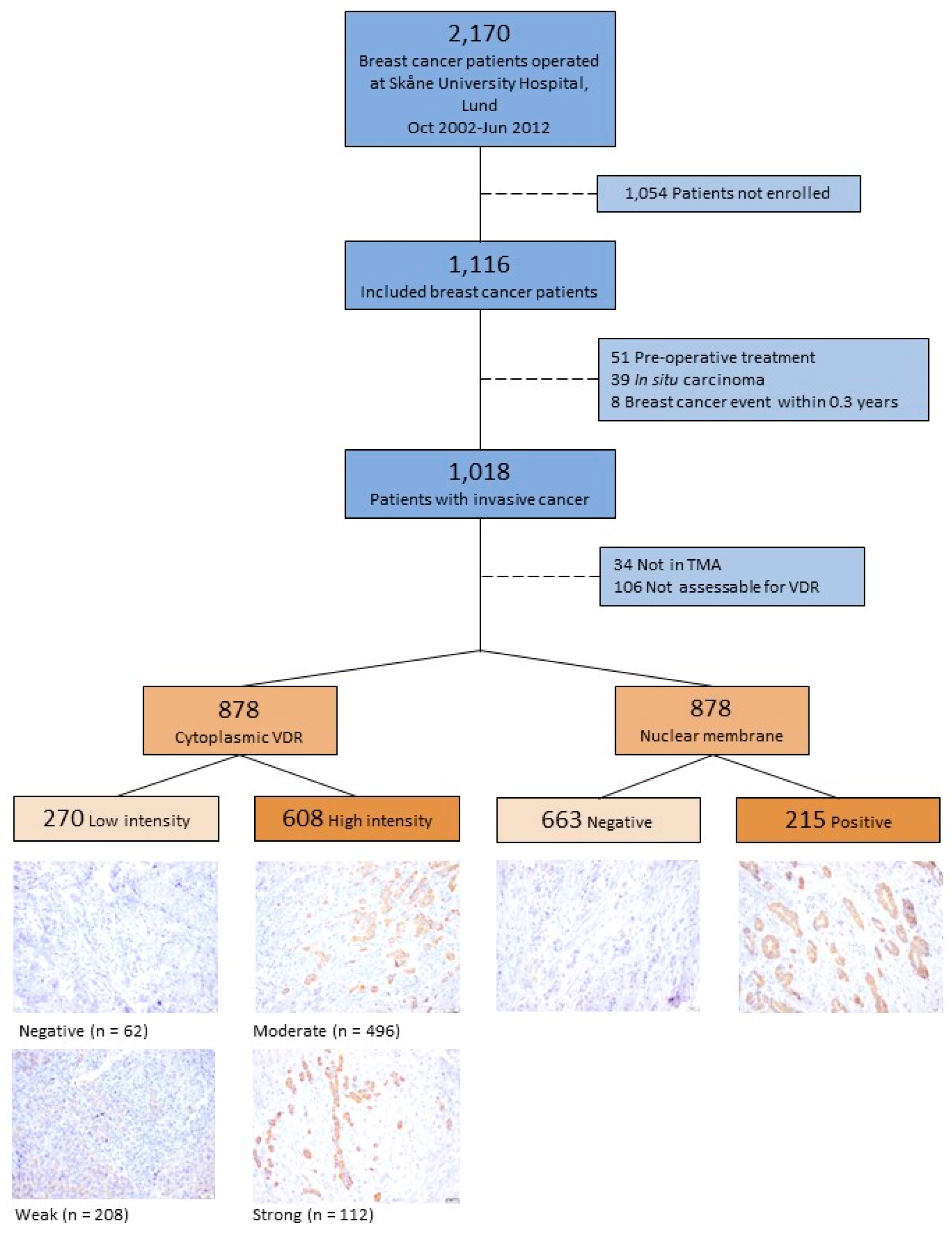
2.1. Study Population
2.2. Clinical Information and Histopathological Analysis
2.3. Tissue Microarray
2.4. Microscopy Assessment
2.5. Endpoint Retrieval
2.6. Statistical Methods
3. Results
3.1. Patient Characteristics
3.2. Tumor Characteristics and Adjuvant Treatment
3.3. VDR Levels and Prognosis
3.4. Effect Modification between VDRnum and Clinicopathological Factors
4. Discussion
4.1. Associations of VDR with Different Types of Breast Cancer
4.2. Using VDR as a Prognostic Factor and Vitamin D Supplements in Breast Cancer Treatment
4.3. Generalizability of the Results
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Variable Included in Cox Model | Missing Number Out of 1018 | Variable Included in MI Model |
|---|---|---|
| VDRnum | 140 | VDRnum |
| BC event/death | 0 | BC event/death |
| Follow-up time | 0 | Log of follow-up time |
| Time between surgery and staining | 0 | Time between surgery and staining |
| Age at inclusion | 0 | Age at inclusion |
| 303 | Age at menopause | |
| 0 | Parity | |
| BMI | 28 | BMI |
| 26 | Height | |
| 26 | Weight | |
| 38 | Waist circumference | |
| 160 | Breast volume | |
| 1 | Use of oral contraceptives | |
| 3 | Menopausal hormone therapy | |
| 7 | Vitamin D supplementation | |
| 2 | Smoking | |
| 3 | Alcohol | |
| 0 | Detection mode | |
| 0 | Month of surgery | |
| Tumor size | 0 | Tumor size |
| Lymph node involvement | 2 | Lymph node involvement |
| Histologic grade | 1 | Histologic grade |
| 0 | Histologic type | |
| ER | 1 | ER |
| 1 | PgR | |
| 63 | HER2 | |
| 7 | Triple-negative tumor | |
| Radiation therapy | 0 | Radiation therapy |
| Chemotherapy | 0 | Chemotherapy |
| Herceptin | 0 | Herceptin |
| Aromatase inhibitors | 0 | Aromatase inhibitors |
| Tamoxifen | 0 | Tamoxifen |
References
- Voutsadakis, I.A. Vitamin D baseline levels at diagnosis of breast cancer: A systematic review and meta-analysis. Hematol. Oncol. Stem Cell Ther. 2021, 14, 16–26. [Google Scholar] [CrossRef]
- Jacobs, E.T.; Kohler, L.N.; Kunihiro, A.G.; Jurutka, P.W. Vitamin D and Colorectal, Breast, and Prostate Cancers: A Review of the Epidemiological Evidence. J. Cancer 2016, 7, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Sucheston, L.E.; Millen, A.E.; Johnson, C.S.; Trump, D.L.; Nesline, M.K.; Davis, W.; Hong, C.C.; McCann, S.E.; Hwang, H.; et al. Pretreatment serum concentrations of 25-hydroxyvitamin D and breast cancer prognostic characteristics: A case-control and a case-series study. PLoS ONE 2011, 6, e17251. [Google Scholar] [CrossRef] [PubMed]
- Chandler, P.D.; Chen, W.Y.; Ajala, O.N.; Hazra, A.; Cook, N.; Bubes, V.; Lee, I.-M.; Giovannucci, E.L.; Willett, W.; Buring, J.E.; et al. Effect of Vitamin D3 Supplements on Development of Advanced Cancer: A Secondary Analysis of the VITAL Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2025850. [Google Scholar] [CrossRef] [PubMed]
- Leyssens, C.; Verlinden, L.; Verstuyf, A. Antineoplastic effects of 1,25(OH)2D3 and its analogs in breast, prostate and colorectal cancer. Endocr.-Relat. Cancer 2013, 20, R31–R47. [Google Scholar] [CrossRef]
- Al-Azhri, J.; Zhang, Y.; Bshara, W.; Zirpoli, G.R.; McCann, S.E.; Khoury, T.; Morrison, C.D.; Edge, S.B.; Ambrosone, C.B.; Yao, S. Tumor Expression of Vitamin D Receptor and Breast Cancer Histopathological Characteristics and Prognosis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 97–103. [Google Scholar] [CrossRef]
- Ditsch, N.; Toth, B.; Mayr, D.; Lenhard, M.; Gallwas, J.; Weissenbacher, T.; Dannecker, C.; Friese, K.; Jeschke, U. The association between vitamin D receptor expression and prolonged overall survival in breast cancer. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2012, 60, 121–129. [Google Scholar] [CrossRef]
- Huss, L.; Butt, S.T.; Borgquist, S.; Elebro, K.; Sandsveden, M.; Rosendahl, A.; Manjer, J. Vitamin D receptor expression in invasive breast tumors and breast cancer survival. Breast Cancer Res. BCR 2019, 21, 84. [Google Scholar] [CrossRef]
- Huss, L.; Butt, S.T.; Borgquist, S.; Elebro, K.; Sandsveden, M.; Manjer, J.; Rosendahl, A. Levels of Vitamin D and Expression of the Vitamin D Receptor in Relation to Breast Cancer Risk and Survival. Nutrients 2022, 14, 3353. [Google Scholar] [CrossRef]
- Xu, H.; Liu, Z.; Shi, H.; Wang, C. Prognostic role of vitamin D receptor in breast cancer: A systematic review and meta-analysis. BMC Cancer 2020, 20, 1051. [Google Scholar] [CrossRef]
- Murray, A.; Madden, S.; Synnott, N.; Klinger, R.; O’Connor, D.; O’Donovan, N.; Gallagher, W.; Crown, J.; Duffy, M. Vitamin D receptor as a target for breast cancer therapy. Endocr.-Relat. Cancer 2017, 24, 181–195. [Google Scholar] [CrossRef]
- Heublein, S.; Mayr, D.; Meindl, A.; Kircher, A.; Jeschke, U.; Ditsch, N. Vitamin D receptor, retinoid X receptor and peroxisome proliferator-activated receptor gamma are overexpressed in BRCA1 mutated breast cancer and predict prognosis. J. Exp. Clin. Cancer Res. 2017, 36, 57. [Google Scholar] [CrossRef][Green Version]
- Vanhevel, J.; Verlinden, L.; Doms, S.; Wildiers, H.; Verstuyf, A. The role of vitamin D in breast cancer risk and progression. Endocr. -Relat. Cancer 2022, 29, R33–R55. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, T.; Zheng, Y.; Fournier, P.G.J.; Murthy, S.; John, S.; Schillo, S.; Dunstan, C.R.; Mohammad, K.S.; Zhou, H.; Seibel, M.J.; et al. The vitamin D receptor is involved in the regulation of human breast cancer cell growth via a ligand-independent function in cytoplasm. Oncotarget 2017, 8, 26687–26701. [Google Scholar] [CrossRef]
- Persson, M.; Simonsson, M.; Markkula, A.; Rose, C.; Ingvar, C.; Jernström, H. Impacts of smoking on endocrine treatment response in a prospective breast cancer cohort. Br. J. Cancer 2016, 115, 382–390. [Google Scholar] [CrossRef]
- Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation; World Health Organization Technical Report Series; WHO: Geneva, Switzerland, 2000; Volume 894, pp. i–xii, 1–253.
- Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation. Available online: https://iris.who.int/bitstream/handle/10665/44583/9789241501491_eng.pdf?ua=1 (accessed on 20 September 2023).
- Strömbeck, J.O.; Malm, M. Priority grouping in a waiting list of patients for reduction mammaplasty. Ann. Plast. Surg. 1986, 17, 498–502. [Google Scholar] [CrossRef]
- Markkula, A.; Bromée, A.; Henningson, M.; Hietala, M.; Ringberg, A.; Ingvar, C.; Rose, C.; Jernström, H. Given breast cancer, does breast size matter? Data from a prospective breast cancer cohort. Cancer Causes Control CCC 2012, 23, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Sandén, E.; Khazaei, S.; Tryggvadottir, H.; Borgquist, S.; Isaksson, K.; Jirström, K.; Jernström, H. Re-evaluation of HER2 status in 606 breast cancers-gene protein assay on tissue microarrays versus routine pathological assessment. Virchows Arch. 2020, 477, 317–320. [Google Scholar] [CrossRef]
- Wang, Y.; Becklund, B.R.; DeLuca, H.F. Identification of a highly specific and versatile vitamin D receptor antibody. Arch. Biochem. Biophys. 2010, 494, 166–177. [Google Scholar] [CrossRef]
- Welsh, J. Vitamin D and breast cancer: Past and present. J. Steroid Biochem. Mol. Biol. 2018, 177, 15–20. [Google Scholar] [CrossRef]
- Lopes, N.; Sousa, B.; Martins, D.; Gomes, M.; Vieira, D.; Veronese, L.A.; Milanezi, F.; Paredes, J.; Costa Jé, L.; Schmitt, F. Alterations in Vitamin D signalling and metabolic pathways in breast cancer progression: A study of VDR, CYP27B1 and CYP24A1 expression in benign and malignant breast lesions Vitamin D pathways unbalanced in breast lesions. BMC Cancer 2010, 10, 483. [Google Scholar] [CrossRef] [PubMed]
- Reichrath, J.; Reichrath, S.; Heyne, K.; Vogt, T.; Roemer, K. Tumor suppression in skin and other tissues via cross-talk between vitamin D- and p53-signaling. Front. Physiol. 2014, 5, 166. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.; Synnott, N.; Crown, J. Mutant p53 in breast cancer: Potential as a therapeutic target and biomarker. Breast Cancer Res. Treat. 2018, 170, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Soljic, M.; Mrklic, I.; Tomic, S.; Omrcen, T.; Sutalo, N.; Bevanda, M.; Vrdoljak, E. Prognostic value of vitamin D receptor and insulin-like growth factor receptor 1 expression in triple-negative breast cancer. J. Clin. Pathol. 2018, 71, 34–39. [Google Scholar] [CrossRef]
- Stambolsky, P.; Tabach, Y.; Fontemaggi, G.; Weisz, L.; Maor-Aloni, R.; Siegfried, Z.; Shiff, I.; Kogan, I.; Shay, M.; Kalo, E.; et al. Modulation of the vitamin D3 response by cancer-associated mutant p53. Cancer Cell 2010, 17, 273–285. [Google Scholar] [CrossRef]
- Lindgren, H.; Ademi, D.; Godina, C.; Tryggvadottir, H.; Isaksson, K.; Jernström, H. Potential interplay between tumor size and vitamin D receptor (VDR) polymorphisms in breast cancer prognosis: A prospective cohort study. Cancer Causes Control CCC 2024, 5. [Google Scholar] [CrossRef]
- Costa, P.L.; Franca, M.M.; Ferraz-de-Souza, B. Nonspecific binding of a frequently used vitamin D receptor (VDR) antibody: Important implications for vitamin D research in human health. Endocrine 2016, 54, 556–559. [Google Scholar] [CrossRef]
- Cuschieri, S. The STROBE guidelines. Saudi J. Anaesth. 2019, 13, S31–S34. [Google Scholar] [CrossRef]
- McShane, L.M.; Altman, D.G.; Sauerbrei, W.; Taube, S.E.; Gion, M.; Clark, G.M. Reporting recommendations for tumor marker prognostic studies (REMARK). J. Natl. Cancer Inst. 2005, 97, 1180–1184. [Google Scholar] [CrossRef]
- Allotey, P.A.; Harel, O. Multiple Imputation for Incomplete Data in Environmental Epidemiology Research. Curr. Environ. Health Rep. 2019, 6, 62–71. [Google Scholar] [CrossRef]



| Eligible Cases | All n = 1018 | ||||
|---|---|---|---|---|---|
| Tumor in Tissue Microarray n (%) | Yes 984 (96.7) | No 34 (3.3) | |||
| VDR Assessable n (%) | Yes 878 (89.2) | No 106 (10.8) | |||
| Nuclear Membrane VDR n (%) | Negative 663 (75.6) | Positive 215 (24.5) | |||
| Factor | n (%) | n (%) | n (%) | n (%) | n (%) |
| Invasive tumor size | |||||
| 1–20 mm | 743 (73.0) | 471 (71.0) | 166 (77.2) | 76 (71.7) | 30 (88.2) |
| >20 mm * | 275 (27.0) | 192 (29.0) | 49 (22.8) | 30 (28.3) | 4 (11.8) |
| Lymph node status | |||||
| Negative | 627 (61.6) | 390 (58.8) | 135 (62.8) | 79 (74.5) | 23 (67.6) |
| Positive | 389 (38.2) | 271 (40.9) | 80 (37.2) | 27 (25.5) | 11 (32.4) |
| Nottingham grade | |||||
| I | 258 (25.3) | 123 (18.6) | 87 (40.5) | 38 (35.8) | 10 (29.4) |
| II | 502 (49.3) | 326 (49.2) | 104 (48.4) | 55 (51.9) | 17 (50.0) |
| III | 257 (25.2) | 214 (32.3) | 24 (11.2) | 12 (11.3) | 7 (20.6) |
| Histological type | |||||
| Ductal | 824 (80.9) | 554 (83.6) | 176 (81.9) | 67 (63.2) | 27 (79.4) |
| Lobular | 116 (11.4) | 60 (9.0) | 24 (11.2) | 28 (26.4) | 4 (11.8) |
| Other/mixed | 78 (7.7) | 49 (7.4) | 15 (7.0) | 11 (10.4) | 3 (8.8) |
| ER status | |||||
| Pos (>10%) | 894 (87.8) | 555 (83.7) | 214 (99.5) | 97 (91.5) | 28 (82.4) |
| PgR status | |||||
| Pos (>10%) | 723 (71.0) | 441 (66.5) | 179 (83.3) | 79 (74.5) | 24 (70.6) |
| HER2 | |||||
| Amplified | 110 (11.5) | 87 (13.5) | 10 (4.7) | 7 (9.0) | 6 (31.6) |
| Unknown | 63 | 18 | 2 | 28 | 15 |
| Triple-negative | |||||
| Yes | 74 (7.3) | 68 (10.3) | 0 (0) | 5 (4.8) | 1 (3.2) |
| Unknown | 7 | 2 | 0 | 2 | 3 |
| Treatment | |||||
| Final type of operation | |||||
| Mastectomy | 410 (40.3) | 271 (40.9) | 85 (39.5) | 40 (37.7) | 14 (41.2) |
| Chemotherapy | 258 (25.3) | 193 (29.1) | 41 (19.1) | 15 (14.2) | 9 (26.5) |
| Radiotherapy | 644 (63.4) | 418 (63.0) | 139 (64.7) | 68 (64.2) | 19 (55.9) |
| Herceptin | 73 (7.2) | 54 (8.1) | 10 (4.7) | 4 (3.8) | 5 (14.7) |
| Endocrine therapy ** | |||||
| Tamoxifen | 572 (64.0) | 382 (68.2) | 119 (55.6) | 58 (59.8) | 13 (46.4) |
| Aromatase inhibitors | 371 (41.5) | 249 (44.9) | 80 (37.4) | 32 (33.0) | 10 (35.7) |
| Event | |||||
| Any breast cancer event | 195 (19.2) | 145 (21.9) | 28 (13.0) | 15 (14.2) | 7 (20.6) |
| Death | 188 (18.5) | 144 (21.7) | 23 (10.7) | 15 (14.2) | 6 (17.6) |
| Eligible Cases | All n = 1018 | ||||
|---|---|---|---|---|---|
| Tumor in Tissue Microarray n (%) | Yes 984 (96.7) | No 34 (3.3) | |||
| VDR Assessable n (%) | Yes 878 (89.2) | No 106 (10.8) | |||
| Nuclear Membrane VDR n (%) | Negative 663 (75.6) | Positive 215 (24.5) | |||
| Factor | n (%) | n (%) | n (%) | n (%) | n (%) |
| Age at diagnosis | |||||
| ≥50 | 816 (80.2) | 535 (80.7) | 168 (78.1) | 89 (84.0) | 24 (70.6) |
| BMI at inclusion | |||||
| ≥25 | 503 (50.8) | 336 (51.9) | 100 (49.0) | 49 (46.7) | 18 (54.5) |
| Unknown | 28 | 15 | 11 | 1 | 1 |
| Waist circumference | |||||
| ≥80 cm | 731 (74.6) | 490 (76.1) | 143 (70.8) | 73 (72.3) | 25 (75.8) |
| Unknown | 38 | 19 | 13 | 5 | 1 |
| Total breast volume | |||||
| ≥850 mL | 492 (57.3) | 335 (58.9) | 87 (50.0) | 54 (62.1) | 16 (57.1) |
| Unknown | 160 | 94 | 41 | 19 | 6 |
| Parity | |||||
| Parous | 896 (88.0) | 576 (86.9) | 196 (91.2) | 93 (87.7) | 31 (91.2) |
| Oral contraceptives | |||||
| Ever | 722 (71.0) | 467 (70.4) | 151 (70.6) | 77 (72.6) | 27 (79.4) |
| Menopausal hormone therapy | |||||
| Ever | 446 (43.9) | 281 (42.4) | 96 (45.1) | 57 (53.8) | 49 (35.3) |
| Alcohol abstainer | |||||
| Yes | 106 (10.4) | 72 (10.9) | 23 (10.7) | 11 (10.4) | 0 (0) |
| Smoking currently | |||||
| Yes | 206 (20.2) | 130 (19.6) | 41 (19.1) | 31 (29.2) | 4 (11.8) |
| Vitamin D supplements | |||||
| Yes | 103 (10.2) | 71 (10.8) | 16 (7.5) | 10 (9.4) | 6 (17.6) |
| Unknown | 7 | 6 | 1 | 0 | 0 |
| Season of operation | |||||
| Winter (January–March) | 251 (24.8) | 160 (24.1) | 58 (27.0) | 28 (26.4) | 5 (14.7) |
| Spring (April–June) | 303 (29.8) | 193 (29.1) | 69 (32.1) | 33 (31.1) | 8 (23.5) |
| Summer (July–September) | 177 (17.0) | 111 (16.7) | 38 (17.7) | 20 (18.9) | 8 (23.5) |
| Fall (October–December) | 287 (28.4) | 199 (30.0) | 50 (23.3) | 25 (23.5) | 13 (38.2) |
| Detection mode (45–74 years) | |||||
| Clinical | 290 (33.8) | 208 (37.8) | 52 (28.0) | 21 (22.1) | 9 (32.1) |
| Screening | 569 (66.2) | 342 (62.2) | 134 (72.0) | 74 (77.9) | 19 (67.9) |
| Breast Cancer Free Interval | |||||||
|---|---|---|---|---|---|---|---|
| Nuclear Membrane VDR Level | Included in Analyses | Total n | Events n | HR 1 (CI 95%) | HR 2 (CI 95%) | HR 3 (CI 95%) | HR 4 (CI 95%) |
| Negative | Complete case * | 663 | 145 | ref | ref | ref | ref |
| Positive | 215 | 28 | 0.61 (0.40–0.91) | 0.71 (0.46–1.07) | 0.72 (0.48–1.10) | 0.70 (0.46–1.07) | |
| Negative | All included ** | 730 | 155 | ref | ref | ref | ref |
| Positive | 288 | 40 | 0.64 (0.44–0.95) | 0.74 (0.49–1.10) | 0.75 (0.50–1.12) | 0.71 (0.47–1.06) | |
| Overall Survival | |||||||
| Negative | Complete case * | 663 | 144 | ref | ref | ref | ref |
| Positive | 215 | 23 | 0.51 (0.33–0.79) | 0.63 (0.40–0.99) | 0.69 (0.42–1.06) | 0.67 (0.42–1.08) | |
| Negative | All included ** | 764 | 161 | ref | ref | ref | ref |
| Positive | 254 | 27 | 0.52 (0.34–0.78) | 0.62 (0.40–0.96) | 0.64 (0.41–0.99) | 0.65 (0.42–1.00) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huss, L.; Gulz-Haake, I.; Nilsson, E.; Tryggvadottir, H.; Nilsson, L.; Nodin, B.; Jirström, K.; Isaksson, K.; Jernström, H. The Vitamin D Receptor as a Prognostic Marker in Breast Cancer—A Cohort Study. Nutrients 2024, 16, 931. https://doi.org/10.3390/nu16070931
Huss L, Gulz-Haake I, Nilsson E, Tryggvadottir H, Nilsson L, Nodin B, Jirström K, Isaksson K, Jernström H. The Vitamin D Receptor as a Prognostic Marker in Breast Cancer—A Cohort Study. Nutrients. 2024; 16(7):931. https://doi.org/10.3390/nu16070931
Chicago/Turabian StyleHuss, Linnea, Igis Gulz-Haake, Emma Nilsson, Helga Tryggvadottir, Linn Nilsson, Björn Nodin, Karin Jirström, Karolin Isaksson, and Helena Jernström. 2024. "The Vitamin D Receptor as a Prognostic Marker in Breast Cancer—A Cohort Study" Nutrients 16, no. 7: 931. https://doi.org/10.3390/nu16070931
APA StyleHuss, L., Gulz-Haake, I., Nilsson, E., Tryggvadottir, H., Nilsson, L., Nodin, B., Jirström, K., Isaksson, K., & Jernström, H. (2024). The Vitamin D Receptor as a Prognostic Marker in Breast Cancer—A Cohort Study. Nutrients, 16(7), 931. https://doi.org/10.3390/nu16070931





