Abstract
There is a growing body of evidence that suggests a connection between traumatic brain injury (TBI) and subsequent post-traumatic stress disorder (PTSD). While the exact mechanism is unknown, we hypothesize that chronic glutamate neurotoxicity may play a role. The consumption of dietary glutamate is a modifiable factor influencing glutamate levels in the blood and, therefore, in the brain. In this systematic review, we explored the relationship between dietary glutamate and the development of post-TBI PTSD. Of the 1748 articles identified, 44 met the inclusion criteria for analysis in this review. We observed that individuals from countries with diets traditionally high in glutamate had greater odds of developing PTSD after TBI (odds ratio = 15.2, 95% confidence interval 11.69 to 19.76, p < 0.01). These findings may support the hypothesis that chronically elevated blood glutamate concentrations caused by high dietary intake invoke neurodegeneration processes that could ultimately result in PTSD. Further studies will clarify whether lowering glutamate via diet would be an effective strategy in preventing or treating post-TBI PTSD.
1. Introduction
Traumatic brain injury (TBI) is a severe public health crisis with more than 50 million cases reported worldwide annually [1], amounting to billions of dollars in associated annual costs [2]. It is widely accepted that depression, dementia, and anxiety are common neuropsychiatric complications of TBI [3,4,5,6,7]. While post-traumatic stress disorder (PTSD) was initially believed to be unrelated to brain injury [8,9,10], more recent investigations have highlighted a relationship between TBI and the development of PTSD [11,12,13,14].
A large body of evidence now confirms a tendency of PTSD occurrence after TBI [15,16,17]. In fact, some studies suggest that post-TBI PTSD is as common a psychiatric complication as post-TBI depression [18]. Several theories explaining post-TBI PTSD in individuals who are unable to recall the traumatic event have been offered [13,19,20,21,22,23,24,25].
PTSD also carries with it a high risk of comorbidities such as major depressive disorder, anxiety disorders, and substance abuse [26,27]. Unfortunately, PTSD often shows a limited response to pharmacologic treatment [28], though clinicians remain optimistic about discovering new therapies to treat its symptoms and control the risk of comorbidities. Despite these efforts, there is still a lack of a unified understanding of the mechanism of PTSD development, especially in cases associated with head trauma [29,30].
Generating and examining new mechanistic hypotheses is necessary for breakthroughs in this field and could result in the development of updated strategies for the treatment of this pathology. A recent related hypothesis points to the development of post-TBI neuropsychiatric sequelae through chronic blood–brain barrier (BBB) destruction, chronic glutamate neurotoxicity, and neurodegeneration [31,32]. In a previous investigation, we examined the effect of dietary glutamate on chronic neurotoxicity and its role in the development of post-TBI depression [33].
In this systematic review, we explore the relationship between dietary glutamate and the development of post-TBI PTSD in 321,057 patients. Specifically, we compare the incidence and prevalence of post-TBI PTSD in Asian countries with traditionally rich-glutamate diets that potentially cause an increase in blood glutamate levels relative to regions with poor-glutamate diets, including in Europe, North America, Australia, and New Zealand. We theorize that following post-TBI damage to the BBB, persistently high blood glutamate levels, worsened by dietary intake, may contribute to further neurodegeneration and potentially result in PTSD.
2. Materials and Methods
2.1. Protocol and Registration
This systematic review was conducted and reported as per the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [34]. The protocol details were registered on the PROSPERO registry for systematic review protocols (CRD42023465270) prior to execution.
2.2. Literature Search
A literature search was conducted between October 2023 and December 2023 using the following electronic databases: ProQuest, PubMed, Web of Science, APA PsycNET, Scopus, and the Cochrane Library. Search terms included the following: (1) Traumatic brain injury; (2) TBI; (3) PTSD; (4) Post-traumatic stress disorder; (5) Posttraumatic stress disorder; (6) Post traumatic stress disorder. Using the terms above, the search included: (#1 OR #2) AND (#3 OR #4 OR #5 OR #6). All identified articles were summarized in Excel spreadsheets. Duplicates were located and removed.
2.3. Inclusion Criteria
Studies were included if they met the following criteria: (1) English language, with full text available; (2) adult participants (16+) from the general population; (3) confirmed TBI; (4) a diagnosis of post-TBI PTSD; (5) TBI sustained 2 months prior to the study to ensure recovery from the acute phase; (6) representation of both sexes; and (7) published between 1 January 1900 and 30 October 2023. Only randomized controlled trials and observational studies were included.
2.4. Exclusion Criteria
Studies were excluded if they had the following: (1) non-physical trauma-caused injuries, including focal stroke, meningitis, drug-induced injury, tumor-engendered changes, or hypoxic injuries/oxygen loss-triggered brain damage; (2) sample size with <10 TBI patients; (3) qualitative in nature; and (4) populations involving military veterans with combat-related injuries.
Populations with combat-related TBIs were excluded in order to maximize the generalizability of the results for the civilian population, since most combat-related TBIs are caused specifically by blasts. Additionally, significant differences in contextual factors between combat injuries and civilian injuries as well as the increased potential for compensation seeking in the service sector [16,35,36] potentially affect their generalizability. Moreover, publications with combat-related TBI cases use a single database, which houses data that can be reused in multiple studies. Also, repetitive TBI and psychological stress that regularly accompany combatants can also affect outcomes. Nonetheless, we also reviewed military studies for the potential that they included disparate studies with only civilian samples.
Non-English publications, conference and poster abstracts, reviews, case reports, publications with combat-recruited study populations, comments, and letters to the editor were excluded.
2.5. Study Screening and Selection
The titles and abstracts of studies collected through the search process were screened independently by two contributors (AO and MB) to identify studies that were appropriate for inclusion following the stated criteria. The two contributors independently assessed the full texts of these potentially eligible studies; any disagreement between the two contributors was resolved through dialogue with a third contributor (AZ). The methodology of each paper was qualitatively assessed using the Newcastle–Ottawa Scale [37].
2.6. Statistical Analysis
The primary outcome was the number of cases of PTSD among TBI patients. The odds ratio was calculated as previously described [38,39,40].
3. Results
3.1. Search Results
The database searches yielded 352 hits in PubMed, 264 in ProQuest, 629 in Web of Science, 288 in APA PsycNET, 150 in Scopus, and 65 in Cochrane Library (Figure 1).
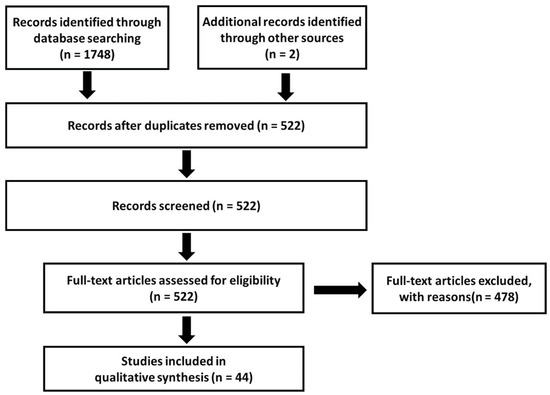
Figure 1.
A flow chart of the study selection process.
Two additional publications were identified through a manual search of the reference lists from the included publications. After the removal of duplicates, the search was left with 522 unique records. The exclusion of publications describing duplicate cohorts and groups of combatants or children reduced the total number of publications to 48. Three different publications by Bryant, R.A., et al. [19,41,42] and two publications by Bryant, R.A., et al. [43,44] published in 2000–2001 and 1998–1999, respectively, containing similar incidence rates of TBI and PTSD, were identified. Because it was likely that these papers used the same dataset, with the same patient population, only one [41] out of the three publications in the first group of publications and one [43] out of two in the second group of publications were included for analysis after consultation among the authors.
After we analyzed the data, a notice of retraction of an article by Xu et al. [45] was published. The reason for the retraction was as follows: “The investigation found evidence of systematic manipulation of the publication and review process”. Although the reason for the retraction was not falsification of data, it was clear that the article did not meet the criteria for publication in that journal. Thus, we removed the article from our review.
Consequently, the final number of publications was 44, and the final number of cases contained therein was 321,057. A full list of references of the identified publications is provided in Table S1.
3.2. Comparison of Study Groups
We grouped the cases by region of origin. The calculated odds ratio (OR) in the Asian group was 15.2 (95% CI [11.69, 19.76]; Z = 20.33; p < 0.01) in comparison to the European, American, Australian, UK, and Israeli populations in the first year following TBI, supporting our hypothesis that there is a higher incidence of PTSD after TBI in Asian countries (Figure 2). As we note in the subsequent section, the differences in the assessment and diagnosis of post-TBI PTSD are significant; however, it is unlikely that these differences can completely account for the enormous discrepancy in the incidence of post-TBI PTSD among non-Asian countries relative to Asian countries (see Supplementary Materials).
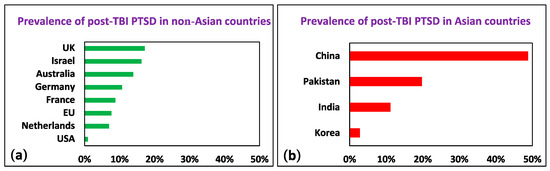
Figure 2.
Prevalence of post-TBI PTSD in (a) non-Asian and (b) Asian countries.
3.3. Article Quality
Each article considered in this study underwent review for potential bias and methodological integrity using the Newcastle–Ottawa Scale [37]. This scale assesses the risk of selection bias, the comparability of comparison groups, and the validity of outcome/exposure ascertainment for observational, non-randomized investigations. The scale has specific evaluation criteria for case–control and cohort studies, and we labeled each study accordingly. Bias for every article was scored as high, low, or unclear. The results of the evaluation are summarized in Figure 3. Each article was scored independently by two reviewers (AO and FM). Any disagreement between the two reviewers was resolved via dialogue with a third reviewer (MB).
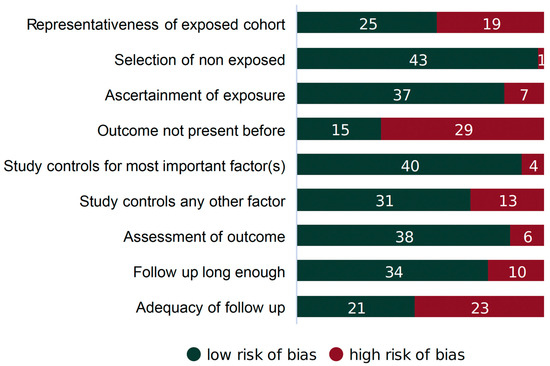
Figure 3.
Sources of potential bias in cohort studies (n = 44).
4. Discussion
Post-TBI PTSD involves the manifestation of typical PTSD symptoms, such as intrusive thoughts or memories, avoidance behaviors, negative alterations in mood or cognition, and heightened arousal or reactivity. In addition to TBI, risk factors of PTSD include a low education level, Black ethnicity, and injury type [4]. Other risk factors are repeated head trauma [46], being a combatant [14,46], and a history of mental illness [14]. Mild TBI demonstrably raises predicted PTSD symptoms by a factor of 1.23, while moderate or severe TBI amplifies predicted symptoms by a factor of 1.71 [18,47]. The mechanisms of post-TBI PTSD development are unclear, but it is apparent that they have multifaceted aspects. The hypothesis of the current investigation points to neurodegeneration due to the destruction of BBB and chronic glutamate neurotoxicity as one of the possible key mechanisms for the development of PTSD. Confirming this hypothesis could enable improved therapies for the treatment of this severe illness.
4.1. The Relationship between PTSD and TBI
The relationship between PTSD and TBI is complex and significantly impacts pathways for diagnosis and treatment. The interplay between PTSD and TBI can complicate recovery and has important implications for clinical management. Research has highlighted the co-occurrence of these conditions, particularly mild TBI, and the challenges in differentiating their symptoms. The overlap in symptoms and the bidirectional influences of PTSD and TBI on recovery have been the subject of extensive research, especially in the context of war-related injuries [46]. This research focus considerably impacts the assessment and treatment of individuals with a history of TBI, given that PTSD can exacerbate or mask many of the symptoms attributed to post-concussive symptoms. Because symptoms attributed to TBI are potentially linked to PTSD [11] or represent conditions known as “persistent symptoms after TBI” [48] and “acute stress disorder”, the coexistence of these conditions can complicate diagnosis [10]. Additionally, different approaches exist to diagnose PTSD, which can produce inconsistent diagnoses of the illness [49].
Post-TBI PTSD development is a multifaceted process. Several theories on how to elucidate the putative mechanisms underlying the development of PTSD after TBI have been suggested, including neurobiological [22,30], psychological [11], and cognitive factors [50], reflecting a potentially complex interaction between the physiological and psychological consequences of TBI [30].
Despite some understanding about the reasons for post-TBI PTSD, PTSD in general is poorly amenable to pharmacologic treatment [28]. And while clinicians retain hope of unearthing new drugs against the illness, there remains no clear consensus in the understanding of the mechanism of PTSD development that is specifically associated with head trauma [29,30]. The present inquiry provides dietary evidence that supports our hypothesis that BBB dysfunction has an important role in the development of PTSD through the mechanisms of neurodegeneration and chronic glutamate neurotoxicity.
4.2. The Relationship between BBB and PTSD
The association between PTSD with BBB permeability has been a subject of growing interest. BBB disruption seems to significantly influence the pathophysiology of PTSD, and PTSD is associated with increased BBB permeability, possibly contributing to the development of cognitive dysfunction and other symptoms associated with the disorder [51,52,53]. Multiple mechanisms clarifying the link between PTSD and BBB permeability have been advanced. For instance, molecular mechanisms and inflammatory signaling pathways are potentially responsible for stress-induced BBB disruption, with evidence pointing to increased BBB permeability as a crucial factor in the development of PTSD-related behavioral abnormalities [53,54]. Additionally, the role of microglia in BBB impairment and cognitive dysfunction in a PTSD-like rodent model has been highlighted, further emphasizing the potential impact of BBB integrity on the pathogenesis of PTSD [52].
Furthermore, the association between PTSD and dysfunctional endothelia has been implicated, implying that increased BBB permeability likely predisposes individuals to the development of PTSD, potentially linking the disorder to vascular and cerebrovascular conditions [51]. Moreover, the breakdown of the BBB may be involved in the early cognitive dysfunction associated with PTSD, suggesting that BBB impairment underlies the common pathological foundation of PTSD and cognitive dysfunction [52].
In summary, there is compelling evidence of a relationship between PTSD and BBB permeability as evidenced from recent studies. Increased BBB permeability conceivably has a critical impact on the incidence and manifestation of PTSD-related symptoms, including cognitive and psychiatric dysfunction. The causal connection between BBB and PTSD is still unclear. This study focused on the hypothesis that BBB dysfunction critically affects the development of PTSD. Further investigation into the mechanisms underlying BBB disruption in the context of PTSD must be conducted to advance our understanding of the disorder and explore potential therapeutic targets.
4.3. TBI and the Disruption of the BBB
The dysfunction of the BBB in the acute and subacute stages, which occur in the first days and weeks after TBI, is well documented [55,56]. However, the chronic stage of the TBI-related destruction of the BBB requires clarification, as it is a relatively new theory. Here, we clarify the role of long-term BBB dysfunction in the development of neurodegenerative processes after TBI.
4.3.1. BBB Regulation in CNS Disorders
Provably, BBB dysregulation correlates with the development of cerebrovascular, neurodegenerative, and neuroinflammatory diseases, including stroke, TBI, brain tumor, multiple sclerosis, Alzheimer’s disease, Parkinson’s disease, epilepsy, edema, glaucoma, amyotrophic lateral sclerosis, depression, anxiety, and dementia [57,58,59,60]. Environmental factors can also affect BBB permeability [31,61]. The proposed pathway for the development of neuropsychiatric diseases, which includes the chronic impairment of BBB permeability, glutamate neurotoxicity, and neurodegeneration, is shown in Figure 4.
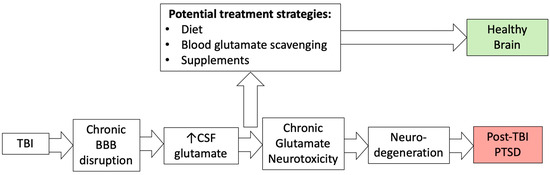
Figure 4.
The relationship between TBI, blood–brain barrier disruption, chronic glutamate neurotoxicity, and PTSD.
Although the relationship between these illnesses and BBB disruption is apparent, its nature has not been clearly elucidated yet. In particular, it is unclear if a dysfunctional BBB stems from the pathological condition or if damage to the BBB is the main pathogenic factor that occurs before the disease onset [57]. This inquiry relies on the causal link between neurodegenerative processes in the context of the long-term impairment of the BBB permeability.
4.3.2. Mechanisms of the BBB Dysfunction in TBI
BBB destruction after brain injury is referred to as biphasic [62]. The first phase reaches its zenith at 5 h after the injury [62] and the second phase at 72 h after the injury [63,64,65] in rats and occurs in humans on day 3 after the injury [66,67,68]. The recovery of a healthy BBB has been shown to take 1–3 months [69] and up to 10 months in rats [70] and up to years after injury to the brain in humans [55,71,72]. Damage to the BBB impedes the transfer of cerebral glutamate from the extracellular fluid to the bloodstream [73]. The integrity of the BBB naturally limits the pathological increase in extracellular fluid and cerebrospinal fluid glutamate levels during neuronal death and crucially maintains the upper levels of extracellular fluid and cerebrospinal fluid glutamate concentrations in the brain after TBI. In addition, BBB constantly modulates the lower threshold of brain glutamate content of the healthy as well as the impacted brain following TBI. Recently, we identified BBB permeability as a key to regulating both the high and low limits of brain glutamate in healthy and injured brains [32].
4.4. The Relationship between Neurodegeneration and the BBB
The relationship between neurodegeneration and BBB permeability is gaining substantial attention. Investigations have highlighted a close association between BBB dysfunction and neurodegenerative diseases, indicating a bidirectional relationship. Reportedly, a well-functioning BBB is crucial to maintaining healthy brain tissues [74]. A disruption in the BBB integrity, often linked to increased permeability, occurs in various neurodegenerative conditions, such as Alzheimer’s disease and Parkinson’s disease. This impairment can lead to the entry of harmful substances, inflammatory factors, and oxidative stress into the brain, potentially contributing to neuronal damage [75].
4.5. The Relationship between Neurodegeneration and PTSD
Recent research has found that the association between PTSD and neurodegenerative disorders is significant. Demonstrably, chronic PTSD is linked to a higher risk of neurodegeneration, including conditions such as dementia, Alzheimer’s disease, and Parkinson’s disease [76,77,78]. This association is supported by evidence of elevated levels of markers associated with neurodegeneration in individuals with PTSD [24]. Furthermore, clinical structural neuroimaging studies have uncovered links between PTSD and the loss of neural integrity in key brain regions, such as the hippocampus, amygdala, and prefrontal cortex, pointing to a potential connection between PTSD and neurodegeneration [79]. The duration and severity of PTSD could be linked to the extent of neurodegenerative changes [79]. Although evidence suggests a strong association between PTSD and neurodegeneration, the causal relationship between these conditions is unclear.
4.6. The Relationship between Neurodegeneration and Glutamate Neurotoxicity
The relationship between glutamate-induced neuronal degeneration and neurotoxicity is well established [80,81,82]. Recent discoveries about its influence on mood disorders has propelled research on updated therapies, proposing that drugs targeting the glutamatergic systems could serve as antidepressants [5,6,83,84,85,86].
Higher levels of extracellular glutamate can induce excitotoxicity by excessively activating ionotropic glutamate receptors following acute brain insults [82,87,88,89,90,91,92]. Chronic glutamate neurotoxicity has also been considered to play a role in many neurodegenerative conditions [90]. In these conditions, chronic excitotoxicity possibly appears as part of diseases where nerve cell death occurs more gradually, during which neurons encountering glutamate at higher-than-normal concentrations can eventually result in cell death [90]. Therefore, we propose that management of these neurological conditions should focus on restoring glutamatergic balance by promoting the update of glutamate and the release of extracellular glutamate.
4.7. Impaired BBB Permeability Disturbance in the Balance of Glutamate Concentration between the Blood and Brain Compartments
Glutamate, the brain’s most common free amino acid [93], has concentrations ranging from 50–100 μM/L in plasma and 150–300 μM/L in whole blood [31]. In the brain, concentrations can reach 10,000–12,000 μM/kg [94], while in extracellular fluids, it is much lower, between 1–10 μM/L [31]. The stable gradient between brain cells, the blood, and extracellular fluid is maintained through the facilitative and active transport mechanisms of the BBB [94]. When the BBB is healthy, it can impede the transport of glutamate between the intraparenchymal compartment and the blood compartment [73]. Several factors can contribute to an increase in post-TBI brain glutamate level, such as neuronal death [95], inflammation [96,97,98], disruptions in glutamate recycling and signaling [99], chronic stress [80], the astrocytic release of adenosine triphosphate [100], and other reasons [73,101]. We argue that the mechanisms of BBB destruction are the most important factors of increased brain glutamate as it relates to TBI [102].
4.8. The Role of Diet on Blood Glutamate Concentration: The Involvement of Glutamate in Neurocognitive Processes
Several factors can disrupt and cause the normally stable levels of blood glutamate concentrations to fluctuate. One of these factors may be dietary intake, caused by the presence of glutamate in many foods [33]. Traditional diets in Asian countries include high levels of glutamate because monosodium glutamate (MSG) is a popular flavoring in many cuisines, especially in East and Southeast Asian cuisine [103,104].
Many pharmacokinetic studies have pointed to the role of blood glutamate levels following dietary intake. An MSG intake of 16.0 mg/kg of body weight is a safe level; the average daily consumption in European countries and the United States sits at around 0.3–0.5 g/day [104], and up to 1.2–4 g/day in Asian countries [105]. The potential of MSG to lead to neurotoxicity, due to its role in chronic impairment of BBB permeability, is worrisome. Studies of MSG administration in food have found that blood and plasma glutamate levels rose from 1.4 to 19 times, depending on the amount of MSG consumed [106,107,108,109,110,111,112]. While MSG is considered safe for consumption by the Food and Drug Administration, several animal studies have identified potentially dangerous effects possibly associated with chronic MSG consumption [113].
Glutamate’s importance as an excitatory neurotransmitter in the central nervous system means that, when it exceeds normal levels, it can lead to excitotoxicity, associated with severe neuronal damage and disruptions to cognitive and behavioral health [104,114,115,116,117,118,119]. Recent studies have proposed relationships between the gut microbiota and the brain neurotransmitters dopamine, serotonin, GABA, and glutamate [120]. These relationships imply a correlation between dietary factors, neurotransmitter functions, and psychiatric disorders, such as PTSD. Certain studies have posited that diets with high levels of sodium glutamate raise blood glutamate and glutamic acid levels, resulting in hyper-glutamatergic neurotransmission. Hyper-glutamatergic neurotransmission has been shown with an association with several different psychiatric conditions, including PTSD [120,121,122,123,124,125]. In several studies, rodents on a diet with a high intake of sodium glutamate were observed with more depressive behaviors, such as less social interaction, anhedonia, and behavioral despair [120,126,127]. A preclinical analysis shows a possible link between sodium glutamate intake and the development of anxious and depressive-like phenotypes [120]. Animal models administered sodium glutamate showed higher rates of anxiety and behavioral dysfunction [128,129,130,131,132]. Rats with post-traumatic depressive-like symptoms and with anxiety who were treated for elevated blood and cerebrospinal fluid glutamate levels showed no difference in behavior post-treatment from that of naïve rats [5,6]. These data imply that dietary glutamate consumption and the development of PTSD have a close correlation.
In addition, glutamate dysregulation affects the pathophysiology of PTSD, which is especially relevant to glutamatergic dysfunction in trauma-related disorders. Similarly, the role of glutamate in synaptic plasticity, memory formation, and the development of PTSD-related behaviors has been hypothesized [121,122,125]. Furthermore, altered metabotropic glutamate receptor 5 (mGluR5) markers have been noted in patients with PTSD, with patient brains containing increased mGluR5 levels. This could have consequences for the treatment of PTSD, as the overstimulation of mGluR5 is linked to fear and stress-related behaviors, and drugs that moderate mGluR5 function could improve these symptoms [125]. Therefore, the relationship between consumption of dietary glutamate and the development of PTSD has been clearly outlined.
4.9. Current and Potential Therapeutic Strategies for Post-TBI PTSD
Treatment success rates for post-TBI PTSD vary across studies. In some studies, the success rates range extensively, with rates as high as 24% to 50% [44,133,134]. In other reports, the success rates are as low as 3% [135] or do not register the presence of PTSD at all [9,136]. Several approaches to post-TBI PTSD treatment, including cognitive processing therapy [137], prolonged exposure [137], selective serotonin reuptake inhibitors [21,138], cognitive behavioral therapy [139], eye movement desensitization and reprocessing, antidepressants, risperidone, topiramate, and even stem cells, have been met with scrutiny [30]. However, the treatment of comorbid TBI and PTSD is a complex issue, and the most effective approaches may differ depending on the individual’s specific condition and needs.
Our previous hypothesis that the integrity of the BBB is critical in controlling glutamate levels in the cerebrospinal fluid after brain injury [102] is particularly relevant after TBI, in which neurotoxicity associated with high glutamate levels induces neurodegenerative processes that ultimately lead to psychiatric disorders and, in particular, PTSD [32]. Consequently, a targeted control of elevated glutamate levels could impact the neuropsychological symptoms of acute and chronic brain diseases significantly. Therefore, chances are high that future strategies to find new therapeutic approaches will focus on the role of BBB and chronic glutamate neurotoxicity as central factors in the development of neuropsychiatric sequelae of TBI.
4.10. Limitations
As a systematic review, this study cannot study all the parameters of the conditions described here, since it is constrained by the methods and results of the literature it reviews. In addition, we do not explore the potential consequences of diet-related changes as a therapeutic approach for the reduction of glutamate levels. Therefore, it is our hope that future research will investigate topics that we could not include, such as comorbidities of PTSD and their relationships to glutamate, diet as a treatment for PTSD and/or glutamate dysregulation more generally, and the specific glutamate receptors that may play a role in the development of PTSD.
5. Conclusions
Excessive levels of blood glutamate are closely associated with the onset of PTSD following TBI. We hypothesize that the permeability of the BBB is crucial in facilitating neurodegenerative processes due to glutamate neurotoxicity, as well as the emergence of neuropsychiatric disorders. Therefore, strategies aimed at reducing blood glutamate concentrations and restoring the integrity of the BBB might aid in treating and preventing PTSD that arises after TBI. Various factors can influence blood glutamate levels, yet a functional BBB effectively shields the brain from glutamate’s neurotoxic effects. Among these factors, dietary glutamate intake appears to significantly contribute to increased blood glutamate levels. In cases of chronic conditions with BBB damage post-TBI, sustained high levels of blood glutamate, driven by high glutamate intake, can lead to brain neurotoxicity and neurodegeneration, potentially culminating in PTSD. A diet low in glutamate, along with targeted treatments for the glutamate system and dietary supplements to reduce blood glutamate, might offer effective approaches for managing PTSD following TBI.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu16060901/s1. Table S1. Prevalence of post-TBI PTSD in Asian countries (rich-glutamate diets) vs. non-Asian countries (poor-glutamate diets). Table S2. Prevalence of post-TBI PTSD in no Asian countries. Table S3. Prevalence of post-TBI PTSD in Asian countries. References [140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177] are cited in the supplementary materials.
Author Contributions
Conceptualization, M.B. and B.F.G.; methodology, A.F.; software, A.F.; validation, A.O., F.M. and M.B.; formal analysis, F.M.; investigation, A.O.; resources, A.F.; data curation, F.M.; writing—original draft preparation, H.N.S.; writing—review and editing, B.F.G.; supervision, M.B.; project administration, A.Z.; funding acquisition, A.Z. All authors have read and agreed to the published version of the manuscript.
Funding
Funding was provided by a departmental grant from the Department of Anesthesiology and Critical Care at Soroka Medical Center, Ben-Gurion University of the Negev.
Data Availability Statement
Data is contained within the article and Supplementary Materials.
Acknowledgments
We express our gratitude to Igor Merzlikin from the Department of Biology and Methods of Teaching Biology at the A. S. Makarenko Sumy State Pedagogical University, UA, Ukraine, for his valuable support and insightful contributions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Maas, A.I.R.; Menon, D.K.; Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Büki, A.; Chesnut, R.M. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar] [CrossRef]
- Peterson, C.; Miller, G.F.; Barnett, S.B.L.; Florence, C. Economic cost of injury—United States, 2019. Morb. Mortal. Wkly. Rep. 2021, 70, 1655. [Google Scholar] [CrossRef]
- Ahmed, S.; Venigalla, H.; Mekala, H.M.; Dar, S.; Hassan, M.; Ayub, S. Traumatic brain injury and neuropsychiatric complications. Indian J. Psychol. Med. 2017, 39, 114–121. [Google Scholar] [CrossRef]
- Torregrossa, W.; Raciti, L.; Rifici, C.; Rizzo, G.; Raciti, G.; Casella, C.; Naro, A.; Calabrò, R.S. Behavioral and Psychiatric Symptoms in Patients with Severe Traumatic Brain Injury: A Comprehensive Overview. Biomedicines 2023, 11, 1449. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.; Gruenbaum, B.F.; Shelef, I.; Severynovska, O.; Gal, R.; Dubilet, M.; Zlotnik, A.; Kofman, O.; Boyko, M. Blood glutamate scavenging with pyruvate as a novel preventative and therapeutic approach for depressive-like behavior following traumatic brain injury in a rat model. Front. Neurosci. 2022, 16, 832478. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.; Gruenbaum, B.F.; Shelef, I.; Zvenigorodsky, V.; Severynovska, O.; Fleidervish, I.; Knyazer, B.; Frenkel, A.; Zlotnik, A.; Kofman, O. Blood glutamate scavenging as a novel glutamate-based therapeutic approach for post-traumatic brain injury anxiety and social impairment. Transl. Psychiatry 2023, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Boyko, M.; Gruenbaum, B.F.; Shelef, I.; Zvenigorodsky, V.; Severynovska, O.; Binyamin, Y.; Knyazer, B.; Frenkel, A.; Frank, D.; Zlotnik, A. Traumatic brain injury-induced submissive behavior in rats: Link to depression and anxiety. Transl. Psychiatry 2022, 12, 239. [Google Scholar] [CrossRef] [PubMed]
- King, N.S. PTSD and traumatic brain injury: Folklore and fact? Brain Inj. 2008, 22, 1–5. [Google Scholar] [CrossRef]
- Sbordone, R.J.; Liter, J.C. Mild traumatic brain injury does not produce post-traumatic stress disorder. Brain Inj. 1995, 9, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Sbordone, R.J.; Ruff, R.M. Re-examination of the controversial coexistence of traumatic brain injury and posttraumatic stress disorder: Misdiagnosis and self-report measures. Psychol. Inj. Law 2010, 3, 63–76. [Google Scholar] [CrossRef]
- Bryant, R. Post-traumatic stress disorder vs traumatic brain injury. Dialogues Clin. Neurosci. 2011, 13, 251–262. [Google Scholar] [CrossRef]
- Villalobos, D.; Bivona, U. Post-traumatic stress disorder after severe traumatic brain injury: A systematic review. Arch. Clin. Neuropsychol. 2022, 37, 583–594. [Google Scholar] [CrossRef]
- Iljazi, A.; Ashina, H.; Al-Khazali, H.M.; Ashina, M.; Winther Schytz, H.; Ashina, S. Post-traumatic stress disorder attributed to traumatic brain injury in children–a systematic review. Brain Inj. 2020, 34, 857–863. [Google Scholar] [CrossRef]
- Iljazi, A.; Ashina, H.; Al-Khazali, H.M.; Lipton, R.B.; Ashina, M.; Schytz, H.W.; Ashina, S. Post-traumatic stress disorder after traumatic brain injury—A systematic review and meta-analysis. Neurol. Sci. 2020, 41, 2737–2746. [Google Scholar] [CrossRef]
- Esagoff, A.I.; Stevens, D.A.; Kosyakova, N.; Woodard, K.; Jung, D.; Richey, L.N.; Daneshvari, N.O.; Luna, L.P.; Bray, M.J.C.; Bryant, B.R. Neuroimaging Correlates of Post-Traumatic Stress Disorder in Traumatic Brain Injury: A Systematic Review of the Literature. J. Neurotrauma 2023, 40, 1029–1044. [Google Scholar] [CrossRef]
- Uiterwijk, D.; Stargatt, R.; Humphrey, S.; Crowe, S.F. The relationship between cognitive functioning and symptoms of depression, anxiety, and post-traumatic stress disorder in adults with a traumatic brain injury: A meta-analysis. Neuropsychol. Rev. 2022, 32, 758–806. [Google Scholar] [CrossRef]
- Van Praag, D.L.G.; Cnossen, M.C.; Polinder, S.; Wilson, L.; Maas, A.I.R. Post-traumatic stress disorder after civilian traumatic brain injury: A systematic review and meta-analysis of prevalence rates. J. Neurotrauma 2019, 36, 3220–3232. [Google Scholar] [CrossRef]
- Stein, M.B.; Jain, S.; Giacino, J.T.; Levin, H.; Dikmen, S.; Nelson, L.D.; Vassar, M.J.; Okonkwo, D.O.; Diaz-Arrastia, R.; Robertson, C.S. Risk of posttraumatic stress disorder and major depression in civilian patients after mild traumatic brain injury: A TRACK-TBI study. JAMA Psychiatry 2019, 76, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Bryant, R.A.; Marosszeky, J.E.; Crooks, J.; Gurka, J.A. Posttraumatic stress disorder after severe traumatic brain injury. Am. J. Psychiatry 2000, 157, 629–631. [Google Scholar] [CrossRef] [PubMed]
- Heim, C.; Schultebraucks, K.; Marmar, C.R.; Nemeroff, C.B. Neurobiological pathways involved in fear, stress, and PTSD. In Post-Traumatic Stress Disorder; Oxford University Press: Oxford, UK, 2018; pp. 331–352. [Google Scholar]
- Vasterling, J.J.; Jacob, S.N.; Rasmusson, A. Traumatic brain injury and posttraumatic stress disorder: Conceptual, diagnostic, and therapeutic considerations in the context of co-occurrence. J. Neuropsychiatry Clin. Neurosci. 2018, 30, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Sherin, J.E.; Nemeroff, C.B. Post-traumatic stress disorder: The neurobiological impact of psychological trauma. Dialogues Clin. Neurosci. 2011, 13, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Risbrough, V.B.; Vaughn, M.N.; Friend, S.F. Role of inflammation in traumatic brain injury–associated risk for neuropsychiatric disorders: State of the evidence and where do we go from here. Biol. Psychiatry 2022, 91, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, R.P. Biomarkers of Neurodegeneration in Post-Traumatic Stress Disorder: An Integrative Review. Biomedicines 2023, 11, 1465. [Google Scholar] [CrossRef] [PubMed]
- Malejko, K.; Abler, B.; Plener, P.L.; Straub, J. Neural correlates of psychotherapeutic treatment of post-traumatic stress disorder: A systematic literature review. Front. Psychiatry 2017, 8, 85. [Google Scholar] [CrossRef] [PubMed]
- Brady, K.T.; Killeen, T.K.; Brewerton, T.; Lucerini, S. Comorbidity of psychiatric disorders and posttraumatic stress disorder. J. Clin. Psychiatry 2000, 61, 22–32. [Google Scholar] [PubMed]
- Qassem, T.; Aly-ElGabry, D.; Alzarouni, A.; Abdel-Aziz, K.; Arnone, D. Psychiatric co-morbidities in post-traumatic stress disorder: Detailed findings from the adult psychiatric morbidity survey in the English population. Psychiatr. Q. 2021, 92, 321–330. [Google Scholar] [CrossRef]
- Hoskins, M.D.; Bridges, J.; Sinnerton, R.; Nakamura, A.; Underwood, J.F.G.; Slater, A.; Lee, M.R.D.; Clarke, L.; Lewis, C.; Roberts, N.P. Pharmacological therapy for post-traumatic stress disorder: A systematic review and meta-analysis of monotherapy, augmentation and head-to-head approaches. Eur. J. Psychotraumatol. 2021, 12, 1802920. [Google Scholar] [CrossRef]
- Williamson, J.B.; Heilman, K.M.; Porges, E.C.; Lamb, D.G.; Porges, S.W. A possible mechanism for PTSD symptoms in patients with traumatic brain injury: Central autonomic network disruption. Front. Neuroeng. 2013, 6, 13. [Google Scholar] [CrossRef]
- Monsour, M.; Ebedes, D.; Borlongan, C.V. A review of the pathology and treatment of TBI and PTSD. Exp. Neurol. 2022, 351, 114009. [Google Scholar] [CrossRef]
- Gruenbaum, B.F.; Zlotnik, A.; Frenkel, A.; Fleidervish, I.; Boyko, M. Glutamate Efflux across the Blood–Brain Barrier: New Perspectives on the Relationship between Depression and the Glutamatergic System. Metabolites 2022, 12, 459. [Google Scholar] [CrossRef]
- Gruenbaum, B.F.; Zlotnik, A.; Fleidervish, I.; Frenkel, A.; Boyko, M. Glutamate Neurotoxicity and Destruction of the Blood–Brain Barrier: Key Pathways for the Development of Neuropsychiatric Consequences of TBI and Their Potential Treatment Strategies. Int. J. Mol. Sci. 2022, 23, 9628. [Google Scholar] [CrossRef]
- Boyko, M.; Gruenbaum, B.F.; Oleshko, A.; Merzlikin, I.; Zlotnik, A. Diet’s Impact on Post-Traumatic Brain Injury Depression: Exploring Neurodegeneration, Chronic Blood–Brain Barrier Destruction, and Glutamate Neurotoxicity Mechanisms. Nutrients 2023, 15, 4681. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Lamberty, G.J.; Nelson, N.W.; Yamada, T. Effects and outcomes in civilian and military traumatic brain injury: Similarities, differences, and forensic implications. Behav. Sci. Law 2013, 31, 814–832. [Google Scholar] [CrossRef]
- Denning, J.H.; Shura, R.D. Cost of malingering mild traumatic brain injury-related cognitive deficits during compensation and pension evaluations in the veterans benefits administration. Appl. Neuropsychol. Adult 2019, 26, 1–16. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. Newcastle-Ottawa Quality Assessment Scale Cohort Studies; University of Ottawa: Ottawa, ON, USA, 2014. [Google Scholar]
- Stolze, T.; Franke, S.; Haybaeck, J.; Moehler, M.; Grimminger, P.P.; Lang, H.; Roth, W.; Gockel, I.; Kreuser, N.; Bläker, H. Mismatch repair deficiency, chemotherapy and survival for resectable gastric cancer: An observational study from the German staR cohort and a meta-analysis. J. Cancer Res. Clin. Oncol. 2023, 149, 1007–1017. [Google Scholar] [CrossRef]
- Petousis, S.; Christidis, P.; Margioula-Siarkou, C.; Liberis, A.; Vavoulidis, E.; Margioula-Siarkou, G.; Vatopoulou, A.; Papanikolaou, A.; Mavromatidis, G.; Dinas, K. Axillary lymph node dissection vs. sentinel node biopsy for early-stage clinically node-negative breast cancer: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2022, 306, 1221–1234. [Google Scholar] [CrossRef] [PubMed]
- Bhutta, M.S.; Shechter, O.; Gallo, E.S.; Martin, S.D.; Jones, E.; Doncel, G.F.; Borenstein, R. Ginkgolic acid inhibits herpes simplex virus type 1 skin infection and prevents zosteriform spread in mice. Viruses 2021, 13, 86. [Google Scholar] [CrossRef]
- Bryant, R.A.; Marosszeky, J.E.; Crooks, J.; Baguley, I.; Gurka, J. Coping style and post-traumatic stress disorder following severe traumatic brain injury. Brain Inj. 2000, 14, 175–180. [Google Scholar] [PubMed]
- Bryant, R.A.; Marosszeky, J.E.; Crooks, J.; Baguley, I.J.; Gurka, J.A. Posttraumatic stress disorder and psychosocial functioning after severe traumatic brain injury. J. Nerv. Ment. Dis. 2001, 189, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Bryant, R.A.; Harvey, A.G. Relationship between acute stress disorder and posttraumatic stress disorder following mild traumatic brain injury. Am. J. Psychiatry 1998, 155, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Bryant, R.A.; Harvey, A.G. The influence of traumatic brain injury on acute stress disorder and post-traumatic stress disorder following motor vehicle accidents. Brain Inj. 1999, 13, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Li, Q.; Gao, Y.; Huo, H.; Zhang, W. Changes and influencing factors of stress disorder in patients with mild traumatic brain injury stress disorder. BioMed Res. Int. 2022, 2022, 9082946. [Google Scholar] [CrossRef] [PubMed]
- Dieter, J.N.I.; Engel, S.D. Traumatic brain injury and posttraumatic stress disorder: Comorbid consequences of war. Neurosci. Insights 2019, 14, 1179069519892933. [Google Scholar] [CrossRef] [PubMed]
- Ashman, T.A.; Spielman, L.A.; Hibbard, M.R.; Silver, J.M.; Chandna, T.; Gordon, W.A. Psychiatric challenges in the first 6 years after traumatic brain injury: Cross-sequential analyses of Axis I disorders. Arch. Phys. Med. Rehabil. 2004, 85, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, R.C.; Schindler, A.G.; Pagulayan, K.F. Untangling PTSD and TBI: Challenges and strategies in clinical care and research. Curr. Neurol. Neurosci. Rep. 2018, 18, 106. [Google Scholar] [CrossRef] [PubMed]
- Sumpter, R.E.; McMillan, T.M. Errors in self-report of post-traumatic stress disorder after severe traumatic brain injury. Brain Inj. 2006, 20, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Glaesser, J.; Neuner, F.; Lütgehetmann, R.; Schmidt, R.; Elbert, T. Posttraumatic stress disorder in patients with traumatic brain injury. BMC Psychiatry 2004, 4, 5. [Google Scholar] [CrossRef]
- Sfera, A.; Osorio, C.; Rahman, L.; Zapata-Martín del Campo, C.M.; Maldonado, J.C.; Jafri, N.; Cummings, M.A.; Maurer, S.; Kozlakidis, Z. PTSD as an endothelial disease: Insights from COVID-19. Front. Cell. Neurosci. 2021, 15, 770387. [Google Scholar] [CrossRef]
- Ni, K.; Zhu, J.; Xu, X.; Liu, Y.; Yang, S.; Huang, Y.; Xu, R.; Jiang, L.; Zhang, J.; Zhang, W. Hippocampal Activated Microglia May Contribute to Blood–Brain Barrier Impairment and Cognitive Dysfunction in Post-Traumatic Stress Disorder-Like Rats. J. Mol. Neurosci. 2022, 72, 975–982. [Google Scholar] [CrossRef]
- Taghadosi, Z.; Zarifkar, A.; Razban, V.; Owjfard, M.; Aligholi, H. Effect of chronically electric foot shock stress on spatial memory and hippocampal blood brain barrier permeability. Behav. Brain Res. 2021, 410, 113364. [Google Scholar] [CrossRef]
- Welcome, M.O.; Mastorakis, N.E. Stress-induced blood brain barrier disruption: Molecular mechanisms and signaling pathways. Pharmacol. Res. 2020, 157, 104769. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, H.; Guo, X.; Pluimer, B.; Zhao, Z. Blood–brain barrier dysfunction in mild traumatic brain injury: Evidence from preclinical murine models. Front. Physiol. 2020, 11, 1030. [Google Scholar] [CrossRef]
- Price, L.; Wilson, C.; Grant, G. Blood–brain barrier pathophysiology following traumatic brain injury. In Translational Research in Traumatic Brain Injury; CRC Press/Taylor and Francis Group: Boca Raton, FL, USA, 2016. [Google Scholar]
- Archie, S.R.; Al Shoyaib, A.; Cucullo, L. Blood-Brain Barrier Dysfunction in CNS Disorders and Putative Therapeutic Targets: An Overview. Pharmaceutics 2021, 13, 1779. [Google Scholar] [CrossRef] [PubMed]
- Kealy, J.; Greene, C.; Campbell, M. Blood-brain barrier regulation in psychiatric disorders. Neurosci. Lett. 2020, 726, 133664. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Xiao, Z.J.; Yang, B.; Lan, Z.; Fang, F. Blood-brain barrier: More contributor to disruption of central nervous system homeostasis than victim in neurological disorders. Front. Neurosci. 2020, 14, 764. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Manaenko, A. Blood-brain Barrier Dysfunction in Cerebrovascular Diseases. Curr. Neuropharmacol. 2020, 18, 1166. [Google Scholar] [CrossRef] [PubMed]
- Segarra, M.; Aburto, M.R.; Acker-Palmer, A. Blood–brain barrier dynamics to maintain brain homeostasis. Trends Neurosci. 2021, 44, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Chodobski, A.; Zink, B.J.; Szmydynger-Chodobska, J. Blood–brain barrier pathophysiology in traumatic brain injury. Transl. Stroke Res. 2011, 2, 492–516. [Google Scholar] [CrossRef]
- Toyota, S.; Graf, R.; Valentino, M.; Yoshimine, T.; Heiss, W.-D. Malignant Infarction in Cats After Prolonged Middle Cerebral Artery Occlusion: Glutamate Elevation Related to Decrease of Cerebral Perfusion Pressure. Stroke 2002, 33, 1383–1391. [Google Scholar] [CrossRef][Green Version]
- Hone, E.A.; Hu, H.; Sprowls, S.A.; Farooqi, I.; Grasmick, K.; Lockman, P.R.; Simpkins, J.W.; Ren, X. Biphasic blood-brain barrier openings after stroke. Neurol. Disord. Stroke Int. 2018, 1, 1011. [Google Scholar]
- Başkaya, M.K.; Rao, A.M.; Doğan, A.; Donaldson, D.; Dempsey, R.J. The biphasic opening of the blood–brain barrier in the cortex and hippocampus after traumatic brain injury in rats. Neurosci. Lett. 1997, 226, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Chamoun, R.; Suki, D.; Gopinath, S.P.; Goodman, J.C.; Robertson, C. Role of extracellular glutamate measured by cerebral microdialysis in severe traumatic brain injury. J. Neurosurg. 2010, 113, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Bullock, R.; Zauner, A.; Woodward, J.J.; Myseros, J.; Choi, S.C.; Ward, J.D.; Marmarou, A.; Young, H.F. Factors affecting excitatory amino acid release following severe human head injury. J. Neurosurg. 1998, 89, 507–518. [Google Scholar] [CrossRef]
- Vespa, P.; Prins, M.; Ronne-Engstrom, E.; Caron, M.; Shalmon, E.; Hovda, D.A.; Martin, N.A.; Becker, D.P. Increase in extracellular glutamate caused by reduced cerebral perfusion pressure and seizures after human traumatic brain injury: A microdialysis study. J. Neurosurg. 1998, 89, 971–982. [Google Scholar] [CrossRef]
- Yu, M.; Wang, M.; Yang, D.; Wei, X.; Li, W. Dynamics of blood brain barrier permeability and tissue microstructure following controlled cortical impact injury in rat: A dynamic contrast-enhanced magnetic resonance imaging and diffusion kurtosis imaging study. Magn. Reson. Imaging 2019, 62, 1–9. [Google Scholar] [CrossRef]
- van Vliet, E.A.; Ndode-Ekane, X.E.; Lehto, L.J.; Gorter, J.A.; Andrade, P.; Aronica, E.; Gröhn, O.; Pitkänen, A. Long-lasting blood-brain barrier dysfunction and neuroinflammation after traumatic brain injury. Neurobiol. Dis. 2020, 145, 105080. [Google Scholar] [CrossRef]
- Hay, J.; Johnson, V.; Young, A.; Smith, D.; Stewart, W. Blood-Brain Barrier Disruption Is an Early Event That May Persist for Many Years After Traumatic Brain Injury in Humans. J. Neuropathol. Exp. Neurol. 2015, 74, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Cash, A.; Theus, M.H. Mechanisms of blood–brain barrier dysfunction in traumatic brain injury. Int. J. Mol. Sci. 2020, 21, 3344. [Google Scholar] [CrossRef]
- Bai, W.; Zhou, Y.-G. Homeostasis of the Intraparenchymal-Blood Glutamate Concentration Gradient: Maintenance, Imbalance, and Regulation. Front. Mol. Neurosci. 2017, 10, 400. [Google Scholar] [CrossRef]
- Wu, S.; Yin, Y.; Du, L. Blood–brain barrier dysfunction in the pathogenesis of major depressive disorder. Cell. Mol. Neurobiol. 2022, 42, 2571–2591. [Google Scholar] [CrossRef]
- Knox, E.G.; Aburto, M.R.; Clarke, G.; Cryan, J.F.; O’Driscoll, C.M. The blood-brain barrier in aging and neurodegeneration. Mol. Psychiatry 2022, 27, 2659–2673. [Google Scholar] [CrossRef]
- Neylan, T.C. Post-traumatic Stress Disorder and Neurodegeneration. Am. J. Geriatr. Psychiatry 2020, 28, 61–63. [Google Scholar] [CrossRef]
- Neylan, T.C. Posttraumatic Stress Disorder and Risk For Dementia. Alzheimer’s Dement. 2022, 18, e060705. [Google Scholar] [CrossRef]
- Chan, Y.-L.E.; Bai, Y.-M.; Hsu, J.-W.; Huang, K.-L.; Su, T.-P.; Li, C.-T.; Lin, W.-C.; Pan, T.-L.; Chen, T.-J.; Tsai, S.-J. Post-traumatic stress disorder and risk of parkinson disease: A nationwide longitudinal study. Am. J. Geriatr. Psychiatry 2017, 25, 917–923. [Google Scholar] [CrossRef]
- Miller, M.W.; Sadeh, N. Traumatic stress, oxidative stress and post-traumatic stress disorder: Neurodegeneration and the accelerated-aging hypothesis. Mol. Psychiatry 2014, 19, 1156–1162. [Google Scholar] [CrossRef]
- McGrath, T.; Baskerville, R.; Rogero, M.; Castell, L. Emerging Evidence for the Widespread Role of Glutamatergic Dysfunction in Neuropsychiatric Diseases. Nutrients 2022, 14, 917. [Google Scholar] [CrossRef]
- Lau, A.; Tymianski, M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflügers Arch. Eur. J. Physiol. 2010, 460, 525–542. [Google Scholar] [CrossRef]
- Al-Nasser, M.N.; Mellor, I.R.; Carter, W.G. Is L-Glutamate Toxic to Neurons and Thereby Contributes to Neuronal Loss and Neurodegeneration? A Systematic Review. Brain Sci. 2022, 12, 577. [Google Scholar] [CrossRef] [PubMed]
- Borbély, É.; Simon, M.; Fuchs, E.; Wiborg, O.; Czéh, B.; Helyes, Z. Novel drug developmental strategies for treatment-resistant depression. Br. J. Pharmacol. 2022, 179, 1146–1186. [Google Scholar] [CrossRef] [PubMed]
- Berman, R.M.; Cappiello, A.; Anand, A.; Oren, D.A.; Heninger, G.R.; Charney, D.S.; Krystal, J.H. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 2000, 47, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Blaylock, R.L.; Faria, M. New concepts in the development of schizophrenia, autism spectrum disorders, and degenerative brain diseases based on chronic inflammation: A working hypothesis from continued advances in neuroscience research. Surg. Neurol. Int. 2021, 12, 556. [Google Scholar] [PubMed]
- Frank, D.; Kuts, R.; Tsenter, P.; Gruenbaum, B.F.; Grinshpun, Y.; Zvenigorodsky, V.; Shelef, I.; Natanel, D.; Brotfain, E.; Zlotnik, A. The effect of pyruvate on the development and progression of post-stroke depression: A new therapeutic approach. Neuropharmacology 2019, 155, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Belov Kirdajova, D.; Kriska, J.; Tureckova, J.; Anderova, M. Ischemia-triggered glutamate excitotoxicity from the perspective of glial cells. Front. Cell. Neurosci. 2020, 14, 51. [Google Scholar] [CrossRef] [PubMed]
- Simões, A.P.; Silva, C.G.; Marques, J.M.; Pochmann, D.; Porciúncula, L.O.; Ferreira, S.; Oses, J.P.; Beleza, R.O.; Real, J.I.; Köfalvi, A. Glutamate-induced and NMDA receptor-mediated neurodegeneration entails P2Y1 receptor activation. Cell Death Dis. 2018, 9, 297. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, R.L.; Babu, G.N. Cell death mechanisms in the early stages of acute glutamate neurotoxicity. Neurosci. Res. 2010, 66, 271–278. [Google Scholar] [CrossRef]
- Lewerenz, J.; Maher, P. Chronic glutamate toxicity in neurodegenerative diseases—What is the evidence? Front. Neurosci. 2015, 9, 469. [Google Scholar] [CrossRef]
- Meldrum, B.S. The role of glutamate in epilepsy and other CNS disorders. Neurology 1994, 44 (Suppl. S8), S14–S23. [Google Scholar]
- Meldrum, B.; Garthwaite, J. Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends Pharmacol. Sci. 1990, 11, 379–387. [Google Scholar] [CrossRef]
- Zhou, Y.; Danbolt, N.C. Glutamate as a neurotransmitter in the healthy brain. J. Neural Transm. 2014, 121, 799–817. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.A.; Viña, J.R. How Glutamate Is Managed by the Blood-Brain Barrier. Biology 2016, 5, 37. [Google Scholar] [CrossRef]
- Teichberg, V.I.; Cohen-Kashi-Malina, K.; Cooper, I.; Zlotnik, A. Homeostasis of glutamate in brain fluids: An accelerated brain-to-blood efflux of excess glutamate is produced by blood glutamate scavenging and offers protection from neuropathologies. Neuroscience 2009, 158, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Haroon, E.; Miller, A.H. Inflammation Effects on Brain Glutamate in Depression: Mechanistic Considerations and Treatment Implications. In Inflammation-Associated Depression: Evidence, Mechanisms and Implications; Current Topics in Behavioral Neurosciences; Springer International Publishing: Cham, Switzerland, 2016; Volume 31, pp. 173–198. [Google Scholar]
- Haroon, E.; Chen, X.; Li, Z.; Patel, T.; Woolwine, B.J.; Hu, X.P.; Felger, J.C.; Miller, A.H. Increased inflammation and brain glutamate define a subtype of depression with decreased regional homogeneity, impaired network integrity, and anhedonia. Transl. Psychiatry 2018, 8, 189. [Google Scholar] [CrossRef] [PubMed]
- King, S.; Jelen, L.A.; Horne, C.M.; Cleare, A.; Pariante, C.M.; Young, A.H.; Stone, J.M. Inflammation, Glutamate, and Cognition in Bipolar Disorder Type II: A Proof of Concept Study. Front. Psychiatry 2019, 10, 66. [Google Scholar] [CrossRef]
- Gasiorowska, A.; Wydrych, M.; Drapich, P.; Zadrozny, M.; Steczkowska, M.; Niewiadomski, W.; Niewiadomska, G. The biology and pathobiology of glutamatergic, cholinergic, and dopaminergic signaling in the aging brain. Front. Aging Neurosci. 2021, 391, 654931. [Google Scholar] [CrossRef] [PubMed]
- Ferrini, F.; De Koninck, Y. Microglia control neuronal network excitability via BDNF signalling. Neural Plast. 2013, 2013, 429815. [Google Scholar] [CrossRef] [PubMed]
- Sasaki-Hamada, S.; Sanai, E.; Kanemaru, M.; Kamanaka, G.; Oka, J.-I. Long-term exposure to high glucose induces changes in the expression of AMPA receptor subunits and glutamate transmission in primary cultured cortical neurons. Biochem. Biophys. Res. Commun. 2022, 589, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Boyko, M.; Gruenbaum, B.F.; Frank, D.; Natanel, D.; Negev, S.; Azab, A.N.; Barsky, G.; Knyazer, B.; Kofman, O.; Zlotnik, A. The Integrity of the Blood–Brain Barrier as a Critical Factor for Regulating Glutamate Levels in Traumatic Brain Injury. Int. J. Mol. Sci. 2023, 24, 5897. [Google Scholar] [CrossRef] [PubMed]
- Markits, I.H.S. Monosodium Glutamate (MSG) Chemical Economics Handbook. 2018, pp. 1–88. Available online: https://ihsmarkit.com/products/monosodium-glutamate-chemical-economics-handbook.html (accessed on 1 January 2024).
- Kazmi, Z.; Fatima, I.; Perveen, S.; Malik, S.S. Monosodium glutamate: Review on clinical reports. Int. J. Food Prop. 2017, 20, 1807–1815. [Google Scholar] [CrossRef]
- Insawang, T.; Selmi, C.; Cha’on, U.; Pethlert, S.; Yongvanit, P.; Areejitranusorn, P.; Boonsiri, P.; Khampitak, T.; Tangrassameeprasert, R.; Pinitsoontorn, C. Monosodium glutamate (MSG) intake is associated with the prevalence of metabolic syndrome in a rural Thai population. Nutr. Metab. 2012, 9, 50. [Google Scholar] [CrossRef]
- Rutten, E.P.A.; Engelen, M.P.K.J.; Wouters, E.F.M.; Schols, A.M.W.J.; Deutz, N.E.P. Metabolic effects of glutamine and glutamate ingestion in healthy subjects and in persons with chronic obstructive pulmonary disease. Am. J. Clin. Nutr. 2006, 83, 115–123. [Google Scholar] [PubMed]
- Stegink, L.D.; Filer Jr, L.J.; Baker, G.L. Effect of carbohydrate on plasma and erythrocyte glutamate levels in humans ingesting large doses of monosodium L-glutamate in water. Am. J. Clin. Nutr. 1983, 37, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, J.D.; Cameron, J.L.; Fernstrom, M.H.; McConaha, C.; Weltzin, T.E.; Kaye, W.H. Short-term neuroendocrine effects of a large oral dose of monosodium glutamate in fasting male subjects. J. Clin. Endocrinol. Metab. 1996, 81, 184–191. [Google Scholar] [PubMed]
- Graham, T.E.; Sgro, V.; Friars, D.; Gibala, M.J. Glutamate ingestion: The plasma and muscle free amino acid pools of resting humans. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E83–E89. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.-J.; Huang, P.-C. Circadian variations in plasma and erythrocyte glutamate concentrations in adult men consuming a diet with and without added monosodium glutamate. J. Nutr. 2000, 130, 1002S–1004S. [Google Scholar] [CrossRef] [PubMed]
- Stegink, L.D.; Filer, L.J., Jr.; Baker, G.L. Plasma and erythrocyte amino acid levels in normal adult subjects fed a high protein meal with and without added monosodium glutamate. J. Nutr. 1982, 112, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Loï, C.; Cynober, L. Glutamate: A safe nutrient, not just a simple additive. Ann. Nutr. Metab. 2022, 78, 133–146. [Google Scholar] [CrossRef]
- Hajihasani, M.M.; Soheili, V.; Zirak, M.R.; Sahebkar, A.; Shakeri, A. Natural products as safeguards against monosodium glutamateinduced toxicity. Iran. J. Basic Med. Sci. 2020, 23, 416. [Google Scholar]
- López-Pérez, S.J.; Ureña-Guerrero, M.E.; Morales-Villagrán, A. Monosodium glutamate neonatal treatment as a seizure and excitotoxic model. Brain Res. 2010, 1317, 246–256. [Google Scholar] [CrossRef]
- Beas-Zárate, C.; Pérez-Vega, M.a.I.; González-Burgos, I. Neonatal exposure to monosodium L-glutamate induces loss of neurons and cytoarchitectural alterations in hippocampal CA1 pyramidal neurons of adult rats. Brain Res. 2002, 952, 275–281. [Google Scholar] [CrossRef]
- Dief, A.E.; Kamha, E.S.; Baraka, A.M.; Elshorbagy, A.K. Monosodium glutamate neurotoxicity increases beta amyloid in the rat hippocampus: A potential role for cyclic AMP protein kinase. Neurotoxicology 2014, 42, 76–82. [Google Scholar] [CrossRef]
- Frieder, B.; Grimm, V.E. Prenatal monosodium glutamate (MSG) treatment given through the mother’s diet causes behavioral deficits in rat offspring. Int. J. Neurosci. 1984, 23, 117–126. [Google Scholar] [CrossRef]
- Frieder, B.; Grimm, V.E. Prenatal Monosodium Glutamate Causes Long-Lasting Cholinergic and Adrenergic Changes in Various Brain Regions. J. Neurochem. 1987, 48, 1359–1365. [Google Scholar] [CrossRef]
- Hashem, H.E.; El-Din Safwat, M.D.; Algaidi, S. The effect of monosodium glutamate on the cerebellar cortex of male albino rats and the protective role of vitamin C (histological and immunohistochemical study). J. Mol. Histol. 2012, 43, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Onaolapo, A.Y.; Onaolapo, O.J. Glutamate and depression: Reflecting a deepening knowledge of the gut and brain effects of a ubiquitous molecule. World J. Psychiatry 2021, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Averill, L.A.; Jiang, L.; Purohit, P.; Coppoli, A.; Averill, C.L.; Roscoe, J.; Kelmendi, B.; De Feyter, H.M.; de Graaf, R.A.; Gueorguieva, R. Prefrontal glutamate neurotransmission in PTSD: A novel approach to estimate synaptic strength in vivo in humans. Chronic Stress 2022, 6, 24705470221092734. [Google Scholar] [CrossRef] [PubMed]
- Averill, L.A.; Purohit, P.; Averill, C.L.; Boesl, M.A.; Krystal, J.H.; Abdallah, C.G. Glutamate dysregulation and glutamatergic therapeutics for PTSD: Evidence from human studies. Neurosci. Lett. 2017, 649, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Chambers, R.A.; Bremner, J.D.; Moghaddam, B.; Southwick, S.M.; Charney, D.S.; Krystal, J.H. Glutamate and post-traumatic stress disorder: Toward a psychobiology of dissociation. Semin. Clin. Neuropsychiatry 1999, 4, 274–281. [Google Scholar] [PubMed]
- Fang, Q.; Li, Z.; Huang, G.-D.; Zhang, H.-H.; Chen, Y.-Y.; Zhang, L.-B.; Ding, Z.-B.; Shi, J.; Lu, L.; Yang, J.-L. Traumatic stress produces distinct activations of GABAergic and glutamatergic neurons in amygdala. Front. Neurosci. 2018, 12, 387. [Google Scholar] [CrossRef] [PubMed]
- Holmes, S.E.; Girgenti, M.J.; Davis, M.T.; Pietrzak, R.H.; DellaGioia, N.; Nabulsi, N.; Matuskey, D.; Southwick, S.; Duman, R.S.; Carson, R.E. Altered metabotropic glutamate receptor 5 markers in PTSD: In vivo and postmortem evidence. Proc. Natl. Acad. Sci. USA 2017, 114, 8390–8395. [Google Scholar] [CrossRef] [PubMed]
- Rao, T.S.S.; Asha, M.R.; Ramesh, B.N.; Rao, K.S.J. Understanding nutrition, depression and mental illnesses. Indian J. Psychiatry 2008, 50, 77. [Google Scholar]
- Sharma, S.; Fulton, S. Diet-induced obesity promotes depressive-like behaviour that is associated with neural adaptations in brain reward circuitry. Int. J. Obes. 2013, 37, 382–389. [Google Scholar] [CrossRef]
- Onaolapo, A.Y.; Onaolapo, O.J. Dietary glutamate and the brain: In the footprints of a Jekyll and Hyde molecule. Neurotoxicology 2020, 80, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Onaolapo, O.J.; Onaolapo, A.Y.; Akanmu, M.A.; Olayiwola, G. Changes in spontaneous working-memory, memory-recall and approach-avoidance following “low dose” monosodium glutamate in mice. AIMS Neurosci. 2016, 3, 317–337. [Google Scholar] [CrossRef]
- Baek, J.H.; Vignesh, A.; Son, H.; Lee, D.H.; Roh, G.S.; Kang, S.S.; Cho, G.J.; Choi, W.S.; Kim, H.J. Glutamine supplementation ameliorates chronic stress-induced reductions in glutamate and glutamine transporters in the mouse prefrontal cortex. Exp. Neurobiol. 2019, 28, 270. [Google Scholar] [CrossRef] [PubMed]
- Kraal, A.Z.; Arvanitis, N.R.; Jaeger, A.P.; Ellingrod, V.L. Could dietary glutamate play a role in psychiatric distress? Neuropsychobiology 2020, 79, 13–19. [Google Scholar] [CrossRef]
- Quines, C.B.; Rosa, S.G.; Da Rocha, J.T.; Gai, B.M.; Bortolatto, C.F.; Duarte, M.M.M.F.; Nogueira, C.W. Monosodium glutamate, a food additive, induces depressive-like and anxiogenic-like behaviors in young rats. Life Sci. 2014, 107, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Hickling, E.J.; Blanchard, E.B. Post-traumatic stress disorder and motor vehicle accidents. J. Anxiety Disord. 1992, 6, 285–291. [Google Scholar] [CrossRef]
- Haarbauer-Krupa, J.; Taylor, C.A.; Yue, J.K.; Winkler, E.A.; Pirracchio, R.; Cooper, S.R.; Burke, J.F.; Stein, M.B.; Manley, G.T.; Investigators, T.-T. Screening for post-traumatic stress disorder in a civilian emergency department population with traumatic brain injury. J. Neurotrauma 2017, 34, 50–58. [Google Scholar] [CrossRef]
- Schnyder, U.; Wittmann, L.; Friedrich-Perez, J.; Hepp, U.; Moergeli, H. Posttraumatic stress disorder following accidental injury: Rule or exception in Switzerland? Psychother. Psychosom. 2008, 77, 111–118. [Google Scholar] [CrossRef]
- Mayou, R.; Bryant, B.; Duthie, R. Psychiatric consequences of road traffic accidents. Br. Med. J. 1993, 307, 647–651. [Google Scholar] [CrossRef]
- Mikolić, A.; Polinder, S.; Helmrich, I.R.A.R.; Haagsma, J.A.; Cnossen, M.C. Treatment for posttraumatic stress disorder in patients with a history of traumatic brain injury: A systematic review. Clin. Psychol. Rev. 2019, 73, 101776. [Google Scholar] [CrossRef]
- Yue, J.K.; Burke, J.F.; Upadhyayula, P.S.; Winkler, E.A.; Deng, H.; Robinson, C.K.; Pirracchio, R.; Suen, C.G.; Sharma, S.; Ferguson, A.R. Selective serotonin reuptake inhibitors for treating neurocognitive and neuropsychiatric disorders following traumatic brain injury: An evaluation of current evidence. Brain Sci. 2017, 7, 93. [Google Scholar] [CrossRef]
- Jak, A.J.; Jurick, S.; Crocker, L.D.; Sanderson-Cimino, M.; Aupperle, R.; Rodgers, C.S.; Thomas, K.R.; Boyd, B.; Norman, S.B.; Lang, A.J. SMART-CPT for veterans with comorbid post-traumatic stress disorder and history of traumatic brain injury: A randomised controlled trial. J. Neurol. Neurosurg. Psychiatry 2019, 90, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Marks, M.R.; Dux, M.C.; Rao, V.; Albrecht, J.S. Treatment patterns of anxiety and posttraumatic stress disorder following traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 2022, 34, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, J.S.; Peters, M.E.; Smith, G.S.; Rao, V. Anxiety and post-traumatic stress disorder among medicare beneficiaries following traumatic brain injury. J. Head Trauma Rehabil. 2017, 32, 178. [Google Scholar] [CrossRef] [PubMed]
- van der Vlegel, M.; Polinder, S.; Mikolic, A.; Kaplan, R.; von Steinbuechel, N.; Plass, A.M.; Zeldovich, M.; Van Praag, D.; Bockhop, F.; Cunitz, K. The association of post-concussion and post-traumatic stress disorder symptoms with health-related quality of life, health care use and return-to-work after mild traumatic brain injury. J. Clin. Med. 2021, 10, 2473. [Google Scholar] [CrossRef] [PubMed]
- Zatzick, D.F.; Rivara, F.P.; Jurkovich, G.J.; Hoge, C.W.; Wang, J.; Fan, M.-Y.; Russo, J.; Trusz, S.G.; Nathens, A.; Mackenzie, E.J. Multisite investigation of traumatic brain injuries, posttraumatic stress disorder, and self-reported health and cognitive impairments. Arch. Gen. Psychiatry 2010, 67, 1291–1300. [Google Scholar] [CrossRef]
- Bockhop, F.; Zeldovich, M.; Cunitz, K.; Van Praag, D.; van der Vlegel, M.; Beissbarth, T.; Hagmayer, Y.; von Steinbuechel, N. Measurement invariance of six language versions of the post-traumatic stress disorder checklist for DSM-5 in civilians after traumatic brain injury. Sci. Rep. 2022, 12, 16571. [Google Scholar] [CrossRef] [PubMed]
- Kulbe, J.R.; Jain, S.; Nelson, L.D.; Korley, F.K.; Mukherjee, P.; Sun, X.; Okonkwo, D.O.; Giacino, J.T.; Vassar, M.J.; Robertson, C.S. Association of day-of-injury plasma glial fibrillary acidic protein concentration and six-month posttraumatic stress disorder in patients with mild traumatic brain injury. Neuropsychopharmacology 2022, 47, 2300–2308. [Google Scholar] [CrossRef]
- Van Praag, D.L.G.; Wouters, K.; Van Den Eede, F.; Wilson, L.; Maas, A.I.R.; Åkerlund, C.; Amrein, K.; Andelic, N.; Andreassen, L.; Anke, A. Neurocognitive correlates of probable posttraumatic stress disorder following traumatic brain injury. Brain Spine 2022, 2, 100854. [Google Scholar] [CrossRef]
- Haagsma, J.A.; Scholten, A.C.; Andriessen, T.M.J.C.; Vos, P.E.; Van Beeck, E.F.; Polinder, S. Impact of depression and post-traumatic stress disorder on functional outcome and health-related quality of life of patients with mild traumatic brain injury. J. Neurotrauma 2015, 32, 853–862. [Google Scholar] [CrossRef]
- Stein, M.B.; Jain, S.; Parodi, L.; Choi, K.W.; Maihofer, A.X.; Nelson, L.D.; Mukherjee, P.; Sun, X.; He, F.; Okonkwo, D.O. Polygenic risk for mental disorders as predictors of posttraumatic stress disorder after mild traumatic brain injury. Transl. Psychiatry 2023, 13, 24. [Google Scholar] [CrossRef]
- Lagarde, E.; Salmi, L.-R.; Holm, L.W.; Contrand, B.; Masson, F.; Ribéreau-Gayon, R.; Laborey, M.; Cassidy, J.D. Association of symptoms following mild traumatic brain injury with posttraumatic stress disorder vs postconcussion syndrome. JAMA psychiatry 2014, 71, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Kosaraju, S.; Galatzer-Levy, I.; Schultebraucks, K.; Winters, S.; Hinrichs, R.; Reddi, P.J.; Maples-Keller, J.L.; Hudak, L.; Michopoulos, V.; Jovanovic, T. Associations among civilian mild traumatic brain injury with loss of consciousness, posttraumatic stress disorder symptom trajectories, and structural brain volumetric data. J. Trauma. Stress 2022, 35, 1521–1534. [Google Scholar] [CrossRef] [PubMed]
- Bryant, R.A.; Creamer, M.; O’Donnell, M.; Silove, D.; Clark, C.R.; McFarlane, A.C. Post-traumatic amnesia and the nature of post-traumatic stress disorder after mild traumatic brain injury. J. Int. Neuropsychol. Soc. 2009, 15, 862–867. [Google Scholar] [CrossRef]
- Stein, M.B.; Yuh, E.; Jain, S.; Okonkwo, D.O.; Mac Donald, C.L.; Levin, H.; Giacino, J.T.; Dikmen, S.; Vassar, M.J.; Diaz-Arrastia, R. Smaller regional brain volumes predict posttraumatic stress disorder at 3 months after mild traumatic brain injury. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Creamer, M.; O’Donnell, M.L.; Pattison, P. Amnesia, traumatic brain injury, and posttraumatic stress disorder: A methodological inquiry. Behav. Res. Ther. 2005, 43, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Scheenen, M.E.; Spikman, J.M.; de Koning, M.E.; van der Horn, H.J.; Roks, G.; Hageman, G.; van der Naalt, J. Patients “at risk” of suffering from persistent complaints after mild traumatic brain injury: The role of coping, mood disorders, and post-traumatic stress. J. Neurotrauma 2017, 34, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.M.; Dikmen, S.; Temkin, N.; Bell, K.R. Development of posttraumatic stress disorder after mild traumatic brain injury. Arch. Phys. Med. Rehabil. 2012, 93, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Warren, A.M.; Boals, A.; Elliott, T.R.; Reynolds, M.; Weddle, R.J.; Holtz, P.; Trost, Z.; Foreman, M.L. Mild traumatic brain injury increases risk for the development of posttraumatic stress disorder. J. Trauma Acute Care Surg. 2015, 79, 1062–1066. [Google Scholar] [CrossRef]
- Sawyer, K.; Bell, K.R.; Ehde, D.M.; Temkin, N.; Dikmen, S.; Williams, R.M.; Dillworth, T.; Hoffman, J.M. Longitudinal study of headache trajectories in the year after mild traumatic brain injury: Relation to posttraumatic stress disorder symptoms. Arch. Phys. Med. Rehabil. 2015, 96, 2000–2006. [Google Scholar] [CrossRef]
- Alway, Y.; McKay, A.; Gould, K.R.; Johnston, L.; Ponsford, J. Factors associated with posttraumatic stress disorder following moderate to severe traumatic brain injury: A prospective study. Depress. Anxiety 2016, 33, 19–22. [Google Scholar] [CrossRef]
- Qureshi, K.L.; Upthegrove, R.; Toman, E.; Sawlani, V.; Davies, D.J.; Belli, A. Post-traumatic stress disorder in UK civilians with traumatic brain injury: An observational study of TBI clinic attendees to estimate PTSD prevalence and its relationship with radiological markers of brain injury severity. BMJ Open 2019, 9, e02167. [Google Scholar] [CrossRef] [PubMed]
- Bombardier, C.H.; Fann, J.R.; Temkin, N.; Esselman, P.C.; Pelzer, E.; Keough, M.; Dikmen, S. Posttraumatic stress disorder symptoms during the first six months after traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 2006, 18, 501–508. [Google Scholar] [CrossRef]
- Gil, S.; Caspi, Y.; Ben-Ari, I.Z.; Koren, D.; Klein, E. Does memory of a traumatic event increase the risk for posttraumatic stress disorder in patients with traumatic brain injury? A prospective study. Am. J. Psychiatry 2005, 162, 963–969. [Google Scholar] [CrossRef]
- Caspi, Y.; Gil, S.; Ben-Ari, I.Z.; Koren, D.; Aaron-Peretz, J.; Klein, E. Memory of the traumatic event is associated with increased risk for PTSD: A retrospective study of patients with traumatic brain injury. J. Loss Trauma 2005, 10, 319–335. [Google Scholar] [CrossRef]
- Hickling, E.J.; Gillen, R.; Blanchard, E.B.; Buckley, T.; Taylor, A. Traumatic brain injury and posttraumatic stress disorder: A preliminary investigation of neuropsychological test results in PTSD secondary to motor vehicle accidents. Brain Inj. 1998, 12, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Winkler, E.A.; Yue, J.K.; Ferguson, A.R.; Temkin, N.R.; Stein, M.B.; Barber, J.; Yuh, E.L.; Sharma, S.; Satris, G.G.; McAllister, T.W. COMT Val158Met polymorphism is associated with post-traumatic stress disorder and functional outcome following mild traumatic brain injury. J. Clin. Neurosci. 2017, 35, 109–116. [Google Scholar] [CrossRef]
- Alway, Y.; Gould, K.R.; McKay, A.; Johnston, L.; Ponsford, J. The evolution of post-traumatic stress disorder following moderate-to-severe traumatic brain injury. J. Neurotrauma 2016, 33, 825–831. [Google Scholar] [CrossRef]
- Levin, H.S.; Brown, S.A.; Song, J.X.; McCauley, S.R.; Boake, C.; Contant, C.F.; Goodman, H.; Kotrla, K.J. Depression and posttraumatic stress disorder at three months after mild to moderate traumatic brain injury. J. Clin. Exp. Neuropsychol. 2001, 23, 754–769. [Google Scholar] [CrossRef]
- Bryant, R.A.; Marosszeky, J.E.; Crooks, J.; Gurka, J.A. Elevated resting heart rate as a predictor of posttraumatic stress disorder after severe traumatic brain injury. Psychosom. Med. 2004, 66, 760–761. [Google Scholar] [CrossRef]
- Williams, W.H.; Evans, J.J.; Wilson, B.A.; Needham, P. Brief report: Prevalence of post-traumatic stress disorder symptoms after severe traumatic brain injury in a representative community sample. Brain Inj. 2002, 16, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Sumpter, R.E.; McMillan, T.M. Misdiagnosis of post-traumatic stress disorder following severe traumatic brain injury. Br. J. Psychiatry 2005, 186, 423–426. [Google Scholar] [CrossRef]
- Jones, C.; Harvey, A.G.; Brewin, C.R. Traumatic brain injury, dissociation, and posttraumatic stress disorder in road traffic accident survivors. J. Trauma. Stress Off. Publ. Int. Soc. Trauma. Stress Stud. 2005, 18, 181–191. [Google Scholar] [CrossRef]
- Bryant, R.A.; Harvey, A.G. Postconcussive symptoms and posttraumatic stress disorder after mild traumatic brain injury. J. Nerv. Ment. Dis. 1999, 187, 302–305. [Google Scholar] [CrossRef]
- Roden-Foreman, K.; Solis, J.; Jones, A.; Bennett, M.; Roden-Foreman, J.W.; Rainey, E.E.; Foreman, M.L.; Warren, A.M. Prospective evaluation of posttraumatic stress disorder and depression in orthopaedic injury patients with and without concomitant traumatic brain injury. J. Orthop. Trauma 2017, 31, e275–e280. [Google Scholar] [CrossRef] [PubMed]
- Ohry, A.; Rattok, J.; Solomon, Z. Post-traumatic stress disorder in brain injury patients. Brain Inj. 1996, 10, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, S.K.; Sridhar, S.; Char, S.; Pandya, K.; Mehta, K. Prevalence of comorbidities post mild traumatic brain injuries: A traumatic brain injury model systems study. Front. Hum. Neurosci. 2023, 17, 1158483. [Google Scholar] [CrossRef]
- Zaman, S.; Arouj, K.; Irfan, S. The relationship between post-traumatic stress disorder and acquired brain injury among civilian patients. Clin. Neurol. Neurosurg. 2020, 196, 105981. [Google Scholar] [CrossRef]
- Choi, M.S.; Seo, S.J.; Oh, C.H.; Kim, S.-H.; Cho, J.M. Incidence of post-traumatic stress disorder after a mild traumatic brain injury: Preliminary investigation using the Brief Neuropsychological Screening Test. J. Korean Neurosurg. Soc. 2014, 55, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sun, G.; Liu, K.; Li, M.; Li, B.; Qian, S.-W.; Yu, L.-L. White matter changes in posttraumatic stress disorder following mild traumatic brain injury: A prospective longitudinal diffusion tensor imaging study. Chin. Med. J. 2016, 129, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).