A Novel Tetrapeptide Ala-Phe-Phe-Pro (AFFP) Derived from Antarctic Krill Prevents Scopolamine-Induced Memory Disorder by Balancing Lipid Metabolism of Mice Hippocampus
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
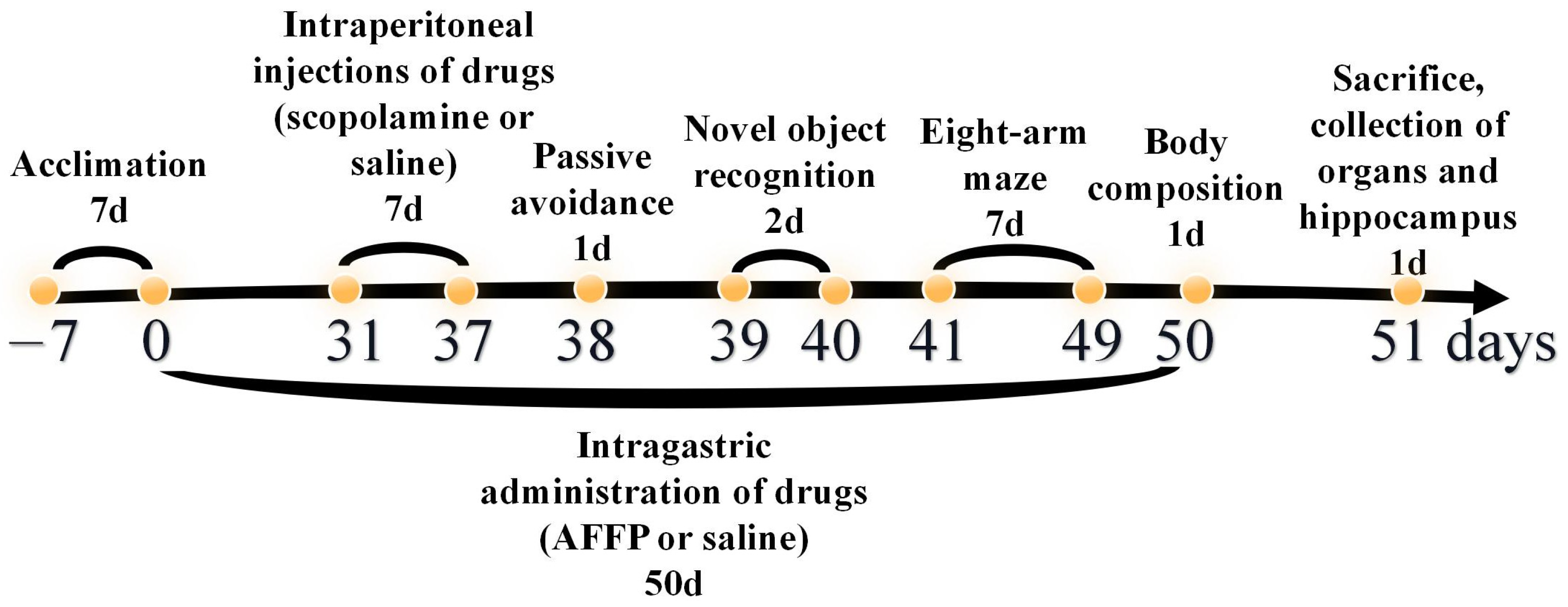
2.2. Animals Treatment
2.2.1. Animals’ Treatment in Behavior Experiment
2.2.2. Animals’ Treatment in Fluorescence Imaging Experiment
2.3. Analysis of Nuclear Magnetic Resonance (NMR) Spectroscopy
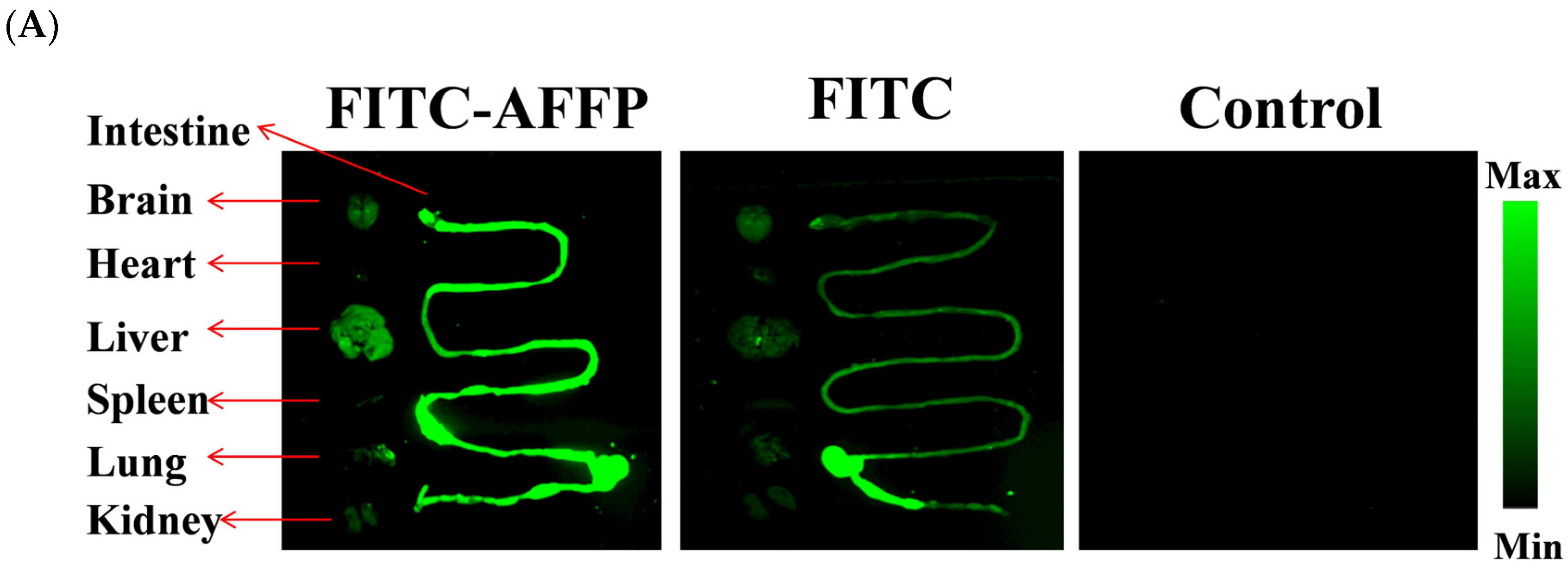
2.4. Analysis of Absorption Distribution of AFFP In Vivo by Fluorescence Imaging
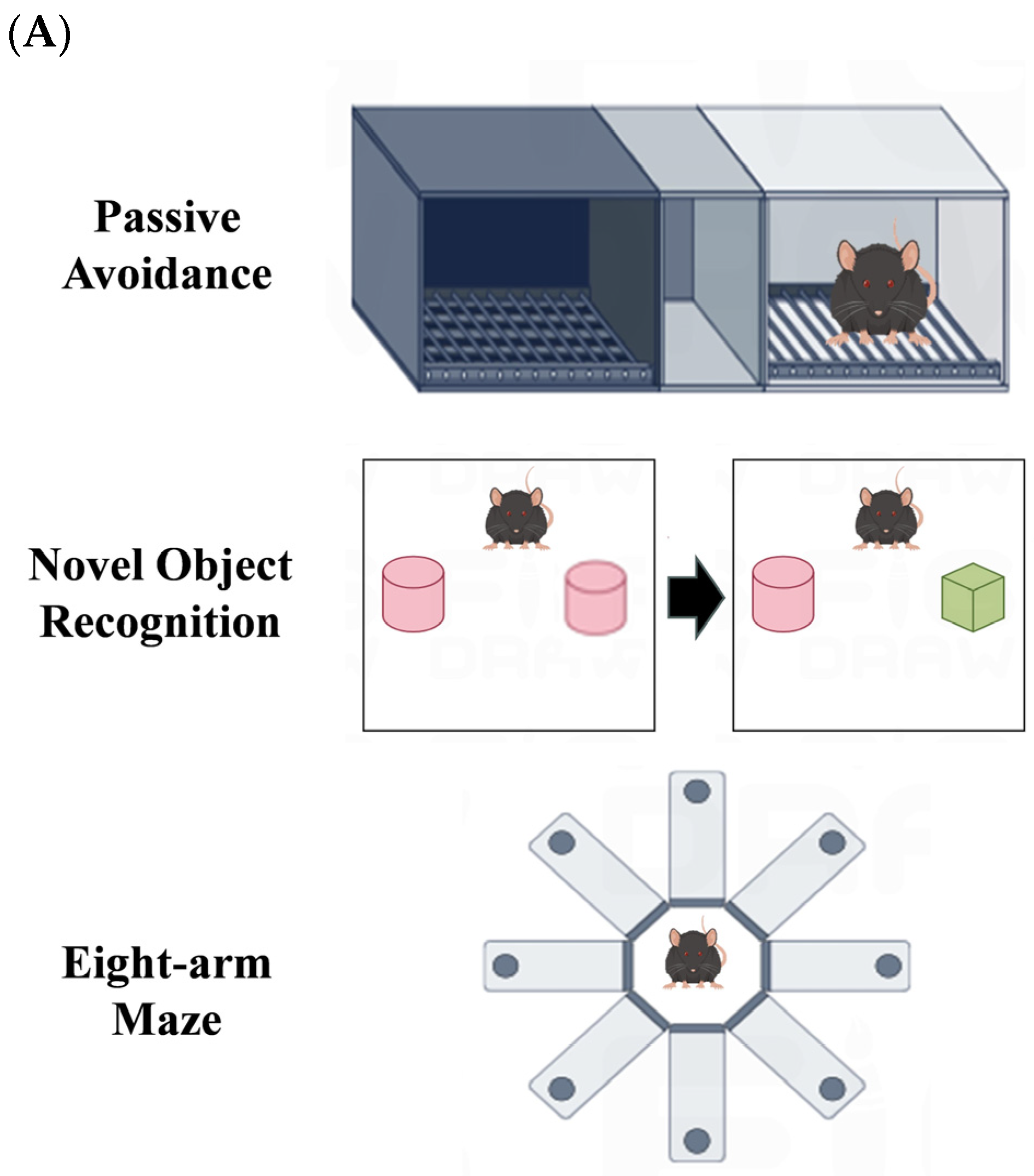
2.5. Passive Avoidance Experiment
2.6. Novel Object Recognition Experiment
2.7. Eight-Arm Maze Experiment
2.8. Body Composition Analysis
2.9. Calculation of Organ Coefficients
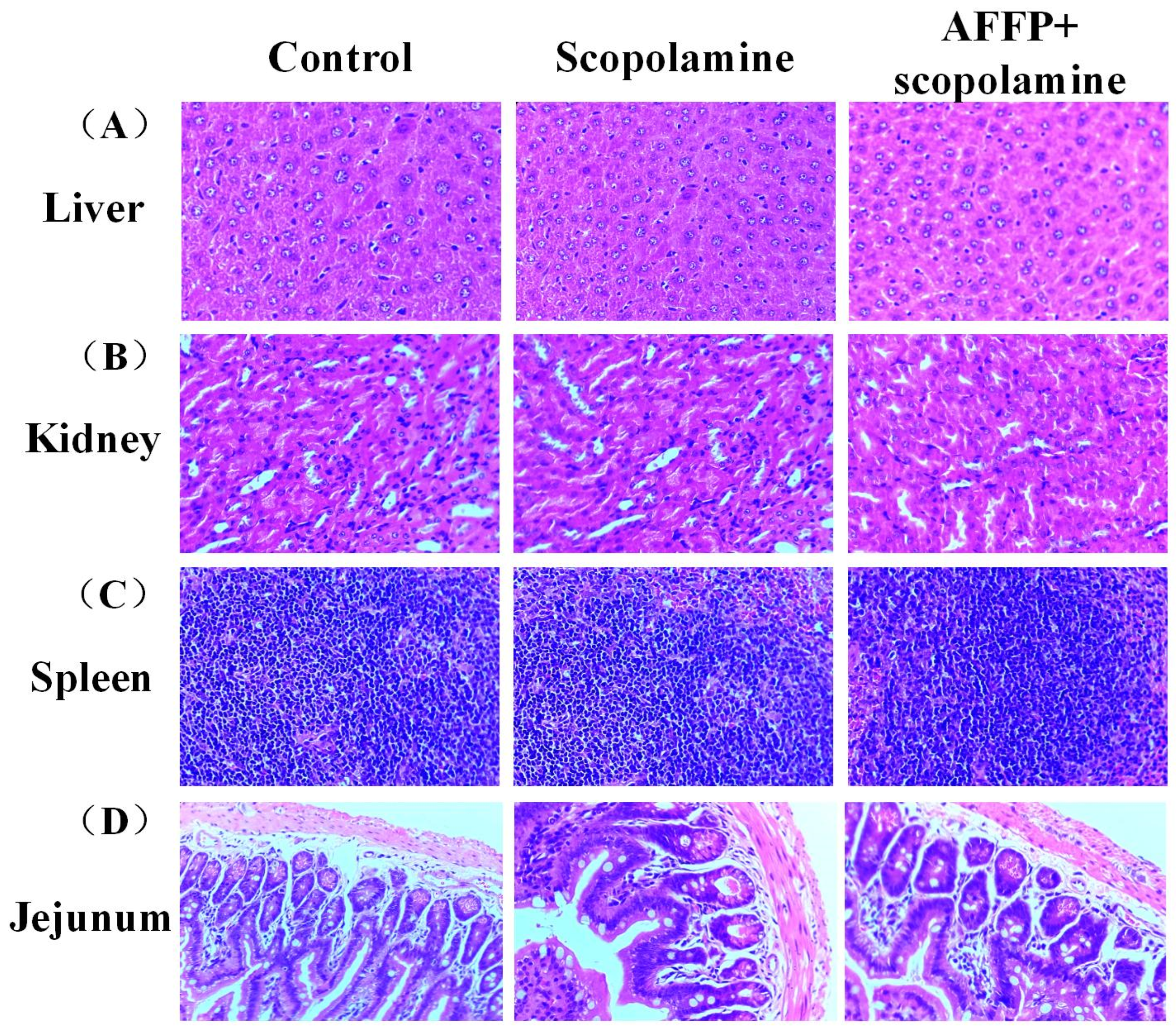
2.10. H&E Staining in Liver, Spleen, Kidney, and Jejunum
2.11. Analysis of Acetylcholine (ACh) and Acetylcholinesterase (AChE) Levels
2.12. Analysis of ROS Levels, SOD Activity, and MDA Level
2.13. Analysis of Saturated and Unsaturated Lipid Contents
2.14. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of Active Hydrogen Atoms in AFFP
3.2. Absorption Distribution of AFFP In Vivo
3.3. Assessment of Adverse Reactions of AFFP to Mice
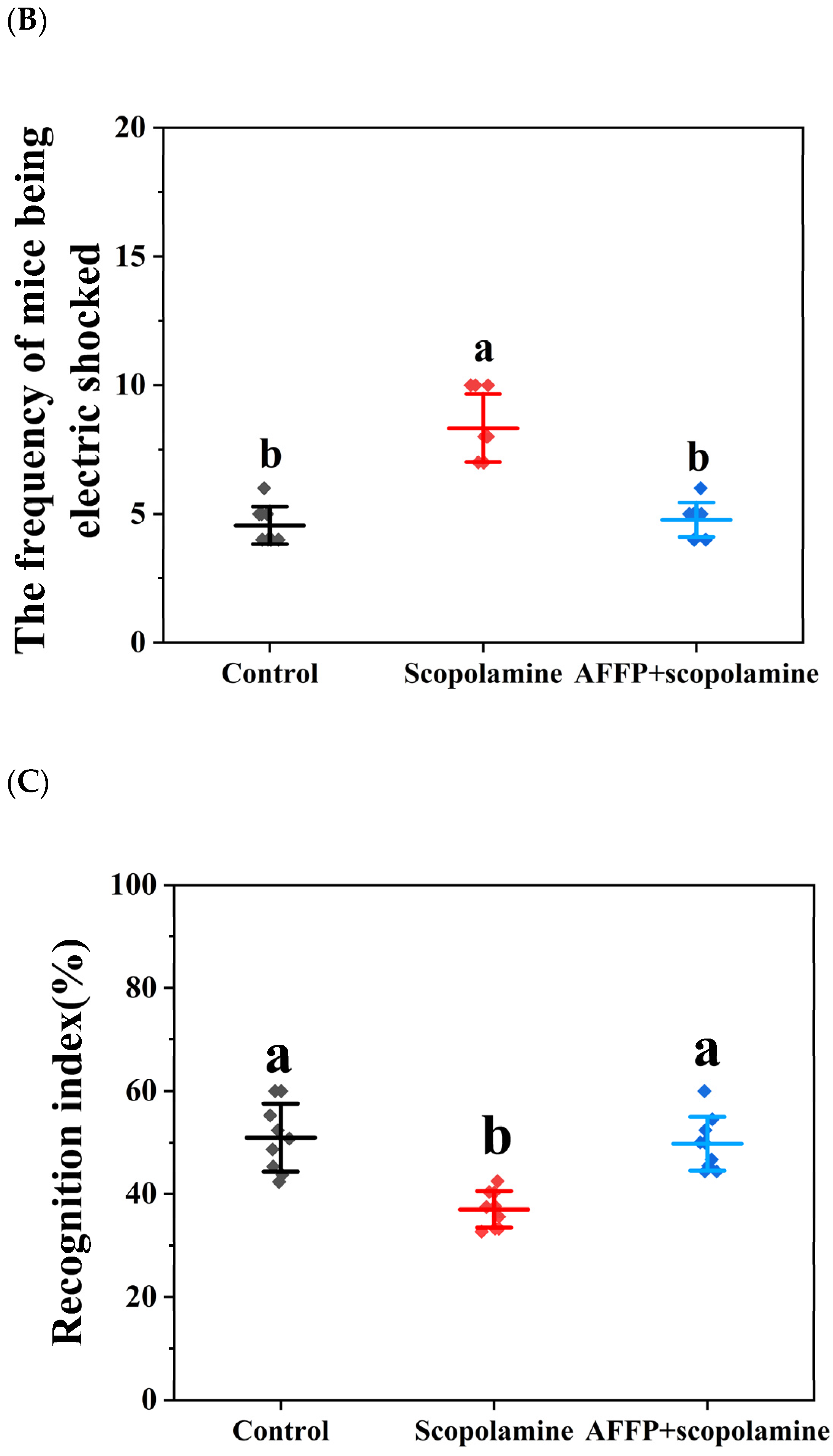
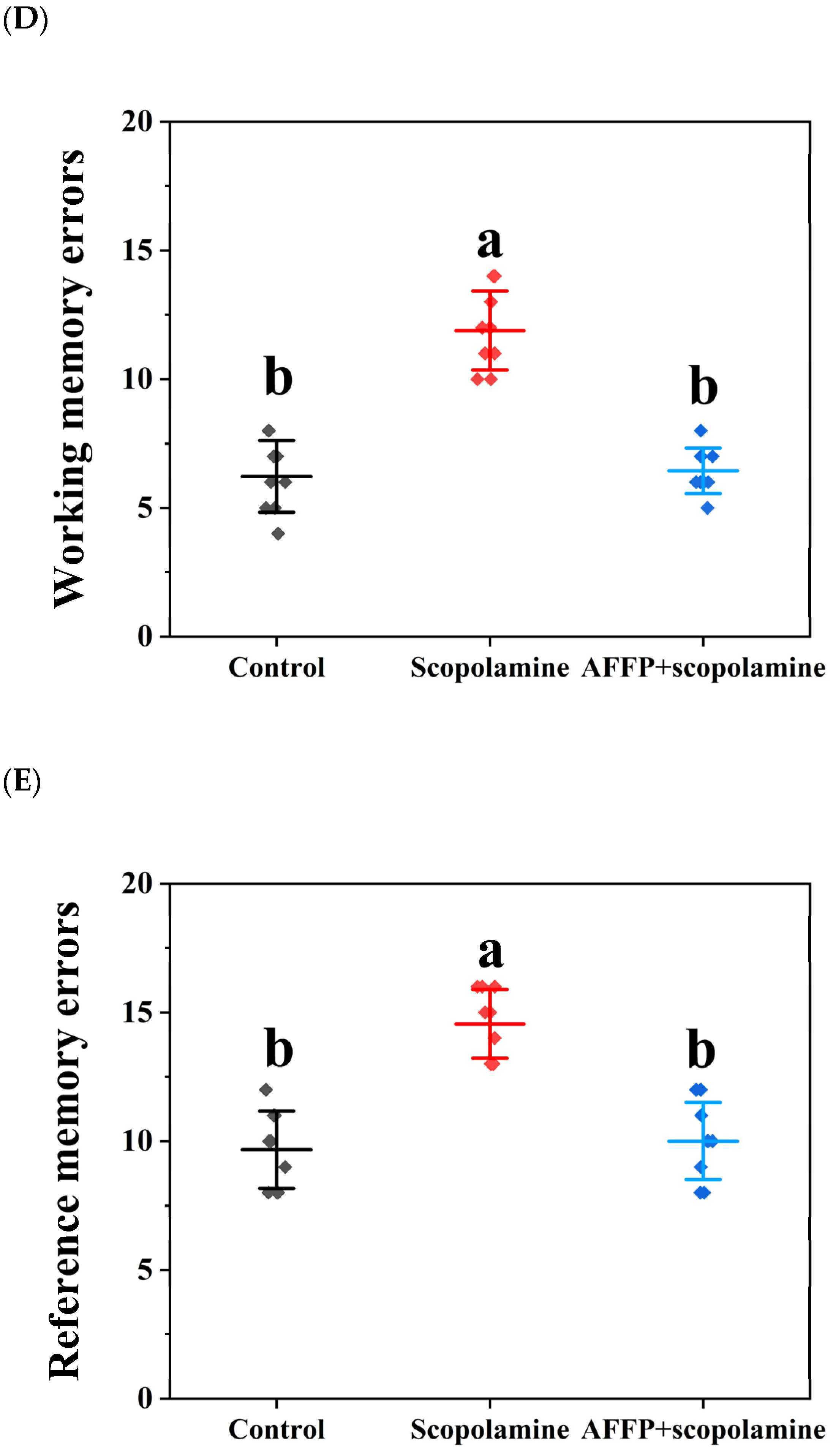
3.4. Effects of AFFP on Behavioral Experiments in Mice
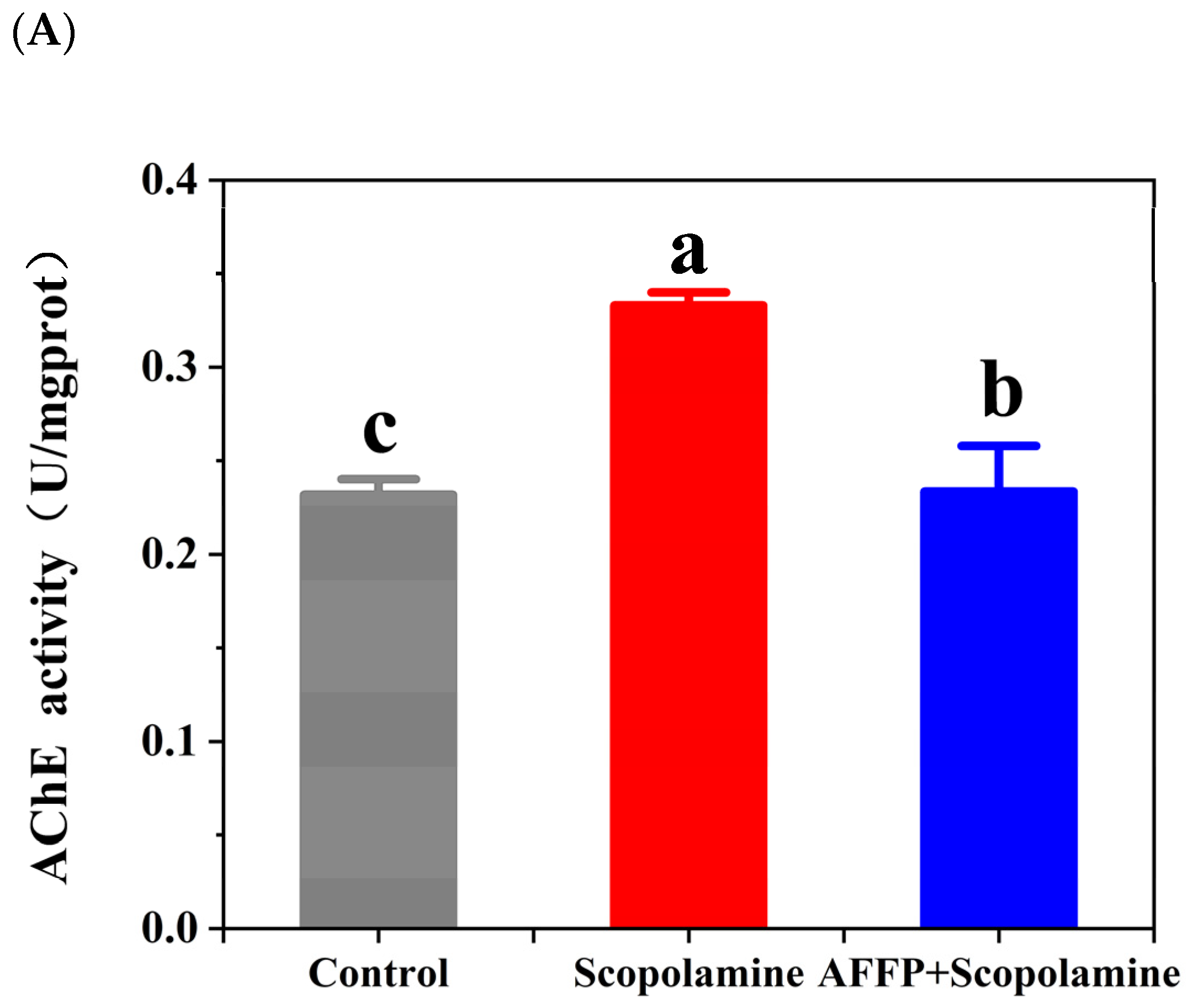
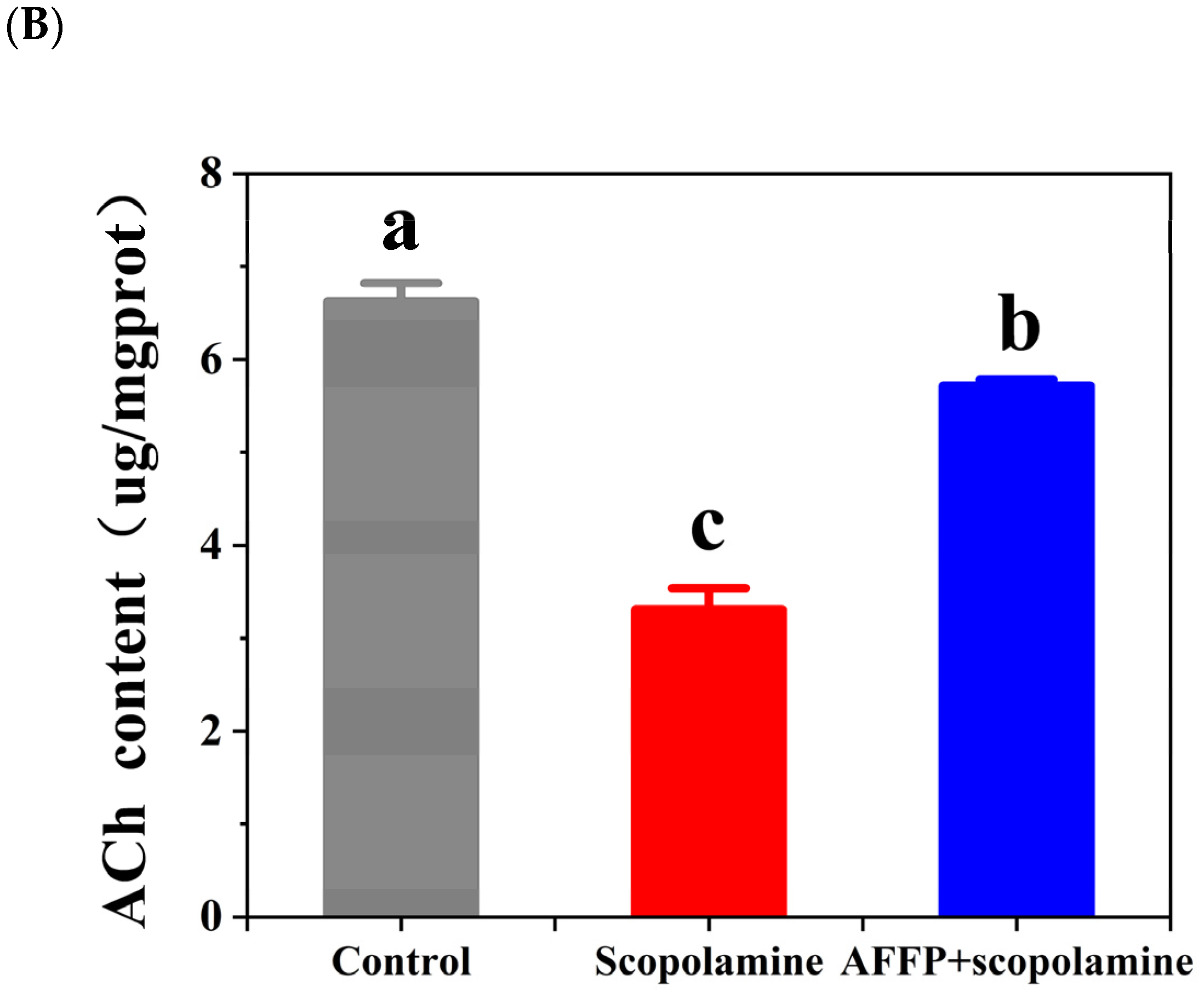
3.5. Effect of AFFP on AChE Activity and ACh Content in the Hippocampus of Scopolamine-Induced Mice
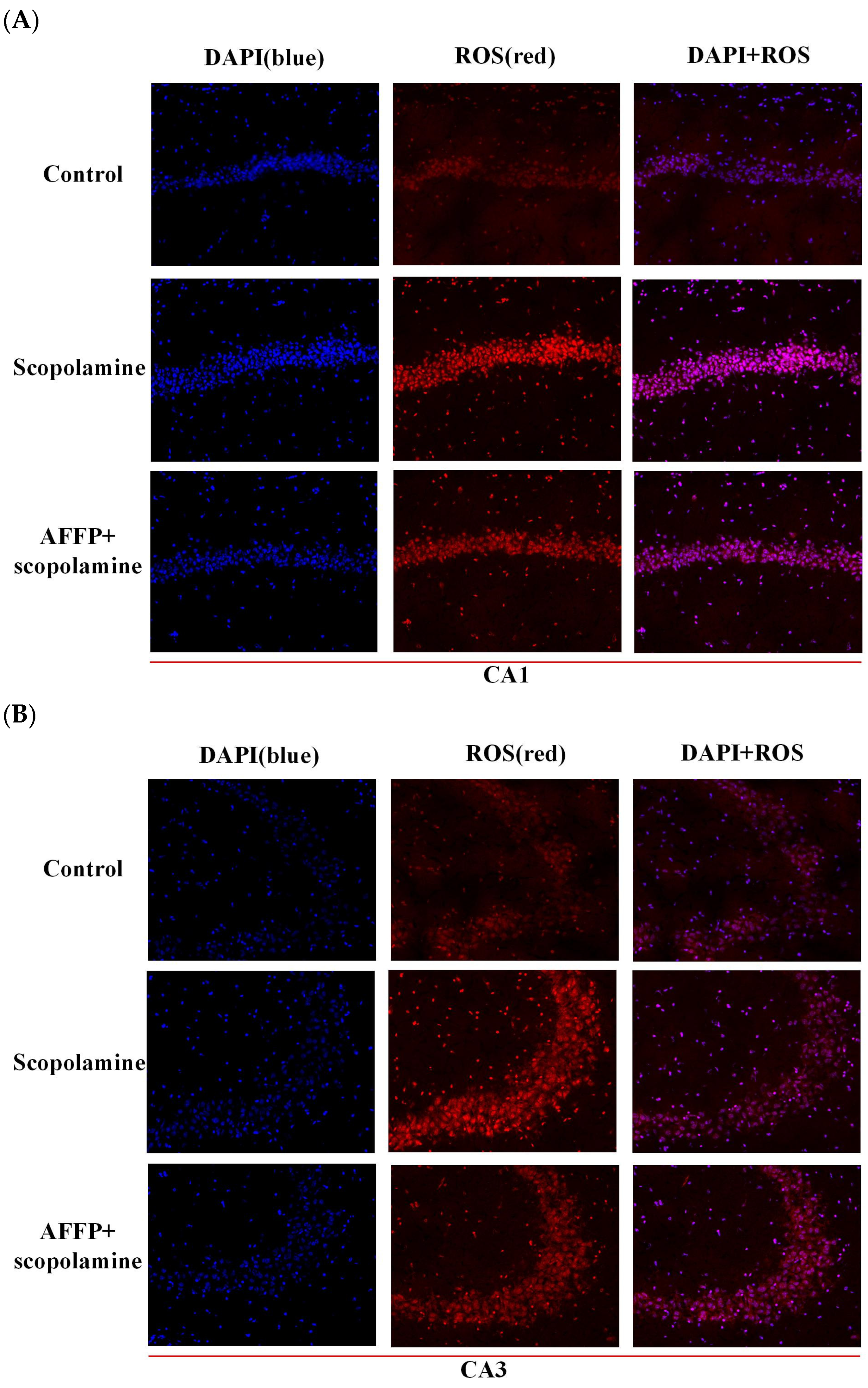
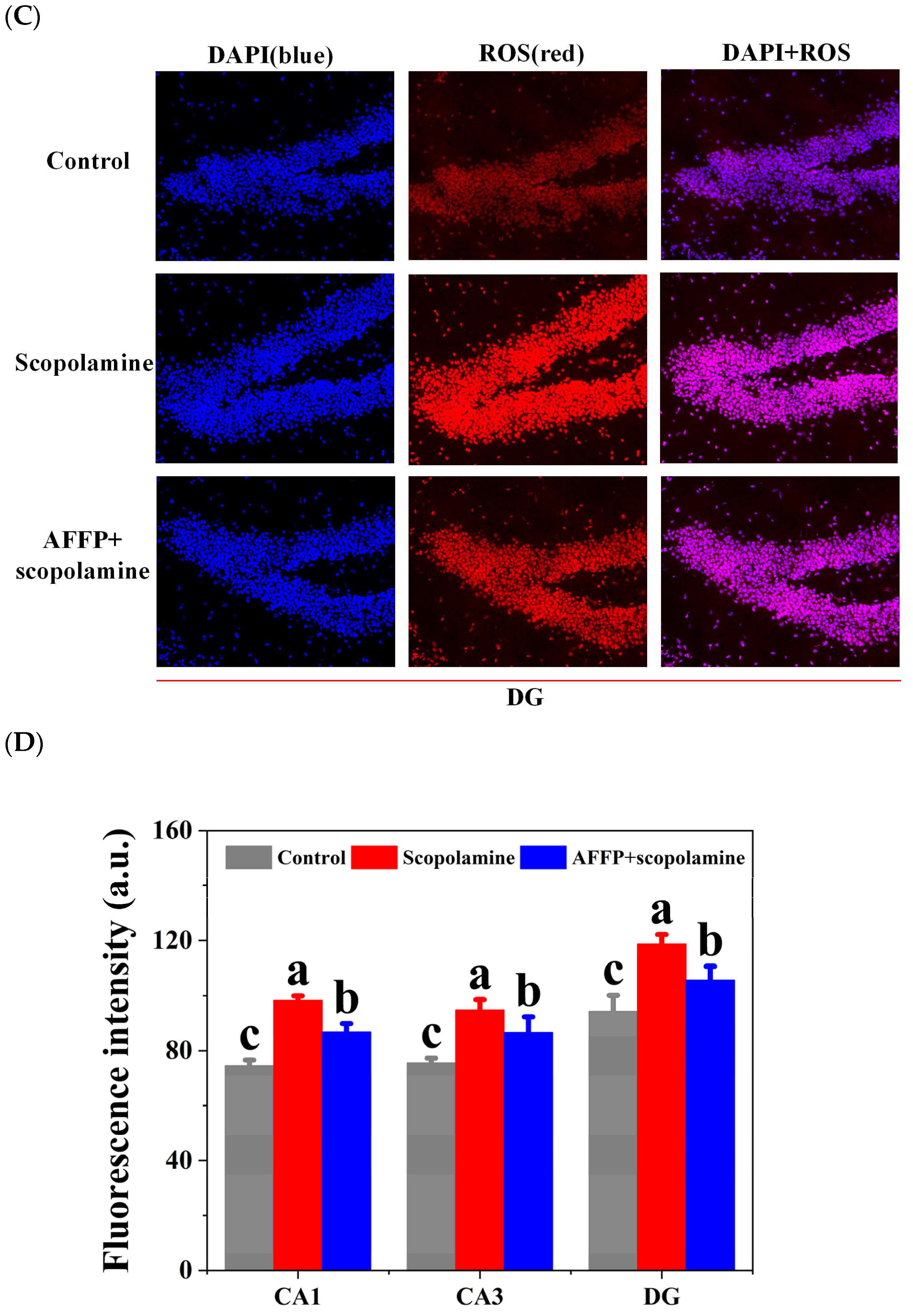
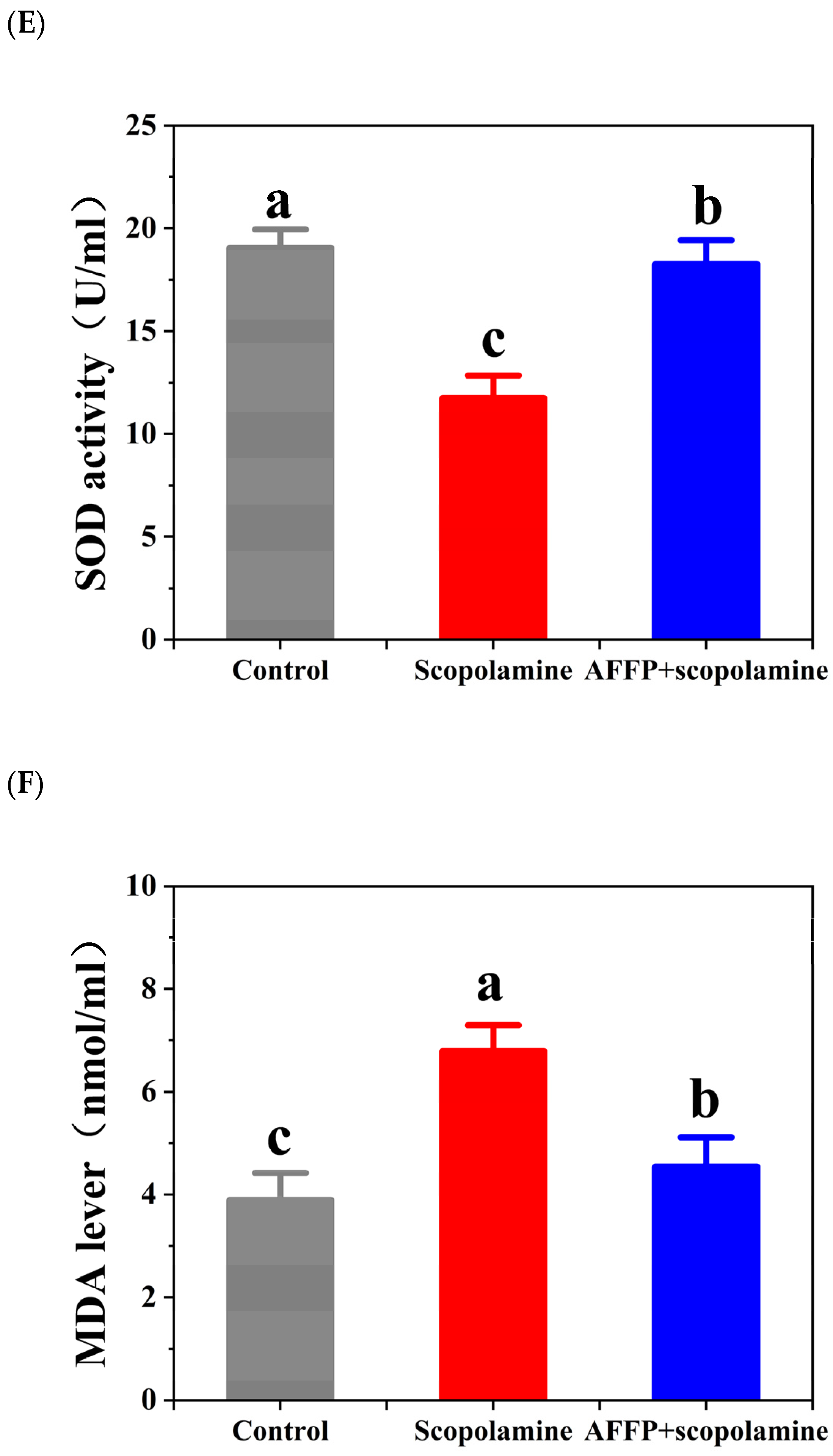
3.6. Effect of AFFP on Scopolamine-Induced Oxidative Stress
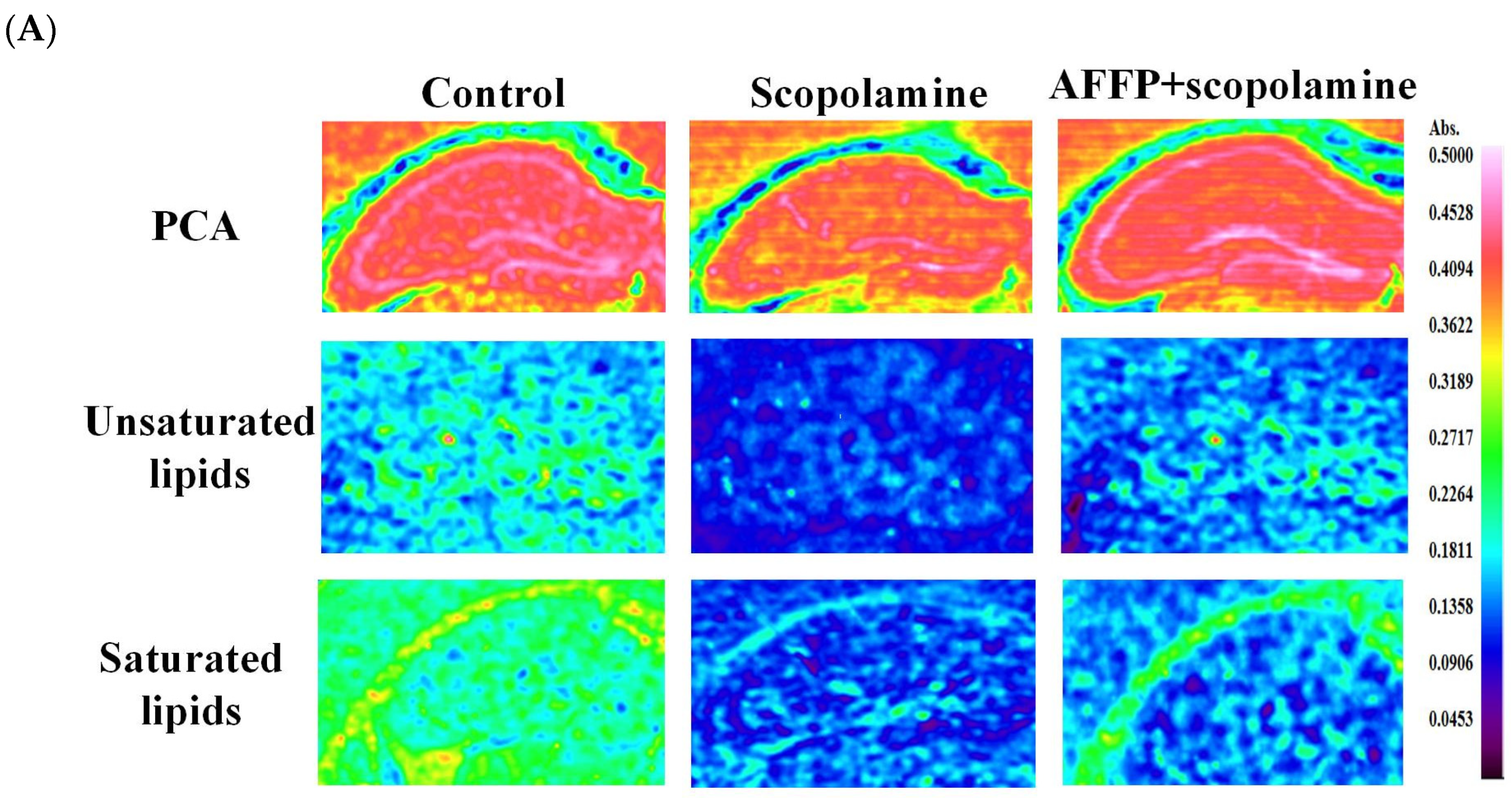
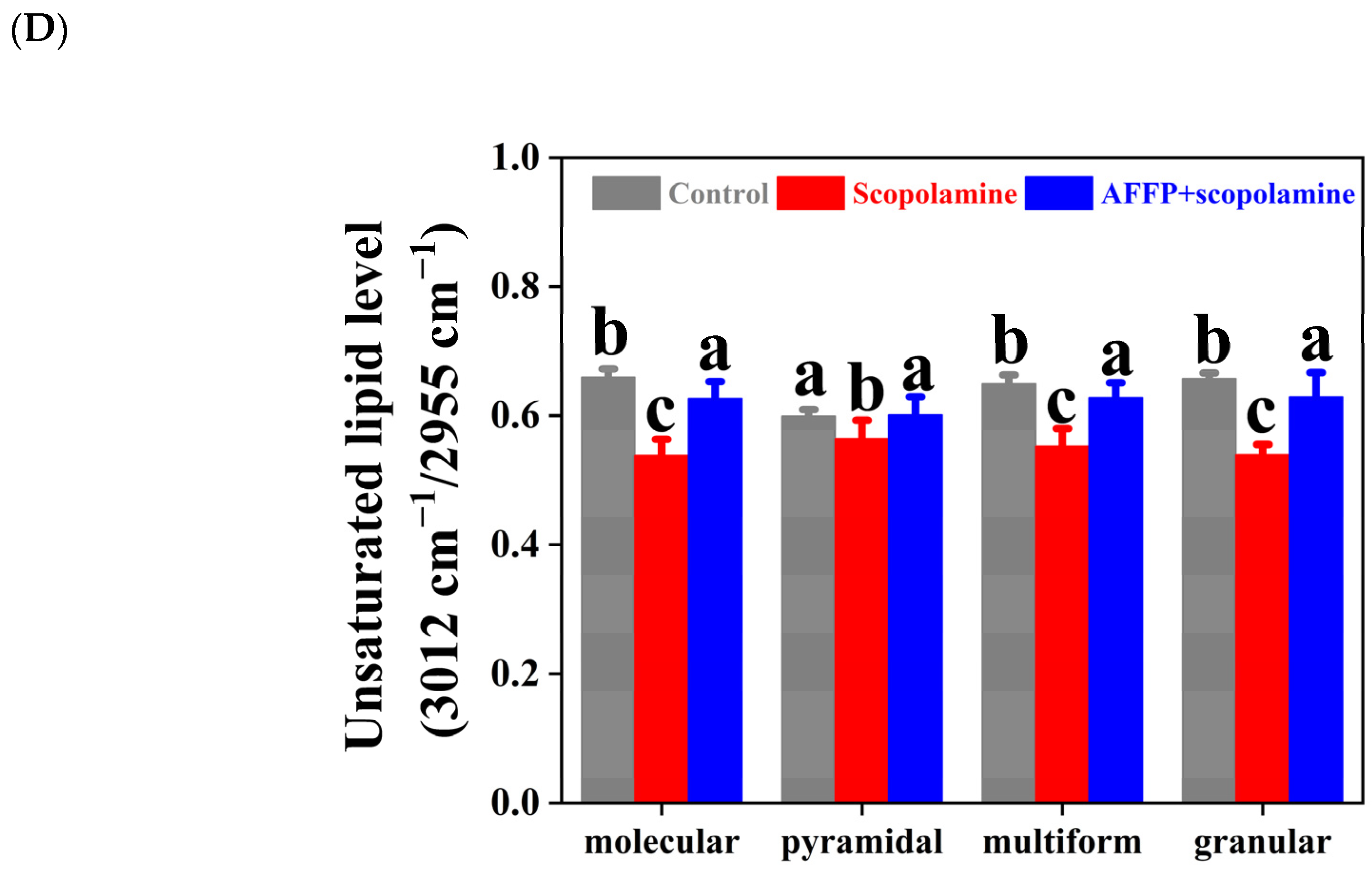
3.7. Effect of AFFP on Unsaturated Lipid Level of Hippocampus
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFFP | Ala-Phe-Phe-Pro |
| SSDAFFPFR | Ser-Ser-Asp-Ala-Phe-Phe-Pro-Phe-Arg |
| ROS | reactive oxygen species |
| MDA | malondialdehyde |
| FITC | fluorescein isothiocyanate |
| NMR | nuclear magnetic resonance |
| DMSO | dimethyl sulfoxide |
| BBFO | broad band fluorine observation |
| D2O | deuterium oxide |
| FITC-AFFP | fluorescein isothiocyanate labelled AFFP |
| WMEs | working memory errors |
| RMEs | reference memory errors |
| MRI | magnetic resonance imaging |
| H&E | hematoxylin and eosin |
| ACh | acetylcholine |
| AChE | acetylcholinesterase |
| BCA | bicinchoninic acid |
| DHE | dihydroethidium |
| PBS | phosphate buffer saline |
| DAPI | 4′,6-diamidino-2-phenylindole |
| SOD | superoxide dismutase |
| FTIR | fourier transform infrared |
| ANOVA | analysis of variance |
| BBB | blood–brain barrier |
References
- Berry, J.A.; Cervantes-Sandoval, I.; Chakraborty, M.; Davis, R.L. Sleep facilitates memory by blocking dopamine neuron-mediated forgetting. Cell 2015, 161, 1656–1667. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Zhao, T.; Zhao, Y.Q.; Sun-Waterhouse, D.; Qiu, C.; Huang, P.; Zhao, M. Effect of anchovy (Coilia mystus) protein hydrolysate and its Maillard reaction product on combating memoryimpairment in mice. Food Res. Int. 2016, 82, 112–120. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.; Xing, R.; Liu, S.; Chen, X.; Li, P. Effect and mechanism of oyster hydrolytic peptides on spatial learning and memory in mice. RSC Adv. 2018, 8, 6125–6135. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Han, J.; Shimozono, H.; Villareal, M.O.; Isoda, H. Caffeoylquinic Acid-Rich Purple Sweet Potato Extract, with or without Anthocyanin, Imparts Neuroprotection and Contributes to the Improvement of Spatial Learning and Memory of SAMP8 Mouse. J. Agric. Food Chem. 2013, 61, 5037–5045. [Google Scholar] [CrossRef] [PubMed]
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects: The 2017 Revision, Key Findings and Advance Tables. Working Paper No. ESA/P/WP/248. 2017. Available online: https://population.un.org/wpp/Publications/Files/WPP2017_KeyFindings.pdf (accessed on 19 November 2019).
- Lu, H.Y.; Fang, L.; Wang, J.; Zhao, F.R.; Liu, C.L.; Gao, Y.W.; Liu, J.S.; Min, W.H. Pine nut antioxidant peptides ameliorate the memory impairment in a scopolamine-induced mouse scopolamine via sirt3-induced synaptic plasticity. Food Funct. 2021, 12, 8026. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.Q.; Xu, X.M.; Li, D.M.; Sun, N.; Lin, S.Y. Sea cucumber peptides attenuated the scopolamine-induced memory impairment in mice and rats and the underlying mechanism. J. Agric. Food Chem. 2021, 70, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Su, G.; Zhang, X.; Song, G.; Zhang, L.; Zheng, L.; Zhao, M. Characterization and exploration of potential neuroprotective peptides in walnut (Juglans regia) protein hydrolysate against cholinergic system damage and oxidative stress in scopolamine-induced cognitive and memory impairment mice and zebrafish. J. Agric. Food Chem. 2021, 69, 2773–2783. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, X.; Ren, G.; Wu, C.; Qin, P.; Yao, Y. Peptides from extruded lupin (Lupinus albus, L.) regulate inflammatory activity via the p38 MAPK signal transduction pathway in RAW 264.7 cells. J. Agric. Food Chem. 2020, 68, 11702–11709. [Google Scholar] [CrossRef] [PubMed]
- Slemmer, J.E.; Shacka, J.J.; Sweeney, M.I.; Weber, J.T. Antioxidants and free radical scavengers for the treatment of stroke, traumatic brain injury and aging. Curr. Med. Chem. 2008, 15, 404–414. [Google Scholar]
- Zhang, X.; Wu, C.; Tan, W. Brain lipid dynamics in amyloid precursor protein/presenilin 1 mouse scopolamine of early Alzheimer’s disease by desorption electrospray ionization and matrix assisted laser desorption ionization-mass spectrometry imaging techniques. J. Proteome Res. 2021, 20, 2643–2650. [Google Scholar] [CrossRef]
- Albouery, M.; Buteau, B.; Gregoire, S.; Cherbuy, C.; Pais de Barros, J.P.; Martine, L.; Chain, F.; Cabaret, S.; Berdeaux, O.; Bron, A.M.; et al. Age-related changes in the gut microbiota modify brain lipid composition. Front. Cell. Infect. Microbiol. 2020, 9, 444. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Qaing, S.; Yang, B.; Wang, Y.; Wang, J.; Yang, T.; Zhang, Y.; Chen, Y.; Li, S. Cold plasma treatment with alginate oligosaccharide improves the digestive stability and bioavailability of nutrient-delivered particles: An in vitro INFOGEST gastrointestinal study. Int. J. Biol. Macromol. 2023, 232, 123309. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Hu, J.; Li, J.; Yang, Z.; Xin, X.; Wang, J.; Ding, J.; Geng, M. Effect of acidic oligosaccharide sugar chain on scopolamine-induced memory impairment in rats and its related mechanisms. Neuroence Lett. 2006, 374, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Kantham, S.; Rao, V.M.; Palanivelu, M.K.; Pham, H.L.; Shaw, P.N.; McGeary, R.P.; Ross, B.P. Metal chelation, radical scavenging and inhibition of Aβ42 fibrillation by food constituents in relation to Alzheimer’s disease. Food Chem. 2016, 199, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Gao, Y.; Ding, J.; Sun, N.; Lin, S. Antarctic krill peptides improve scopolamine-induced memory impairment in mice. Food Biosci. 2022, 49, 101987. [Google Scholar] [CrossRef]
- Zheng, J.; Li, M.; Wang, C.; Sun, N.; Lin, S. A novel nonapeptide SSDAFFPFR from Antarctic krill exerts a protective effect on PC12 cells through the BCL-XL/Bax/Caspase-3/p53 signaling pathway. Food Biosci. 2021, 43, 101345. [Google Scholar] [CrossRef]
- Rivero-Pino, F.; Espejo-Carpio, F.J.; Guadix, E.M. Antidiabetic food-derived peptides for functional feeding: Production, functionality and in vivo evidences. Foods 2020, 9, 983. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.A.; Pintado, M.E. Bioactive peptides derived from marine sources: Biological and functional properties. Trends Food Sci. Technol. 2022, 119, 348–370. [Google Scholar] [CrossRef]
- Kita, M.; Obara, K.; Kondo, S.; Umeda, S.; Ano, Y. Effect of supplementation of a whey peptide rich in tryptophan-tyrosine-related peptides on cognitive performance in healthy adults: A randomized, double-blind, placebo-controlled study. Nutrients 2018, 10, 899. [Google Scholar] [CrossRef]
- Kita, M.; Kobayashi, K.; Obara, K.; Koikeda, T.; Umeda, S.; Ano, Y. Supplementation with whey peptide rich in β-lactolin improves cognitive performance in healthy older adults: A randomized, double-blind, placebo-controlled study. Front. Neurosci. 2019, 13, 448995. [Google Scholar] [CrossRef]
- Ohsawa, K.; Nakamura, F.; Uchida, N.; Mizuno, S.; Yokogoshi, H. Lactobacillus helveticus-fermented milk containing lactononadecapeptide (NIPPLTQTPVVVPPFLQPE) improves cognitive function in healthy middle-aged adults: A randomised, double-blind, placebo-controlled trial. Int. J. Food Sci. Nutr. 2018, 69, 369–376. [Google Scholar] [CrossRef]
- Wu, D.; Xu, X.; Sun, N.; Li, D.; Zhu, B.; Lin, S. AGLPM and QMDDQ peptides exert a synergistic action on memory improvement against scopolamine-induced amnesiac mice. Food Funct. 2020, 11, 10925–10935. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, Z.; Xu, X.; Sun, N.; Lin, S. Sea cucumber-derived peptide attenuates scopolamine-induced cognitive impairment by preventing hippocampal cholinergic dysfunction and neuronal cell death. J. Agric. Food Chem. 2022, 70, 567–576. [Google Scholar] [CrossRef]
- Zhang, S.; Qi, L.; Li, D.; Zhong, L.; Wu, D.; Lin, S. The regulatory mechanism of pulsed electric field (PEF) targeting at C-terminal glutamine of shrimp antioxidant peptide QMDDQ based on MD simulation. LWT 2021, 141, 110930. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, X.; Hua, Z.; Xing, S.; Li, J.; Fei, S.; Tan, M. ROS-triggered self-disintegrating and pH-responsive astaxanthin nanoparticles for regulating the intestinal barrier and colitis. Biomaterials 2023, 292, 121937. [Google Scholar] [CrossRef]
- Erika, A.; Benno, R. The inhibitory avoidance discrimination task to investigate accuracy of memory. Front. Behav. Neurosci. 2015, 9, 60. [Google Scholar]
- Puzzo, D.; Bizzoca, A.; Privitera, L.; Furnari, D.; Giunta, S.; Girolamo, F.; Pinto, M.; Gennarini, G.; Palmeri, A. F3/Contactin promotes hippocampal neurogenesis, synaptic plasticity, and memory in adult mice. Hippocampus 2014, 23, 1367–1382. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Li, C.; Xie, Y.; He, L.L.; Xiao, F.; Zhan, K.B.; Tang, Y.Y.; Li, X.; Tang, X.Q. Hippocampal ornithine decarboxylase/spermidine pathway mediates H2S-alleviated cognitive impairment in diabetic rats: Involving enhancment of hippocampal autophagic flux. J. Adv. Res. 2021, 27, 31–40. [Google Scholar] [CrossRef]
- Tian, Q.; Tang, H.L.; Tang, Y.Y.; Zhang, P.; Kang, X.; Zou, W.; Tang, X.Q. Hydrogen Sulfide Attenuates the Cognitive Dysfunction in Parkinson’s Disease Rats via Promoting Hippocampal Microglia M2 Polarization by Enhancement of Hippocampal Warburg Effect. Oxid Med. Cell Longev. 2022, 2022, 2792348. [Google Scholar] [CrossRef] [PubMed]
- Olton, D.S.; Samuelson, R.J. Remembrance of places passed: Spatial memory in rats. J. Exp. Psychol. Anim. Behav. Process. 1976, 2, 97–116. [Google Scholar] [CrossRef]
- Takayoshi, M.; Yoko, F.; Chiaki, K. Participation of the hippocampal theta rhythm in memory formation for an eight-arm radial maze task in rats. Brain Res. 2006, 1103, 159–163. [Google Scholar]
- Zhu, Z.J.; Zhu, B.W.; Sun, Y.J.; Ai, C.Q.; Wang, L.L.; Wen, C.R.; Yang, J.F.; Song, S.; Liu, X.L. Sulfated Polysaccharide from Sea Cucumber and its Depolymerized Derivative Prevent Obesity in Association with Modification of Gut Microbiota in High-Fat Diet-Fed Mice. Mol. Nutr. Food Res. 2018, 62, 1800446. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Cui, X.J.; Lin, Q.L.; Cai, J.; Tang, L.H.; Liang, Y. Active peptide KF-8 from rice bran attenuates oxidative stress in a mouse scopolamine of aging induced by D-galactose. J. Agric. Food Chem. 2020, 68, 12271–12283. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Fang, J.; Tong, Z.Y.; He, S.S.; Luo, Y.Y. Transcranial direct current stimulation ameliorates cognitive impairment via modulating oxidative stress, inflammation, and autophagy in a rat scopolamine of vascular dementia. Front. Neurosci. 2020, 14, 28–37. [Google Scholar] [CrossRef]
- Ko, Y.H.; Kwon, S.H.; Ma, S.X.; Seo, J.Y.; Lee, B.R.; Kim, K.; Kim, S.Y.; Lee, S.Y.; Jang, C.G. The memory-enhancing effects of 7,8,4′-trihydroxyisoflavone, a major metabolite of daidzein, are associated with activation of the cholinergic system and BDNF signaling pathway in mice. Brain Res. 2018, 142, 197–206. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Ma, Q. Redox regulation by nuclear factor erythroid 2-related factor 2: Gatekeeping for the basal and diabetes-induced expression of thioredoxin-interacting protein. Mol. Pharmacol. 2012, 82, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Huang, Q.; Wang, S. Isolation of a novel luteinprotein complex from Chlorella vulgaris and its functional properties. Food Funct. 2015, 6, 1893–1899. [Google Scholar] [CrossRef] [PubMed]
- Chwiej, J.G.; Ciesielka, S.W.; Skoczen, A.K.; Janeczko, K.J.; Sandt, C.; Planeta, K.L.; Setkowicz, Z.K. Biochemical changes indicate developmental stage in the hippocampal formation. ACS Chem. Neurosci. 2018, 10, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Sun, N.; Zhang, S.; Zheng, J.; Lin, S. A new dual-peptide strategy for enhancing antioxidant activity and exploring the enhancement mechanism. Food Funct. 2019, 10, 7533–7543. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, S.; Ju, H.; Bao, Z.; Lin, S. Tryptophan targeted pulsed electric field treatment for enhanced immune activity in pine nut peptides. J. Food Biochem. 2020, 44, e13224. [Google Scholar] [CrossRef]
- Liang, R.; Cheng, S.; Wang, X. Secondary structure changes induced by pulsed electric field affect antioxidant activity of pentapeptides from pine nut (Pinus koraiensis) protein. Food Chem. 2018, 254, 170–184. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, M.; Xing, J.; Lin, S. A possible mechanism for enhancing the antioxidant activity by pulsed electric field on pine nut peptide Glutamine-Tryptophan-Phenylalanine-Histidine. J. Food Biochem. 2019, 43, 12714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liang, R.; Zhao, Y.; Zhang, S.; Lin, S. Immunomodulatory activity improvement of pine nut peptides by a pulsed electric field and their structure–activity relationships. J. Agric. Food Chem. 2019, 67, 3796–3810. [Google Scholar] [CrossRef] [PubMed]
- Morri, O.; Elsawy, M.A.; Faircloug, M.; Williams, K.J.; Mcmahon, A.; Grigg, J.; Forster, D.; Miller, A.F.; Saiani, A.; Prenant, C. In vivo characterisation of a therapeutically relevant self-assembling 18F-labelled β-sheet forming peptide and its hydrogel using positron emission tomography. J. Label. Compd. Radiopharm. 2017, 60, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Islam, Y.; Leach, A.G.; Smith, J.; Pluchino, S.; Coxon, C.R.; Sivakumaran, M.; Downing, J.; Fatokun, A.A.; Teixidò, M.; Ehtezazi, T. Physiological and pathological factors affecting drug delivery to the brain by nanoparticles. Adv. Sci. 2021, 8, 2002085. [Google Scholar] [CrossRef] [PubMed]
- Abi-Ghanem, C.; Jonnalagadda, D.; Chun, J.; Kihara, Y.; Ranscht, B. CAQK, a peptide associating with extracellular matrix components targets sites of demyelinating injuries. Front. Cell. Neurosci. 2022, 16, 908401. [Google Scholar] [CrossRef] [PubMed]
- Zehra, B.; Sadir, S.; Liaquat, L.; Tabassum, S.; Madiha, S.; Rafiq, S.; Tariq, S.; Batool, T.S.; Saleem, S.; Naqvi, F.; et al. Repeated administration of almonds increases brain acetylcholine levels and enhances memory function in healthy rats while attenuates memory deficits in animal scopolamine of amnesia. Brain Res. Bull. 2016, 120, 63–74. [Google Scholar]
- de Lima, M.N.M.; Presti-Torres, J.; Dornelles, A.; Scalco, F.S.; Roesler, R.; Garcia, V.A.; Schröder, N. Modulatory influence of dopamine receptors on consolidation of object recognition memory. Neurobiol. Learn. Mem. 2011, 95, 305–310. [Google Scholar] [CrossRef]
- Mennenga, S.E.; Baxter, L.C.; Grunfeld, I.S.; Brewer, G.A.; Aiken, L.S.; Engler-Chiurazzi, E.B.; Camp, B.W.; Acosta, J.I.; Braden, B.B.; Schaefer, K.R.; et al. Navigating to new frontiers in behavioral neuroscience: Traditional neuropsychological tests predict human performance on a rodent-inspired radial-arm maze. Front. Behav. Neurosci. 2014, 8, 294. [Google Scholar] [CrossRef]
- Jing, L.; Da, Q.; Zhang, S.; Zhang, J.; Ma, H.; Luo, H. Nitronyl Nitroxide Ameliorates Hypobaric Hypoxia-Induced Cognitive Impairment in Mice by Suppressing the Oxidative Stress, Inflammatory Response and Apoptosis. Neurochem. Res. 2023, 49, 785–799. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kaur, M.; Kukreja, H.; Chugh, R.; Silakari, O.; Singh, D. Acetylcholinesterase inhibitors as Alzheimer therapy: From nerve toxins to neuroprotection. Eur. J. Med. Chem. 2013, 70, 165–188. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Tabassum, S.; Perveen, T. Scopolamine-induced greater alterations in neurochemical profile and increased oxidative stress demonstrated a better scopolamine of dementia: A comparative study. Brain Res. Bull. 2016, 127, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhu, Y.; Wang, Y.; Qi, S.; Wang, Y.; Ma, C.; Li, S.; Jiang, B.; Cheng, X.; Wang, Z.; et al. Anti-amnesic effect of extract and alkaloid fraction from aerial parts of Peganum harmala on scopolamine-induced memory deficits in mice. J. Ethnopharmacol. 2017, 204, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Z.; Liu, B.; Valdez, C.; Chavko, M.; Cancio, L.C. Low-level primary blast induces neuroinflammation and neurodegeneration in rats. Mil. Med. 2019, 184 (Suppl. S1), 265–272. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Li, M.; Ding, J.; Zheng, J.; Zhu, B.; Lin, S. Structure-activity relationship and pathway of antioxidant shrimp peptides in a PC12 cell scopolamine. J. Funct. Foods 2020, 70, 103978. [Google Scholar] [CrossRef]
- Ishii, T.; Takanashi, Y.; Sugita, K.; Miyazawa, M.; Yanagihara, R.; Yasuda, K.; Onouchi, H.; Kawabe, N.; Nakata, M.; Yamamoto, Y.; et al. Endogenous reactive oxygen species cause astrocyte defects and neuronal dysfunctions in the hippocampus: A new scopolamine for aging brain. Aging Cell 2017, 16, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Takahashi, T.; Sumitani, K.; Takatsu, H.; Urano, S. Glucocorticoid generates ROS to induce oxidative injury in the hippocampus, leading to impairment of cognitive function of rats. J. Clin. Biochem. Nutr. 2010, 47, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Han, Y.; Yang, H.; Li, L.; Du, X.; Sun, C. Schisanhenol improves learning and memory in scopolamine-treated mice by reducing acetylcholinesterase activity and attenuating oxidative damage through SIRT1-PGC-1α-Tau signaling pathway. Int. J. Neurosci. 2019, 129, 110–118. [Google Scholar] [CrossRef]
- Valentini, K.J.; Pickens, C.A.; Wiesinger, J.A.; Fenton, J.I. Theeffect of fish oil supplementation on brain DHA and EPA content and fatty acid profile in mice. Int. J. Food Sci. Nutr. 2018, 69, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Wallis, T.P.; Venkatesh, B.G.; Narayana, V.K.; Kvaskoff, D.; Ho, A.; Sullivan, R.K.; Windels, F.; Sah, P.; Meunier, F.A. Saturated free fatty acids and association with memory formation. Nat. Commun. 2021, 12, 3443. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Bi, Y.; Ma, W.; He, L.; Yuan, L.; Feng, J.; Xiao, R. Long-term effects of high lipid and high energy diet on serum lipid, brain fatty acid composition, and memory and learning ability in mice. Int. J. Dev. Neurosci. 2010, 28, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, L. Lipid Peroxidation and Antioxidant Protection. Biomolecules 2023, 13, 1291. [Google Scholar] [CrossRef] [PubMed]
- Chwiej, J.; Skoczen, A.; Janeczko, K.; Kutorasinska, J.; Matusiak, K.; Figiel, H.; Dumas, P.; Sandt, C.; Setkowicz, Z. The biochemical changes in hippocampal formation occurring in normal and seizure experiencing rats as a result of a ketogenic diet. Analyst 2015, 140, 2190–2204. [Google Scholar] [CrossRef] [PubMed]
- Skoczen, A.; Setkowicz, Z.; Janeczko, K.; Sandt, C.; Borondics, F.; Chwiej, J. The influence of high fat diets with different ketogenic ratios on the hippocampal accumulation of creatine—FTIR microspectroscopy study. Spectrochim. Acta Part A. 2017, 184, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Wang, M.F.; Irei, A.V.; Sarukura, N.; Sakai, T.; Hsu, T.F. Effect of dietary lipids on longevity and memory in the SAMP8 mice. J. Nutr. Sci. Vitaminol. 2011, 57, 36–41. [Google Scholar] [CrossRef]
- Carlos, D.H.; Bibiana Roselly, C.R.; Angel, U.L.; Laura, M.A.; Kenya Karina, S.R.; Jose Manuel, C.B.; Alejandra, C.S.; Gabriela, C.C.E.; Estefanía, O.R.; Aracely, S.M. Cognitive improvements in a rat scopolamine with polyunsaturated fatty acids EPA and DHA through α7-nicotinic acetylcholine receptors. Nutr. Neurosci. 2022, 25, 791–800. [Google Scholar] [CrossRef]
- El Fari, R.; Abbaoui, A.; Bourziq, A.; Zroudi, M.; Draoui, A.; El Khiat, A.; Belkouch, M.; Elgot, A.; Gamrani, H. Neuroprotective effects of docosahexaenoic acid against sub-acute manganese intoxication induced dopaminergic and motor disorders in mice. J. Chem. Neuroanat. 2019, 102, 101686. [Google Scholar] [CrossRef]



| Parameter | Control | Scopolamine | AFFP+scopolamine |
|---|---|---|---|
| Body weight (g) | |||
| 7th day | 22.111 ± 0.7607 a | 21.956 ± 0.9825 a | 21.456 ± 1.0026 a |
| 14th day | 22.478 ± 0.7032 a | 22.533 ± 1.0368 a | 22.733 ± 1.2816 a |
| 21th day | 23.344 ± 0.8946 a | 23.067 ± 1.0198 a | 23.111 ± 1.6405 a |
| 28th day | 23.767 ± 0.9862 a | 23.556 ± 1.6501 a | 24.478 ± 1.4567 a |
| 35th day | 24.233 ± 0.9925 a | 24.344 ± 1.7529 a | 24.911 ± 1.412 a |
| 42th day | 23.978 ± 0.9909 a | 24.278 ± 1.4948 a | 24.089 ± 1.3383 a |
| 49th day | 25.133 ± 1.3105 a | 24.756 ± 1.7422 a | 24.511 ± 1.5576 a |
| Body composition (g) | |||
| Heart | 0.539 ± 0.058 a | 0.591 ± 0.0232 a | 0.557 ± 0.0668 a |
| Liver | 3.530 ± 0.3332 a | 3.717 ± 0.2355 a | 3.442 ± 0.2225 a |
| Spleen | 0.251 ± 0.0241 a | 0.271 ± 0.0382 a | 0.242 ± 0.0403 a |
| Lung | 0.687 ± 0.1088 a | 0.668 ± 0.0944 a | 0.690 ± 0.0905 a |
| Kidney | 1.198 ± 0.0901 a | 1.142 ± 0.1775 a | 1.092 ± 0.0935 a |
| Brain | 1.770 ± 0.1639 a | 1.715 ± 0.0825 a | 1.784 ± 0.0915 a |
| Hippocampus | 0.167 ± 0.0125 a | 0.168 ± 0.0146 a | 0.183 ± 0.0124 a |
| Relative organ coefficient (%) | |||
| Lean mass | 23.688 ± 6.9014 a | 23.267 ± 4.668 a | 27.906 ± 3.9113 a |
| Fat | 3.801 ± 0.5677 a | 3.651 ± 0.7943 a | 3.991 ± 0.6571 a |
| Water | 0.907 ± 0.461 a | 0.900 ± 0.2375 a | 0.812 ± 0.2778 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Qi, Y.; Zhu, B.; Lin, S. A Novel Tetrapeptide Ala-Phe-Phe-Pro (AFFP) Derived from Antarctic Krill Prevents Scopolamine-Induced Memory Disorder by Balancing Lipid Metabolism of Mice Hippocampus. Nutrients 2024, 16, 1019. https://doi.org/10.3390/nu16071019
Yang J, Qi Y, Zhu B, Lin S. A Novel Tetrapeptide Ala-Phe-Phe-Pro (AFFP) Derived from Antarctic Krill Prevents Scopolamine-Induced Memory Disorder by Balancing Lipid Metabolism of Mice Hippocampus. Nutrients. 2024; 16(7):1019. https://doi.org/10.3390/nu16071019
Chicago/Turabian StyleYang, Jingqi, Yan Qi, Beiwei Zhu, and Songyi Lin. 2024. "A Novel Tetrapeptide Ala-Phe-Phe-Pro (AFFP) Derived from Antarctic Krill Prevents Scopolamine-Induced Memory Disorder by Balancing Lipid Metabolism of Mice Hippocampus" Nutrients 16, no. 7: 1019. https://doi.org/10.3390/nu16071019
APA StyleYang, J., Qi, Y., Zhu, B., & Lin, S. (2024). A Novel Tetrapeptide Ala-Phe-Phe-Pro (AFFP) Derived from Antarctic Krill Prevents Scopolamine-Induced Memory Disorder by Balancing Lipid Metabolism of Mice Hippocampus. Nutrients, 16(7), 1019. https://doi.org/10.3390/nu16071019





