Association between Consumption of Iodine-Rich Foods and Thyroid Cancer Prevalence: Findings from a Large Population-Based Study
Abstract
1. Introduction
2. Materials and Methods
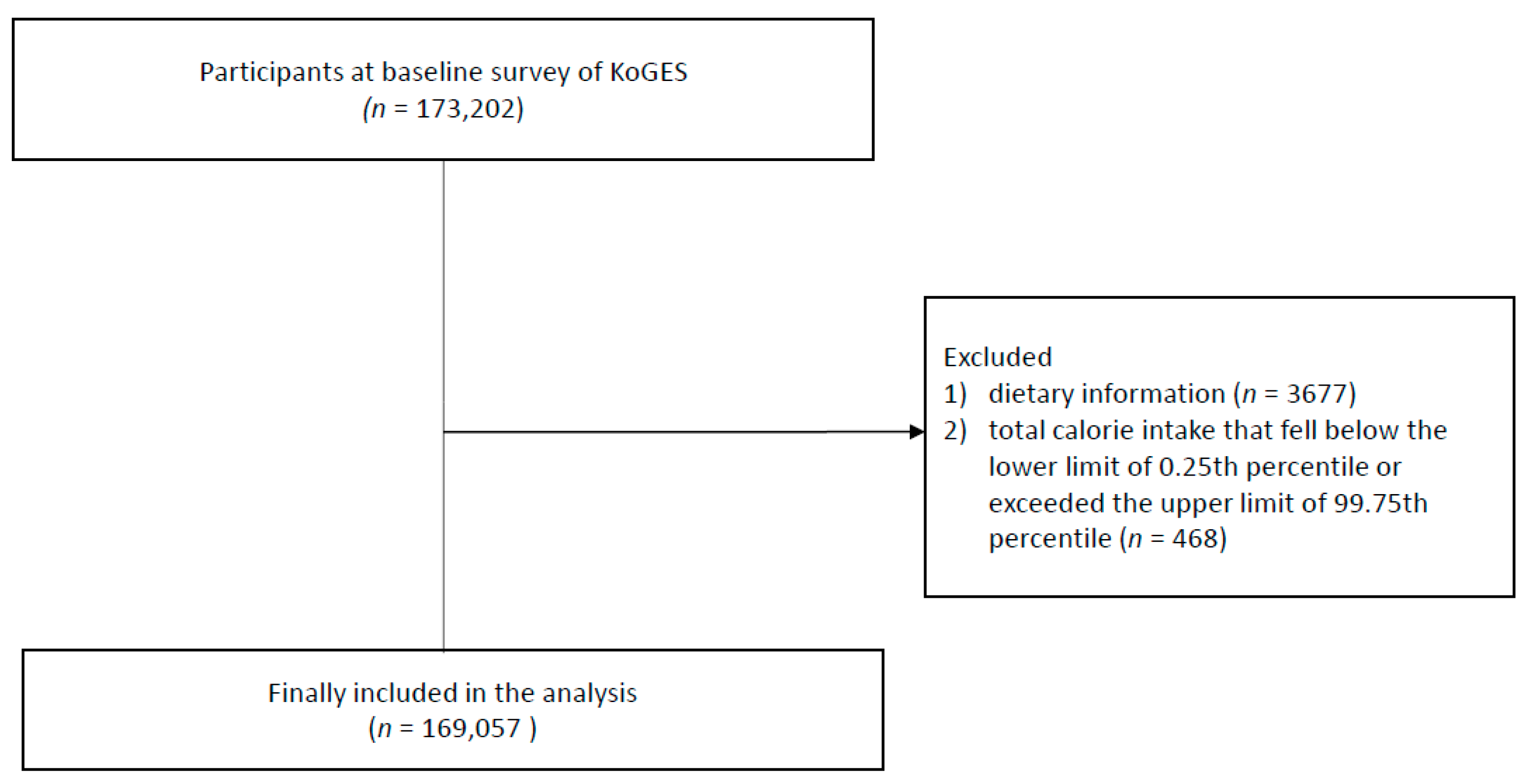
2.1. Participants
2.2. Dietary Assessment
2.3. Covariates
2.4. Study Outcomes
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Study Population
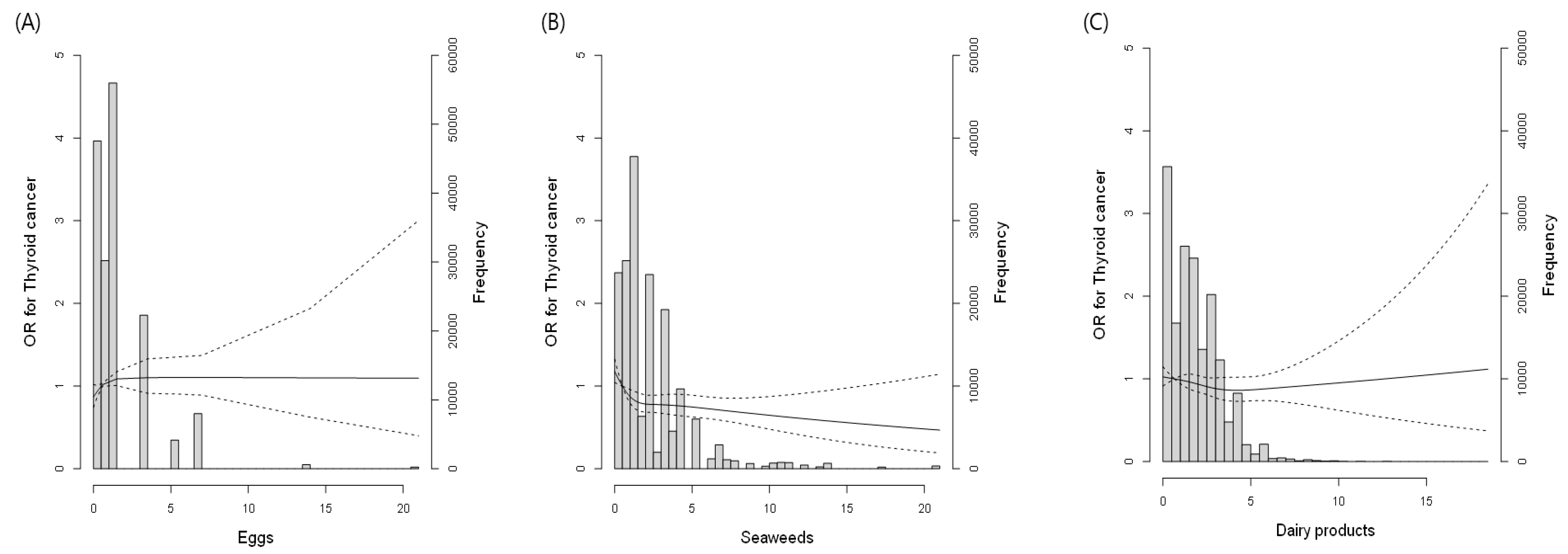
3.2. Association between Iodine-Rich Food Consumption and TC Prevalence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pizzato, M.; Li, M.; Vignat, J.; Laversanne, M.; Singh, D.; La Vecchia, C.; Vaccarella, S. The epidemiological landscape of thyroid cancer worldwide: Globocan estimates for incidence and mortality rates in 2020. Lancet Diabetes Endocrinol. 2022, 10, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.M.; Lee, J.; Kwak, M.K.; Jeon, M.J.; Kim, T.Y.; Hong, E.-G.; Kim, W.B.; Kim, W.G. Recent changes in the incidence of thyroid cancer in Korea between 2005 and 2018: Analysis of Korean national data. Endocrinol. Metab. 2022, 37, 791–799. [Google Scholar] [CrossRef]
- Hyun, M.K.; Kim, J.H.; Kwon, J.W. Incidence of thyroid cancer and medical cost among patients with newly diagnosed thyroid nodules in Korea: A retrospective cohort study using nationwide data. J. Cancer Res. Ther. 2019, 15, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Nettore, I.C.; Colao, A.; Macchia, P.E. Nutritional and environmental factors in thyroid carcinogenesis. Int. J. Environ. Res. Public Health 2018, 15, 1735. [Google Scholar] [CrossRef] [PubMed]
- Bogović Crnčić, T.; Ilić Tomaš, M.; Girotto, N.; Grbac Ivanković, S. Risk factors for thyroid cancer: What do we know so far? Acta Clin. Croat. 2020, 59, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Pugliese, G.; Frias-Toral, E.; Laudisio, D.; Rodriguez, D.; Vitale, G.; Colombo, C.; Colao, A.; Savastano, S.; Muscogiuri, G. Diet as a possible influencing factor in thyroid cancer incidence: The point of view of the nutritionist. Panminerva Medica 2021, 63, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Maso, L.D.; Bosetti, C.; La Vecchia, C.; Franceschi, S. Risk factors for thyroid cancer: An epidemiological review focused on nutritional factors. Cancer Causes Control 2009, 20, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.D.; Gunathilake, M.; Lee, J.; Kim, J. Association between dietary habits and incident thyroid cancer: A prospective cohort study. Front. Nutr. 2023, 10, 1104925. [Google Scholar] [CrossRef]
- Vuong, H.G.; Kondo, T.; Oishi, N.; Nakazawa, T.; Mochizuki, K.; Inoue, T.; Tahara, I.; Kasai, K.; Hirokawa, M.; Tran, T.M.; et al. Genetic alterations of differentiated thyroid carcinoma in iodine-rich and iodine-deficient countries. Cancer Med. 2016, 5, 1883–1889. [Google Scholar] [CrossRef]
- Dijkstra, B.; Prichard, R.S.; Lee, A.; Kelly, L.M.; Smyth, P.P.A.; Crotty, T.; McDermott, E.W.; Hill, A.D.K.; O’higgins, N. Changing patterns of thyroid carcinoma. Ir. J. Med. Sci. 2007, 176, 87–90. [Google Scholar] [CrossRef]
- Leung, A.; Pearce, E.N.E.; Braverman, L. Role of iodine in thyroid physiology. Expert. Rev. Endocrinol. Metab. 2010, 5, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Chen, G.G.; Vlantis, A.C.; van Hasselt, C.A. Iodine mediated mechanisms and thyroid carcinoma. Crit. Rev. Clin. Lab. Sci. 2009, 46, 302–318. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Han, B.G. Cohort profile: The korean genome and epidemiology study (koges) consortium. Int. J. Epidemiol. 2017, 46, e20. [Google Scholar] [CrossRef]
- Ahn, Y.; Kwon, E.; Shim, J.E.; Park, M.K.; Joo, Y.; Kimm, K.; Park, C.; Kim, D.H. Validation and reproducibility of food frequency questionnaire for korean genome epidemiologic study. Eur. J. Clin. Nutr. 2007, 61, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- The Korean Nutrition Society. 2020 Dietary Reference Intakes for Koreans; Ministry of Health and Welfare: Sejong, Republic of Korea, 2020. [Google Scholar]
- Pennington, J.A. A review of iodine toxicity reports. J. Am. Diet. Assoc. 1990, 90, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, H.; Kim, T.H.; Kim, S.W.; Jang, H.W.; Chung, J.H. Trends in childhood thyroid cancer incidence in Korea and Its potential risk factors. Front. Endocrinol. 2021, 12, 681148. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.; Baron-Dubourdieu, D.; Rougier, Y.; Guénel, P. Role of dietary iodine and cruciferous vegetables in thyroid cancer: A countrywide case—Control study in new Caledonia. Cancer Causes Control 2010, 21, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Cléro, É.; Doyon, F.; Chungue, V.; Rachédi, F.; Boissin, J.-L.; Sebbag, J.; Shan, L.; Bost-Bezeaud, F.; Petitdidier, P.; Dewailly, P.; et al. Dietary iodine and thyroid cancer risk in French Polynesia: A case-control study. Thyroid 2012, 22, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Horn-Ross, P.L.; Morris, J.S.; Lee, M.; West, D.W.; Whittemore, A.S.; McDougall, I.R.; Nowels, K.; Stewart, S.L.; Spate, V.L.; Shiau, A.C.; et al. Iodine and thyroid cancer risk among women in a multiethnic population: The bay area thyroid cancer study. Cancer Epidemiol. Biomark. Prev. 2001, 10, 979–985. [Google Scholar]
- Zava, T.T.; Zava, D.T. Assessment of Japanese iodine intake based on seaweed consumption in Japan: A literature-based analysis. Thyroid. Res. 2011, 4, 14. [Google Scholar] [CrossRef]
- Han, M.-R.; Ju, D.L.; Park, Y.J.; Paik, H.-Y.; Song, Y. An Iodine Database for Common Korean foods and the association between iodine intake and thyroid disease in Korean adults. Int. J. Thyroid. 2015, 8, 170–182. [Google Scholar] [CrossRef]
- Chichibu, H.; Yamagishi, K.; Kishida, R.; Maruyama, K.; Hayama-Terada, M.; Shimizu, Y.; Muraki, I.; Umesawa, M.; Cui, R.; Imano, H.; et al. Seaweed intake and risk of cardiovascular disease: The circulatory risk in communities study (CIRCS). J. Atheroscler. Thromb. 2021, 28, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Murai, U.; Yamagishi, K.; Sata, M.; Kokubo, Y.; Saito, I.; Yatsuya, H.; Ishihara, J.; Inoue, M.; Sawada, N.; Iso, H.; et al. Seaweed intake and risk of cardiovascular disease: The Japan Public Health Center–based prospective (JPHC) study. Am. J. Clin. Nutr. 2019, 110, 1449–1455. [Google Scholar] [CrossRef]
- Michikawa, T.; Inoue, M.; Shimazu, T.; Sawada, N.; Iwasaki, M.; Sasazuki, S.; Yamaji, T.; Tsugane, S. Seaweed consumption and the risk of thyroid cancer in women: The Japan public health center-based prospective study. Eur. J. Cancer Prev. 2012, 21, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yatsuya, H.; Li, Y.; Ota, A.; Tamakoshi, K.; Fujino, Y.; Mikami, H.; Iso, H.; Tamakoshi, A. Prospective study of seaweed consumption and thyroid cancer incidence in women: The japan collaborative cohort study. Eur. J. Cancer Prev. 2016, 25, 239–245. [Google Scholar] [CrossRef]
- Murai, U.; Yamagishi, K.; Kishida, R.; Iso, H. Impact of seaweed intake on health. Eur. J. Clin. Nutr. 2021, 75, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Alcalá, L.M.; Castro-Gómez, M.P.; Pimentel, L.L.; Fontecha, J. Milk fat components with potential anticancer ac-tivity—A review. Biosci. Rep. 2017, 37, BSR20170705. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, H.; Zhang, Z.; Zhou, X.; Yao, J.; Zhang, R.; Liao, L.; Dong, J. Vitamin D deficiency as a risk factor for thyroid cancer: A meta-analysis of case-control studies. Nutrition 2019, 57, 5–11. [Google Scholar] [CrossRef]
- Fiore, M.; Cristaldi, A.; Okatyeva, V.; Bianco, S.L.; Conti, G.O.; Zuccarello, P.; Copat, C.; Caltabiano, R.; Cannizzaro, M.; Ferrante, M. Dietary habits and thyroid cancer risk: A hospital-based case–control study in Sicily (South Italy). Food Chem. Toxicol. 2020, 146, 111778. [Google Scholar] [CrossRef]
- Galanti, M.R.; Hansson, L.; Bergström, R.; Wolk, A.; Hjartåker, A.; Lund, E.; Grimelius, L.; Ekbom, A. Diet and the risk of papillary and follicular thyroid carcinoma: A population-based case-control study in Sweden and Norway. Cancer Causes Control 1997, 8, 205–214. [Google Scholar] [CrossRef]
- Tanitame, M.; Sugawara, Y.; Lu, Y.; Matsuyama, S.; Kanemura, S.; Fukao, A.; Tsuji, I. Dairy consumption and incident risk of thyroid cancer in Japan: A pooled analysis of the miyagi cohort study and the ohsaki cohort study. Eur. J. Nutr. 2023, 62, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Réhault-Godbert, S.; Guyot, N.; Nys, Y. The golden egg: Nutritional value, bioactivities, and emerging benefits for human health. Nutrients 2019, 11, 684. [Google Scholar] [CrossRef]
- Richman, E.L.; Kenfield, S.A.; Stampfer, M.J.; Giovannucci, E.L.; Chan, J.M. Egg, red meat, and poultry intake and risk of lethal prostate cancer in the prostate-specific antigen-era: Incidence and survival. Cancer Prev. Res. 2011, 4, 2110–2121. [Google Scholar] [CrossRef] [PubMed]
- Iscovich, J.M.; L’Abbé, K.A.; Castelleto, R.; Calzona, A.; Bernedo, A.; Chopita, N.A.; Jmelnitzsky, A.C.; Kaldor, J. Colon cancer in Argentina. I: Risk from intake of dietary items. Int. J. Cancer 1992, 51, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Filho, A.; Lortet-Tieulent, J.; Bray, F.; Cao, B.; Franceschi, S.; Vaccarella, S.; Dal Maso, L. Thyroid cancer incidence trends by histology in 25 countries: A population-based study. Lancet Diabetes Endocrinol. 2021, 9, 225–234. [Google Scholar] [CrossRef]
- Markou, K.; Georgopoulos, N.; Kyriazopoulou, V.; Vagenakis, A. Iodine-induced hypothyroidism. Thyroid 2001, 11, 501–510. [Google Scholar] [CrossRef]

| Non-Thyroid Cancer | Thyroid Cancer | p | |
|---|---|---|---|
| n | 168,127 | 930 | |
| Men, n (%) | 57,865 (34.4) | 75 (8.06) | <0.001 |
| Age (years) | 53.1 ± 8.4 | 53.0 ± 7.5 | 0.811 |
| Height (cm) | 160.5 ± 8.0 | 158.5 ± 6.2 | <0.001 |
| Weight (kg) | 61.8 ± 9.9 | 59.8 ± 8.5 | <0.001 |
| BMI (kg/m2) | 23.9 ± 2.9 | 23.8 ± 2.9 | 0.134 |
| Waist circumference (cm) | 81.1 ± 8.7 | 79.3 ± 8.0 | <0.001 |
| Systolic BP (mmHg) | 122.7 ± 15.5 | 120.7 ± 14.3 | <0.001 |
| Diastolic BP (mmHg) | 76.2 ± 10.0 | 74.6 ± 9.4 | <0.001 |
| Glucose (mg/dL) | 95.2 ± 21.7 | 93.6 ± 14.8 | 0.021 |
| Total cholesterol (mg/dL) | 197.5 ± 35.6 | 190.0 ± 35.2 | <0.001 |
| HDL-C (mg/dL) | 54.0 ± 12.9 | 53.6 ± 13.1 | 0.387 |
| Triglycerides (mg/dL) | 127.0 ± 89.9 | 113.8 ± 66.0 | <0.001 |
| Smoking status (yes) | 21,881 (13.0) | 15 (1.6) | <0.001 |
| Alcohol intake (yes) | 82,626 (49.1) | 283 (30.4) | <0.001 |
| Regular exercise (yes) | 87,955 (52.3) | 549 (59.0) | |
| Hypertension (yes) | 28,493 (17.0) | 119 (12.8) | 0.001 |
| Diabetes mellitus (yes) | 16,015 (9.5) | 71 (7.6) | 0.050 |
| Dyslipidemia (yes) | 94,744 (56.4) | 448 (48.2) | <0.001 |
| House income (KRW) | 0.016 | ||
| <1.5 million | 30,344 (21.5) | 151 (17.2) | |
| 1.5–3.0 million | 47,539 (33.68) | 282 (32.9) | |
| 3.0–6.0 million | 51,509 (36.49) | 342 (39.9) | |
| >6.0 million | 11,771 (8.34) | 82 (9.6) | |
| hs-CRP | 0.15 ± 0.40 | 0.14 ± 0.3 | 0.434 |
| Egg Consumption | Seaweed Consumption | Dairy Product Consumption | |||||||
|---|---|---|---|---|---|---|---|---|---|
| <1 Time/Week | ≥5 Times/Week | p | <1 Time/Week | ≥5 Times/Week | p | <1 Time/Week | ≥5 Times/Week | p | |
| n | 47,572 | 13,003 | 23,720 | 17,113 | 35,672 | 4870 | |||
| Men, n (%) | 15,114 (31.8) | 4340 (33.4) | * | 9604 (40.5) | 4124 (24.1) | * | 12,145 (34.1) | 1503 (30.9) | * |
| Age (years) | 55.2 ± 8.1 | 52.3 ± 8.6 | * | 53.3 ± 8.3 | 53.5 ± 8.3 | * | 54.2 ± 8.5 | 52.0 ± 8.0 | * |
| BMI (kg/m2) | 24.1 ± 2.9 | 23.7 ± 3.0 | * | 24.0 ± 2.9 | 23.9 ± 2.9 | ns | 24.0 ± 3.0 | 23.8 ± 2.8 | * |
| WC (cm) | 81.7 ± 8.5 | 80.2 ± 8.9 | * | 81.6 ± 8.7 | 80.4 ± 8.6 | * | 81.5 ± 8.7 | 80.1 ± 8.7 | * |
| SBP (mmHg) | 124.1 ± 15.7 | 121.1 ± 15.1 | * | 122.8 ± 15.7 | 122.4 ± 15.7 | * | 123.7 ± 15.8 | 120.7 ± 15.2 | * |
| DBP (mmHg) | 77.0 ± 10.0 | 75.5 ± 9.9 | * | 76.4 ± 10.2 | 76.3 ± 10.1 | ns | 76.7 ± 10.1 | 75.6 ± 9.9 | * |
| Glucose (mg/dL) | 96.2 ± 23.0 | 94.2 ± 19.4 | * | 95.6 ± 22.3 | 94.0 ± 21.2 | * | 96.6 ± 23.5 | 93.3 ± 20.4 | * |
| TC (mg/dL) | 197.8 ± 36.5 | 197.9 ± 35.3 | ns | 196.2 ± 35.5 | 199.4 ± 35.8 | * | 195.0 ± 36.2 | 202.4 ± 35.0 | * |
| HDL-C (mg/dL) | 53.1 ± 12.7 | 55.5 ± 13.6 | * | 53.3 ± 12.9 | 55.0 ± 13.1 | * | 52.9 ± 12.7 | 56.3 ± 13.5 | * |
| TG (mg/dL) | 130.3 ± 90.6 | 122.0 ± 88.5 | * | 129.3 ± 90.8 | 123.0 ± 85.6 | * | 133.2 ± 97.4 | 117.7 ± 82.0 | * |
| hs-CRP | 0.15 ± 0.40 | 0.14 ± 0.41 | * | 0.15 ± 0.42 | 0.14 ± 0.38 | * | 0.16 ± 0.47 | 0.14 ± 0.40 | * |
| Smoking status | 6571 (13.8) | 1547 (11.9) | * | 4037 (17.0) | 1771 (10.4) | * | 4530 (12.3) | 616 (12.7) | * |
| Alcohol intake | 21,130 (44.4) | 6429 (49.4) | * | 12,390 (52.2) | 7292 (42.6) | * | 16,473 (46.2) | 7292 (42.6) | * |
| Regular exercise | 23,977 (50.4) | 7332 (56.4) | * | 11,338 (47.8) | 9999 (58.4) | * | 17,586 (49.4) | 2854 (58.9) | * |
| Hypertension | 9299 (19.6) | 1890 (14.5) | * | 4192 (17.7) | 2990 (17.5) | ns | 6815 (19.1) | 697 (14.3) | * |
| DM | 5455 (11.5) | 1007 (7.7) | * | 2348 (9.9) | 1523 (8.9) | * | 4386 (12.3) | 298 (6.1) | * |
| Dyslipidemia | 27,506 (57.8) | 7166 (55.1) | * | 13,202 (55.7) | 9846 (57.5) | * | 19,810 (55.5) | 2886 (59.3) | * |
| House income (KRW) | * | * | * | ||||||
| <1.5 million | 11,275 (29.6) | 1916 (17.0) | 5052 (26.1) | 2613 (18.9) | 7923 (26.8) | 634 (16.1) | |||
| 1.5–3.0 million | 12,992 (34.2) | 3669 (32.5) | 6476 (33.4) | 4725 (34.2) | 10,108 (34.2) | 1305 (33.2) | |||
| 3.0–6.0 million | 11,319 (29.8) | 4553 (40.4) | 6342 (32.7) | 5221 (37.8) | 9483 (32.1) | 1569 (39.9) | |||
| >6.0 million | 2449 (6.4) | 1145 (10.2) | 1509 (7.8) | 1252 (9.1) | 2042 (6.9) | 424 (10.8) | |||
| Energy (kcal/day) | 1803.9 ± 622.8 | 2461.0 ± 818.7 | * | 1645.8 ± 570.0 | 2527.8 ± 838.8 | * | 1708.7 ± 574.5 | 2859.1 ± 921.1 | * |
| CHO (g/day) | 316.5 ± 100.2 | 386.9 ± 119.2 | * | 292.5 ± 93.2 | 401.0 ± 122.4 | * | 300.9 ± 93.8 | 441.3 ± 132.8 | * |
| Fat (g/day) | 32.7 ± 22.0 | 60.5 ± 32.1 | * | 28.3 ± 20.2 | 61.2 ± 33.0 | * | 29.7 ± 20.2 | 75.3 ± 36.6 | * |
| Protein (g/day) | 59.4 ± 27.8 | 92.6 ± 38.1 | * | 51.0 ± 23.3 | 97.1 ± 41.3 | * | 56.9 ±25.4 | 107.0 ± 45.1 | * |
| CHO (%) | 71.0 ± 7.7 | 63.6 ± 7.4 | * | 71.9 ± 7.8 | 64.3 ± 7.8 | * | 70.6 ± 7.9 | 62.5 ± 7.3 | * |
| Fat (%) | 15.5 ± 0.6 | 21.4 ± 5.6 | * | 14.7 ± 6.3 | 21.1 ± 5.7 | * | 15.5 ± 6.1 | 23.1 ± 5.4 | * |
| Protein (%) | 12.9 ± 2.4 | 14.9 ± 2.2 | * | 12.2 ± 2.2 | 15.2 ± 2.5 | * | 13.2 ± 2.3 | 14.7 ± 2.4 | * |
| Na (mg/day) | 2529.2 ± 1882.7 | 4054.2 ± 2487.1 | * | 1809.9 ± 1399.4 | 5158.8 ± 2818.8 | * | 2422.8 ± 20.2 | 4884.2 ± 3128.5 | * |
| K (mg/day) | 3526.0 ± 1885.9 | 5101.4 ± 2337.6 | * | 2721.8 ± 1474.9 | 5878.6 ± 2520.8 | * | 3237.7 ± 1717.4 | 6065.5 ± 2768.6 | * |
| Ca (mg) | 502.2 ± 303.3 | 789.1 ± 391.7 | * | 381.3 ± 237.7 | 909.6 ± 439.9 | * | 395.3 ± 218.2 | 1229.2 ± 507.1 | * |
| P (mg) | 1077.5 ± 479.7 | 1645.2 ± 637.6 | * | 908.3 ± 397.5 | 1739.5 ± 686.9 | * | 980.4 ± 417.4 | 2037.9 ± 750.3 | * |
| Fe (mg) | 17.2 ± 9.2 | 25.0 ± 12.1 | * | 14.0 ± 7.5 | 28.3 ± 13.2 | * | 16.5 ± 8.5 | 28.5 ± 14.7 | * |
| Vitamin A (R.E.) | 314.1 ± 247.3 | 551.7 ± 333.9 | * | 230.4 ± 184.8 | 631.4 ± 382.0 | * | 284.4 ± 216.8 | 706.7 ± 420.3 | * |
| Niacin (mg) | 12.9 ± 6.2 | 18.5 ± 7.9 | * | 10.6 ± 4.9 | 20.8 ± 8.8 | * | 12.2 ± 5.7 | 21.4 ± 9.6 | * |
| Vitamin C (mg) | 224.3 ± 188.9 | 311.0 ± 211.0 | * | 159.3 ± 156.3 | 366.4 ± 235.9 | * | 201.2 ± 173.2 | 369.5 ± 257.4 | * |
| Zinc (μg) | 11.4 ± 4.7 | 15.5 ± 5.9 | * | 9.7 ± 3.8 | 17.1 ± 6.6 | * | 12.2 ± 5.7 | 18.4 ± 7.1 | * |
| Vitamin B6 (mg) | 2.3 ± 1.7 | 3.3 ± 2.1 | * | 1.7 ± 1.2 | 3.9 ± 2.7 | * | 2.1 ± 1.5 | 3.8 ± 2.7 | * |
| Folate (μg) | 580.3 ± 304.4 | 872.3 ± 374.4 | * | 463.7 ± 247.2 | 956.4 ± 404.4 | * | 561.3 ± 286.0 | 953.6 ± 448.8 | * |
| Retinol (μg) | 55.5 ± 67.0 | 148.8 ± 91.6 | * | 53.1 ± 60.7 | 118.8 ± 108.2 | * | 38.1 ± 47.1 | 234.5 ± 140.8 | * |
| Carotene (μg) | 3103.2 ± 2599.5 | 4834.4 ± 3455.2 | * | 2127.7 ± 1874.4 | 6151.4 ± 3952.7 | * | 2955.4 ± 2367.5 | 5666.4 ± 4218.2 | * |
| Fiber (g) | 29.8 ± 15.6 | 42.7 ±19.4 | * | 23.0 ± 12.1 | 49.9 ± 21.0 | * | 28.6 ± 14.6 | 47.3 ± 23.4 | * |
| Vitamin E (mg) | 12.9 ± 8.2 | 21.9 ± 10.9 | * | 9.8 ± 6.2 | 24.6 ± 12.5 | * | 12.2 ± 7.5 | 24.6 ± 13.5 | * |
| Cholesterol (mg) | 99.2 ± 86.6 | 403.0 ± 167.7 | * | 113.5 ± 104.5 | 258.4 ± 179.0 | * | 127.9 ± 108.8 | 309.2 ± 195.3 | * |
| Unadjusted | Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Egg consumption | ||||||||
| <1 time/week | 0.87 (0.68–1.11) | 0.268 | 0.86 (0.67–1.10) | 0.223 | 0.88 (0.69–1.13) | 0.307 | 0.89 (0.69–1.15) | 0.361 |
| 1–2 times/week | 0.99 (0.77–1.27) | 0.938 | 1.02 (0.79–1.31) | 0.901 | 1.03 (0.80–1.33) | 0.808 | 1.04 (0.81–1.34) | 0.771 |
| 3–4 times/week | 1.03 (0.78–1.37) | 0.84 | 1.05 (0.79–1.39) | 0.759 | 1.05 (0.79–1.40) | 0.713 | 1.06 (0.80–1.41) | 0.696 |
| ≥5 times/week | ref | ref | ref | ref | ||||
| Seaweeds | ||||||||
| <1 time/week | ref | ref | ref | ref | ||||
| 1–2 times/week | 0.78 (0.65–0.93) | 0.006 | 0.74 (0.62–0.88) | <0.001 | 0.72 (0.61–0.86) | <0.001 | 0.68 (0.57–0.82) | <0.001 |
| 3–4 times/week | 0.75 (0.61–0.93) | 0.01 | 0.66 (0.53–0.82) | <0.001 | 0.63 (0.51–0.79) | <0.001 | 0.57 (0.45–0.71) | <0.001 |
| ≥5 times/week | 0.62 (0.47–0.82) | <0.001 | 0.51 (0.39–0.67) | <0.001 | 0.49 (0.37–0.64) | <0.001 | 0.42 (0.32–0.56) | <0.001 |
| Dairy products | ||||||||
| <1 time/week | 1.31 (1.05–1.63) | 0.017 | 1.24 (0.99–1.55) | 0.056 | 1.24 (0.99–1.56) | 0.049 | 1.32 (1.05–1.67) | 0.017 |
| 1–2 times/week | 1.21 (1.00–1.47) | 0.052 | 1.15 (0.95–1.40) | 0.161 | 1.15 (0.94–1.39) | 0.169 | 1.18 (0.97–1.43) | 0.106 |
| 3–4 times/week | ref | ref | ref | ref | ||||
| ≥5 times/week | 1.25 (0.83–1.89) | 0.282 | 1.16 (0.77–1.75) | 0.486 | 1.14 (0.75–1.72) | 0.539 | 1.12 (0.74–1.69) | 0.588 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, Y.-J.; Lee, H.-S.; Kang, S.-W.; Lee, J.-W. Association between Consumption of Iodine-Rich Foods and Thyroid Cancer Prevalence: Findings from a Large Population-Based Study. Nutrients 2024, 16, 1041. https://doi.org/10.3390/nu16071041
Kwon Y-J, Lee H-S, Kang S-W, Lee J-W. Association between Consumption of Iodine-Rich Foods and Thyroid Cancer Prevalence: Findings from a Large Population-Based Study. Nutrients. 2024; 16(7):1041. https://doi.org/10.3390/nu16071041
Chicago/Turabian StyleKwon, Yu-Jin, Hye-Sun Lee, Sang-Wook Kang, and Ji-Won Lee. 2024. "Association between Consumption of Iodine-Rich Foods and Thyroid Cancer Prevalence: Findings from a Large Population-Based Study" Nutrients 16, no. 7: 1041. https://doi.org/10.3390/nu16071041
APA StyleKwon, Y.-J., Lee, H.-S., Kang, S.-W., & Lee, J.-W. (2024). Association between Consumption of Iodine-Rich Foods and Thyroid Cancer Prevalence: Findings from a Large Population-Based Study. Nutrients, 16(7), 1041. https://doi.org/10.3390/nu16071041







