The Association between the Diversity of Coenzyme Q10 Intake from Dietary Sources and the Risk of New-Onset Hypertension: A Nationwide Cohort Study
Abstract
1. Introduction
2. Materials and Methods
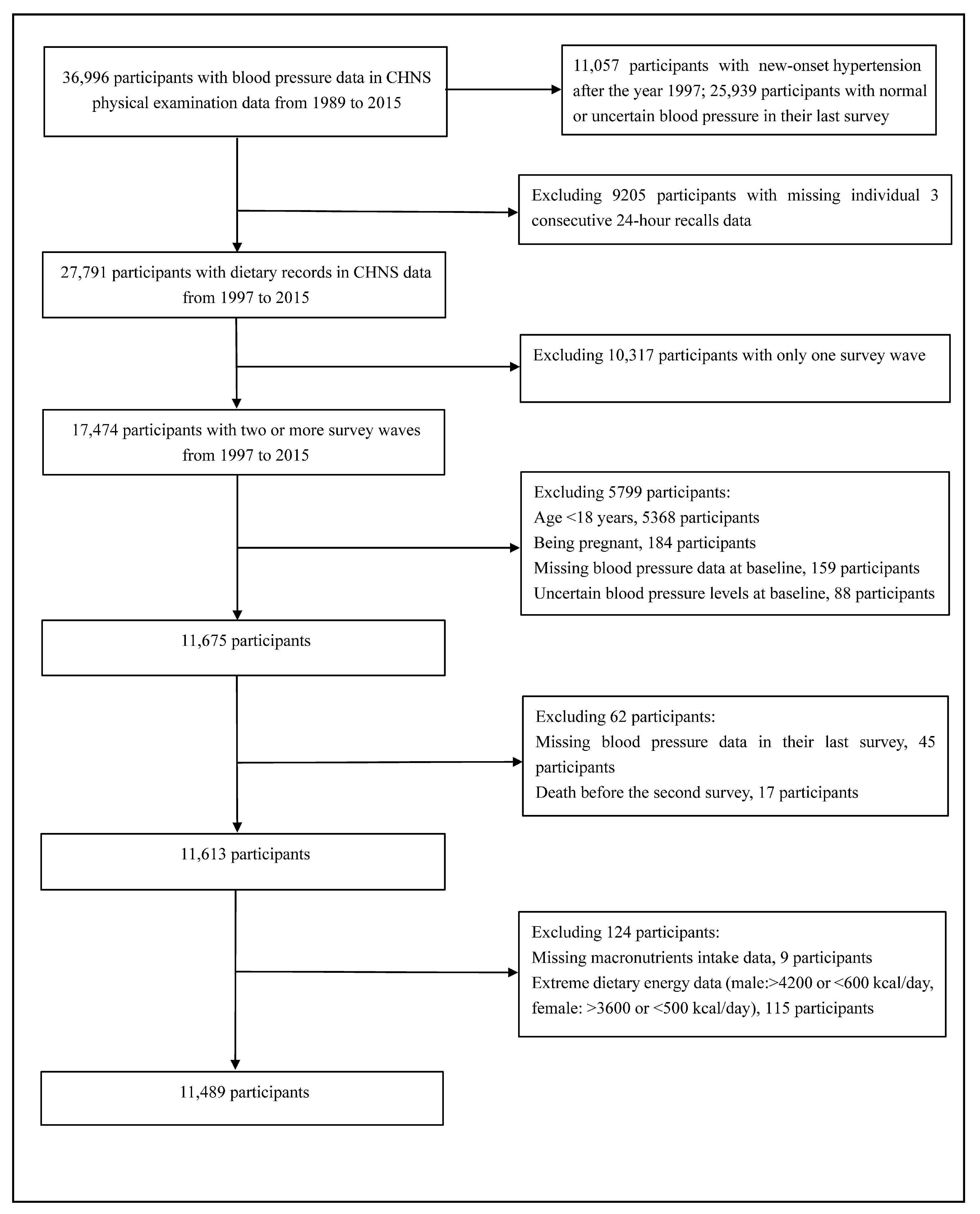
2.1. Study Participants
2.2. Dietary Assessment
2.3. Calculation of Diversity Score of CoQ10 Sources
2.4. Ascertainment of Follow-Up Events
2.5. Basic Information Collection
2.6. Statistical Analyses
3. Results
3.1. Study Participants and Basic Characteristics
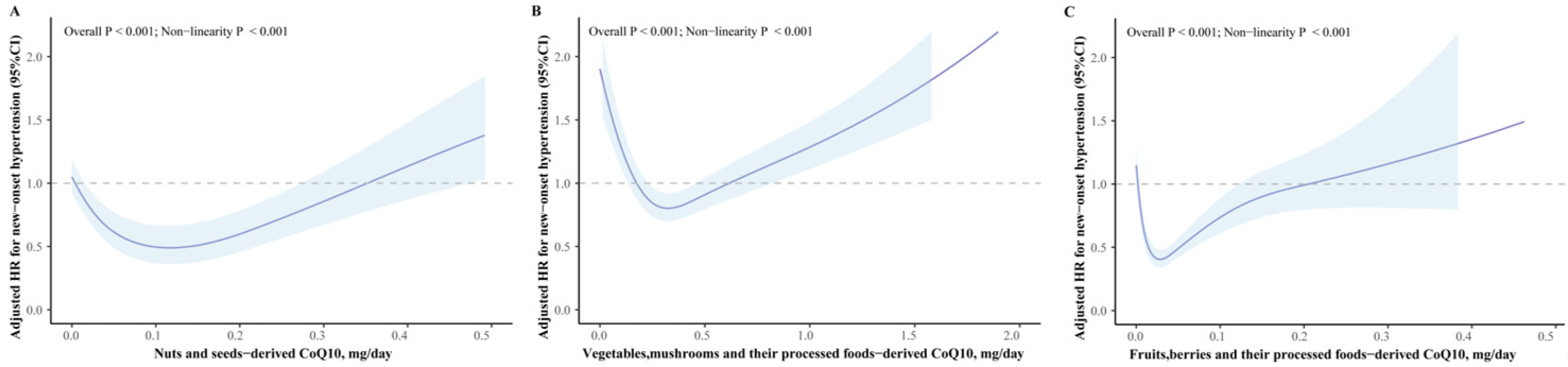
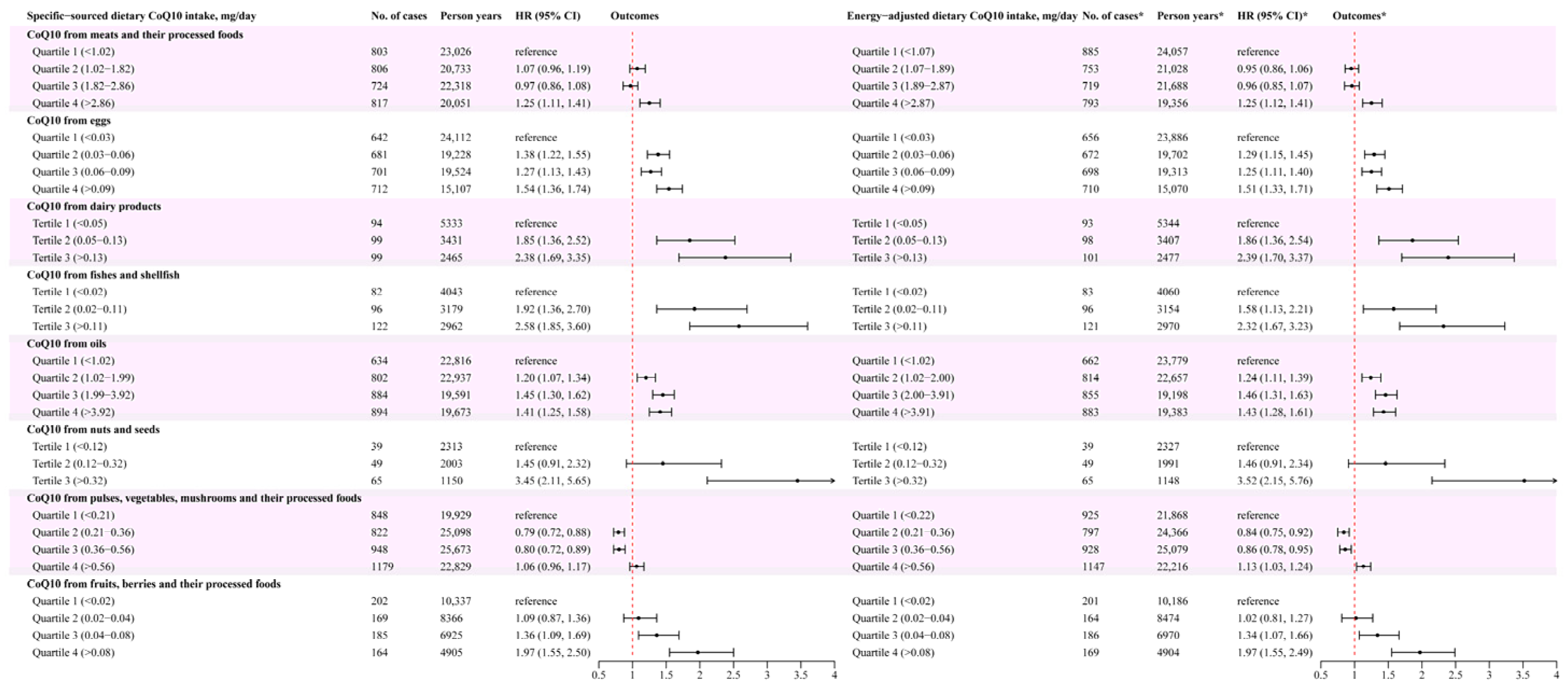
3.2. Association between Different Food Sources of CoQ10 and New-Onset Hypertension Risk
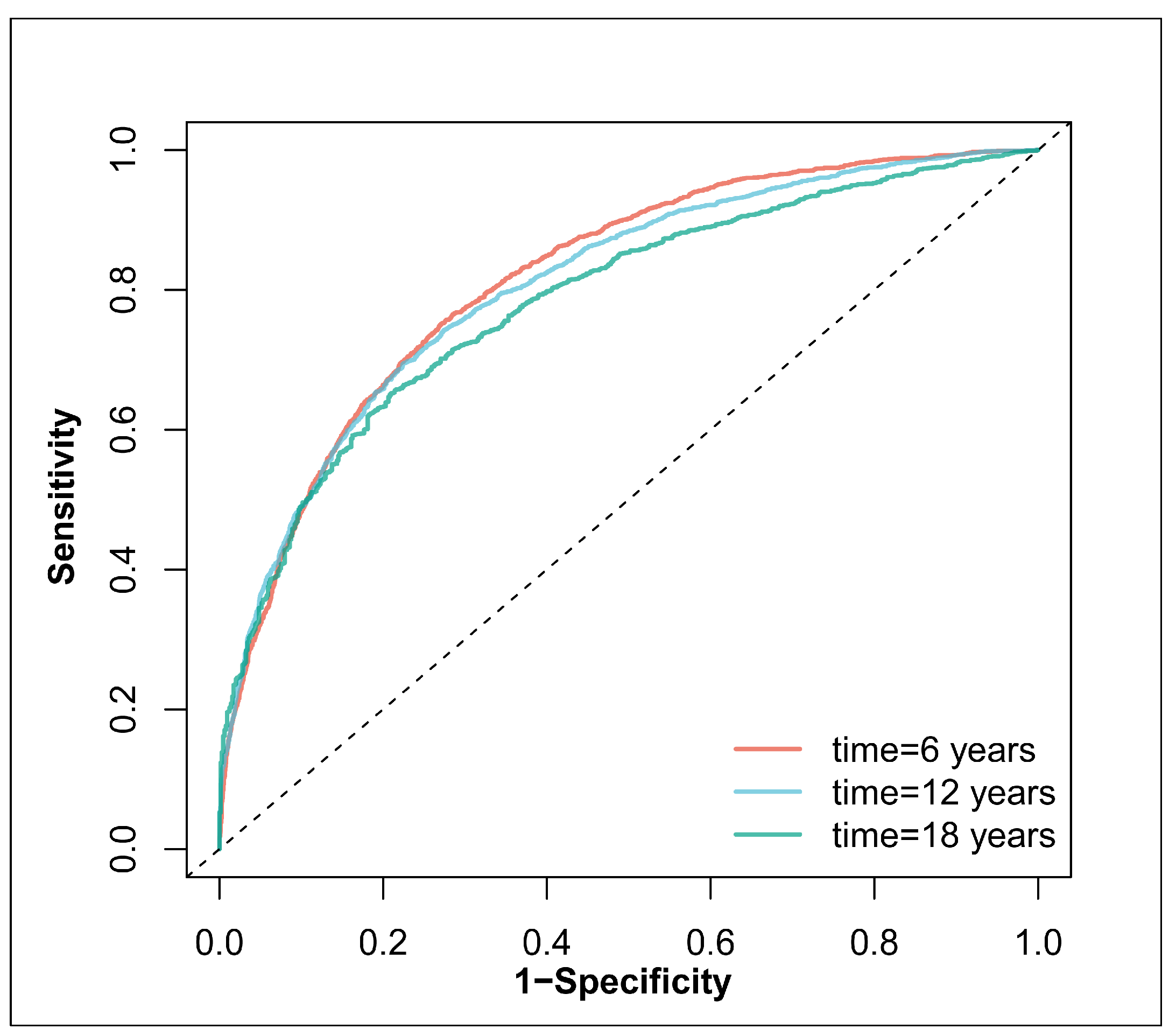
3.3. Relationship of Diversity Score of CoQ10 Sources and New-Onset Hypertension
4. Discussion
4.1. Principal Findings
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Report on Hypertension: The Race against a Silent Killer. Available online: https://www.who.int/publications/i/item/9789240081062 (accessed on 19 September 2023).
- Mensah, G.A.; Fuster, V.; Murray, C.J.L.; Roth, G.A.; Mensah, G.A.; Abate, Y.H.; Abbasian, M.; Abd-Allah, F.; Abdollahi, A.; Abdollahi, M.; et al. Global Burden of Cardiovascular Diseases and Risks, 1990–2022. J. Am. Coll. Cardiol. 2023, 82, 2350–2473. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Zhao, Z.; Kang, Y.; Tian, Y.; Song, Y.; Wang, L.; Zhang, L.; Wang, X.; Chen, Z.; Zheng, C.; et al. The burden of cardiovascular disease attributable to high systolic blood pressure across China, 2005–2018: A population-based study. Lancet Public Health 2022, 7, e1027–e1040. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-G.; Zhang, W.; Li, Y.; Liu, L. Hypertension in China: Epidemiology and treatment initiatives. Nat. Rev. Cardiol. 2023, 20, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Aljuraiban, G.S.; Gibson, R.; Chan, D.S.; Van Horn, L.; Chan, Q. The Role of Diet in the Prevention of Hypertension and Management of Blood Pressure: An Umbrella Review of Meta-Analyses of Interventional and Observational Studies. Adv. Nutr. 2024, 15, 100123. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, S.; Roshanravan, N. The role of nutraceuticals in prevention and treatment of hypertension: An updated review of the literature. Food Res. Int. 2020, 128, 108749. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; He, X.; Zhou, Y.; Peng, W.; Zhao, X.-M.; Jiang, M. Coenzyme Q10 in atherosclerosis. Eur. J. Pharmacol. 2024, 970, 176481. [Google Scholar] [CrossRef] [PubMed]
- Sue-Ling, C.B.; Abel, W.M.; Sue-Ling, K. Coenzyme Q10 as Adjunctive Therapy for Cardiovascular Disease and Hypertension: A Systematic Review. J. Nutr. 2022, 152, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Rabanal-Ruiz, Y.; Llanos-González, E.; Alcain, F.J. The Use of Coenzyme Q10 in Cardiovascular Diseases. Antioxidants 2021, 10, 755. [Google Scholar] [CrossRef]
- Zhao, D.; Liang, Y.; Dai, S.; Hou, S.; Liu, Z.; Liu, M.; Dong, X.; Zhan, Y.; Tian, Z.; Yang, Y. Dose-Response Effect of Coenzyme Q10 Supplementation on Blood Pressure among Patients with Cardiometabolic Disorders: A Grading of Recommendations Assessment, Development, and Evaluation (GRADE)-Assessed Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2022, 13, 2180–2194. [Google Scholar] [CrossRef]
- Podar, A.S.; Semeniuc, C.A.; Ionescu, S.R.; Socaciu, M.-I.; Fogarasi, M.; Fărcaș, A.C.; Vodnar, D.C.; Socaci, S.A. An Overview of Analytical Methods for Quantitative Determination of Coenzyme Q10 in Foods. Metabolites 2023, 13, 272. [Google Scholar] [CrossRef]
- Pravst, I.; Zmitek, K.; Zmitek, J. Coenzyme Q10 contents in foods and fortification strategies. Crit. Rev. Food Sci. Nutr. 2010, 50, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Tian, Z.; Zhao, D.; Liang, Y.; Dai, S.; Xu, Y.; Hou, S.; Yang, Y. L-shaped association between dietary coenzyme Q10 intake and high-sensitivity C-reactive protein in Chinese adults: A national cross-sectional study. Food Funct. 2023, 14, 9815–9824. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Wu, Q.; Yang, S.; Zhang, Y.; Zhou, C.; Liu, M.; Zhang, Z.; He, P.; Zhang, Y.; Li, R.; et al. Variety and quantity of dietary insoluble fiber intake from different sources and risk of new-onset hypertension. BMC Med. 2023, 21, 61. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Q.; Ye, Z.; Liu, M.; Zhang, Z.; Zhang, Y.; Li, H.; He, P.; Li, Q.; Liu, C.; et al. Inverse Association Between Variety of Proteins with Appropriate Quantity from Different Food Sources and New-Onset Hypertension. Hypertension (Dallas Tex. 1979) 2022, 79, 1017–1027. [Google Scholar] [CrossRef]
- Zhang, B.; Zhai, F.Y.; Du, S.F.; Popkin, B.M. The China Health and Nutrition Survey, 1989–2011. Obes. Rev. 2014, 15 (Suppl. S1), 2–7. [Google Scholar] [CrossRef]
- Zhai, F.; Guo, X.; Popkin, B.M.; Ma, L.; Wang, Q.; Yu, W.; Jin, S.; Ge, K. Evaluation of the 24-Hour Individual Recall Method in China. Food Nutr. Bull. 1996, 17, 1–7. [Google Scholar] [CrossRef]
- Mattila, P.; Kumpulainen, J. Coenzymes Q9and Q10: Contents in Foods and Dietary Intake. J. Food Compos. Anal. 2001, 14, 409–417. [Google Scholar] [CrossRef]
- Weber, C.; Bysted, A.; Hølmer, G. Coenzyme Q10 in the diet-daily intake and relative bioavailability. Mol. Asp. Med. 1997, 18, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, C.; Zhang, Z.; Zhou, C.; Li, Q.; He, P.; Zhang, Y.; Li, H.; Qin, X. Quantity and variety of food groups consumption and the risk of diabetes in adults: A prospective cohort study. Clin. Nutr. 2021, 40, 5710–5717. [Google Scholar] [CrossRef]
- Writing Group of Chinese Guidelines for the Management. 2010 Chinese guidelines for the management of hypertension. Zhonghua Xin Xue Guan Bing Za Zhi 2011, 39, 579–615. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Tomova, G.D.; Arnold, K.F.; Gilthorpe, M.S.; Tennant, P.W.G. Adjustment for energy intake in nutritional research: A causal inference perspective. Am. J. Clin. Nutr. 2022, 115, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.F.; Zhang, Y.B.; Liu, G.; Pan, A. The application of energy adjustment models in nutritional epidemiology. Zhonghua Yu Fang Yi Xue Za Zhi [Chin. J. Prev. Med.] 2020, 54, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Schwedhelm, C.; Hoffmann, G.; Knüppel, S.; Iqbal, K.; Andriolo, V.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food Groups and Risk of Hypertension: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Adv. Nutr. 2017, 8, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Madsen, H.; Sen, A.; Aune, D. Fruit and vegetable consumption and the risk of hypertension: A systematic review and meta-analysis of prospective studies. Eur. J. Nutr. 2023, 62, 1941–1955. [Google Scholar] [CrossRef]
- Livingstone, K.M.; Lovegrove, J.A.; Cockcroft, J.R.; Elwood, P.C.; Pickering, J.E.; Givens, D.I. Does Dairy Food Intake Predict Arterial Stiffness and Blood Pressure in Men? Hypertension 2013, 61, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Villaverde, P.; Lajous, M.; MacDonald, C.-J.; Fagherazzi, G.; Boutron-Ruault, M.-C.; Bonnet, F. Dairy product consumption and hypertension risk in a prospective French cohort of women. Nutr. J. 2020, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhao, Y.; Liu, J.; Huang, Z.; Yang, X.; Qin, P.; Chen, C.; Luo, X.; Li, Y.; Wu, Y.; et al. Consumption of Dairy Products and the Risk of Overweight or Obesity, Hypertension, and Type 2 Diabetes Mellitus: A Dose-Response Meta-Analysis and Systematic Review of Cohort Studies. Adv. Nutr. 2022, 13, 2165–2179. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, C.; Yoruk, A.; Wang, L.; Gaziano, J.M.; Sesso, H.D. Fish and omega-3 fatty acid consumption and risk of hypertension. J. Hypertens. 2019, 37, 1223–1229. [Google Scholar] [CrossRef]
- MacDonald, C.-J.; Madika, A.-L.; Bonnet, F.; Fagherazzi, G.; Lajous, M.; Boutron-Ruault, M.-C. Cholesterol and Egg Intakes, and Risk of Hypertension in a Large Prospective Cohort of French Women. Nutrients 2020, 12, 1350. [Google Scholar] [CrossRef]
- Sanlier, N.; Üstün, D. Egg consumption and health effects: A narrative review. J. Food Sci. 2021, 86, 4250–4261. [Google Scholar] [CrossRef] [PubMed]
- Kolahdouz-Mohammadi, R.; Malekahmadi, M.; Clayton, Z.S.; Sadat, S.Z.; Pahlavani, N.; Sikaroudi, M.K.; Soltani, S. Effect of Egg Consumption on Blood Pressure: A Systematic Review and Meta-analysis of Randomized Clinical Trials. Curr. Hypertens. Rep. 2020, 22, 24. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.S.; Bhatia, H.S.; Wood, A.C.; Momin, S.R.; Allison, M.A. State-of-the-Art Review: Evidence on Red Meat Consumption and Hypertension Outcomes. Am. J. Hypertens. 2022, 35, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Woo, H.W.; Shin, J.; Kim, Y.-M.; Shin, M.-H.; Koh, S.-B.; Kim, H.C.; Kim, M.K. Cumulative average nut consumption in relation to lower incidence of hypertension: A prospective cohort study of 10,347 adults. Eur. J. Nutr. 2022, 61, 1571–1583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ritonja, J.A.; Zhou, N.; Chen, B.E.; Li, X. Omega-3 Polyunsaturated Fatty Acids Intake and Blood Pressure: A Dose-Response Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2022, 11, e025071. [Google Scholar] [CrossRef]
- Atefi, M.; Entezari, M.H.; Vahedi, H.; Hassanzadeh, A. The effects of sesame oil on metabolic biomarkers: A systematic review and meta-analysis of clinical trials. J. Diabetes Metab. Disord. 2022, 21, 1065–1080. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Scoditti, E.; Carluccio, M.A.; Calabriso, N.; Santarpino, G.; Verri, T.; De Caterina, R. Effects of Olive Oil on Blood Pressure: Epidemiological, Clinical, and Mechanistic Evidence. Nutrients 2020, 12, 1548. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Martínez-González, M.Á. Olive oil consumption and reduced incidence of hypertension: The SUN study. Lipids 2004, 39, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Medina-Remón, A.; Kirwan, R.; Lamuela-Raventós, R.M.; Estruch, R. Dietary patterns and the risk of obesity, type 2 diabetes mellitus, cardiovascular diseases, asthma, and neurodegenerative diseases. Crit. Rev. Food Sci. Nutr. 2018, 58, 262–296. [Google Scholar] [CrossRef]
- Mente, A.; Dehghan, M.; Rangarajan, S.; O’Donnell, M.; Hu, W.; Dagenais, G.; Wielgosz, A.; A Lear, S.; Wei, L.; Diaz, R.; et al. Diet, cardiovascular disease, and mortality in 80 countries. Eur. Heart J. 2023, 44, 2560–2579. [Google Scholar] [CrossRef]
- Filippou, C.D.; Tsioufis, C.P.; Thomopoulos, C.G.; Mihas, C.C.; Dimitriadis, K.S.; Sotiropoulou, L.I.; Chrysochoou, C.A.; Nihoyannopoulos, P.I.; Tousoulis, D.M. Dietary Approaches to Stop Hypertension (DASH) Diet and Blood Pressure Reduction in Adults with and without Hypertension: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2020, 11, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef] [PubMed]
- Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Kirkpatrick, S.I.; Lerman, J.L.; Tooze, J.A.; Wilson, M.M.; Reedy, J. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 2018, 118, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Theodoridis, X.; Chourdakis, M.; Chrysoula, L.; Chroni, V.; Tirodimos, I.; Dipla, K.; Gkaliagkousi, E.; Triantafyllou, A. Adherence to the DASH Diet and Risk of Hypertension: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 3261. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Zhang, J.; Zhao, C.; Wang, Y.; Qi, Y.; Zhang, B. Adherence to a healthy lifestyle and a DASH-style diet and risk of hypertension in Chinese individuals. Hypertens. Res. 2017, 40, 196–202. [Google Scholar] [CrossRef]
- Gao, M.; Wang, F.; Shen, Y.; Zhu, X.; Zhang, X.; Sun, X. Trajectories of Mediterranean Diet Adherence and Risk of Hypertension in China: Results from the CHNS Study, 1997–2011. Nutrients 2018, 10, 2014. [Google Scholar] [CrossRef]


| Diversity Score | Model 1 a | Model 2 b | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| As continuous variable | ||||
| 0.65 (0.63, 0.67) | <0.001 | 0.66 (0.64, 0.69) | <0.001 | |
| As categorical variable | ||||
| Group 1 (<2) | Ref | Ref | ||
| Group 2 (2) | 0.49 (0.46, 0.54) | <0.001 | 0.51 (0.47, 0.55) | <0.001 |
| Group 3 (3) | 0.41 (0.36, 0.46) | <0.001 | 0.43 (0.38, 0.49) | <0.001 |
| Group 4 (≥4) | 0.29 (0.22, 0.37) | <0.001 | 0.28 (0.21, 0.39) | <0.001 |
| p for trend | <0.001 | <0.001 | ||
| Adjusted Model | |||
|---|---|---|---|
| Subgroups | HR (95% CI) | p Value | p for Interaction |
| Age, years | 0.625 | ||
| <60 | 0.66 (0.64, 0.69) | <0.001 | |
| ≥60 | 0.63 (0.58, 0.70) | <0.001 | |
| Sex | 0.043 | ||
| Male | 0.64 (0.61, 0.68) | <0.001 | |
| Female | 0.68 (0.65, 0.72) | <0.001 | |
| BMI, kg/m2 | 0.557 | ||
| <24 | 0.67 (0.63, 0.70) | <0.001 | |
| ≥24 | 0.66 (0.62, 0.69) | <0.001 | |
| Physical activity, Mets⸱h/wk | 0.102 | ||
| Low | 0.69 (0.64, 0.73) | <0.001 | |
| Moderate | 0.68 (0.64, 0.72) | <0.001 | |
| High | 0.61 (0.57, 0.65) | <0.001 | |
| Smoking status | 0.173 | ||
| No | 0.67 (0.64, 0.70) | <0.001 | |
| Yes | 0.64 (0.60, 0.68) | <0.001 | |
| Drinking status | 0.014 | ||
| No | 0.68 (0.65, 0.71) | <0.001 | |
| Yes | 0.63 (0.60, 0.67) | <0.001 | |
| Baseline SBP stages | 0.403 | ||
| <120 | 0.66 (0.63, 0.70) | <0.001 | |
| ≥120 | 0.66 (0.62, 0.70) | <0.001 | |
| Abdominal obesity | 0.766 | ||
| No | 0.66 (0.64, 0.69) | <0.001 | |
| Yes | 0.66 (0.62, 0.71) | <0.001 | |
| Unknown | 0.70 (0.52, 0.93) | 0.014 | |
| Total energy intake, kcal | 0.013 | ||
| <2110 (median) | 0.68 (0.64, 0.71) | <0.001 | |
| ≥2110 | 0.63 (0.60, 0.66) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, S.; Tian, Z.; Zhao, D.; Liang, Y.; Zhong, Z.; Xu, Y.; Hou, S.; Yang, Y. The Association between the Diversity of Coenzyme Q10 Intake from Dietary Sources and the Risk of New-Onset Hypertension: A Nationwide Cohort Study. Nutrients 2024, 16, 1017. https://doi.org/10.3390/nu16071017
Dai S, Tian Z, Zhao D, Liang Y, Zhong Z, Xu Y, Hou S, Yang Y. The Association between the Diversity of Coenzyme Q10 Intake from Dietary Sources and the Risk of New-Onset Hypertension: A Nationwide Cohort Study. Nutrients. 2024; 16(7):1017. https://doi.org/10.3390/nu16071017
Chicago/Turabian StyleDai, Suming, Zezhong Tian, Dan Zhao, Ying Liang, Zepei Zhong, Yixuan Xu, Shanshan Hou, and Yan Yang. 2024. "The Association between the Diversity of Coenzyme Q10 Intake from Dietary Sources and the Risk of New-Onset Hypertension: A Nationwide Cohort Study" Nutrients 16, no. 7: 1017. https://doi.org/10.3390/nu16071017
APA StyleDai, S., Tian, Z., Zhao, D., Liang, Y., Zhong, Z., Xu, Y., Hou, S., & Yang, Y. (2024). The Association between the Diversity of Coenzyme Q10 Intake from Dietary Sources and the Risk of New-Onset Hypertension: A Nationwide Cohort Study. Nutrients, 16(7), 1017. https://doi.org/10.3390/nu16071017







