Effects of Saffron Extract (Affron®) with 100 mg/kg and 200 mg/kg on Hypothalamic–Pituitary–Adrenal Axis and Stress Resilience in Chronic Mild Stress-Induced Depression in Wistar Rats
Abstract
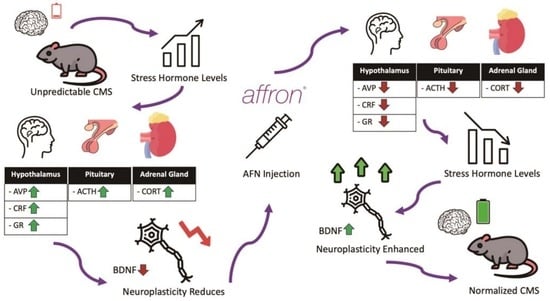
:1. Introduction
2. Materials and Methods
2.1. Test Substance
2.2. Animals and Experimental Groups
- Group 1 (control): Rats in this group received no specific treatment and were solely administered the vehicle.
- Group 2 (stress-induced): Rats in this group were subjected to unpredictable CMS and subsequently administered the vehicle.
- Group 3 (AFN 100 mg/kg): Rats in this group were exposed to unpredictable CMS and administered AFN at a dosage of 100 mg/kg (CMS + AFN 100 mg/kg/day).
- Group 4 (AFN 200 mg/kg): This group consisted of rats that underwent unpredictable CMS and were given AFN at a dose of 200 mg/kg (CMS + AFN 200 mg/kg/day).
2.3. Experimental Models
2.3.1. Unpredictable Chronic Mild Stress (CMS)
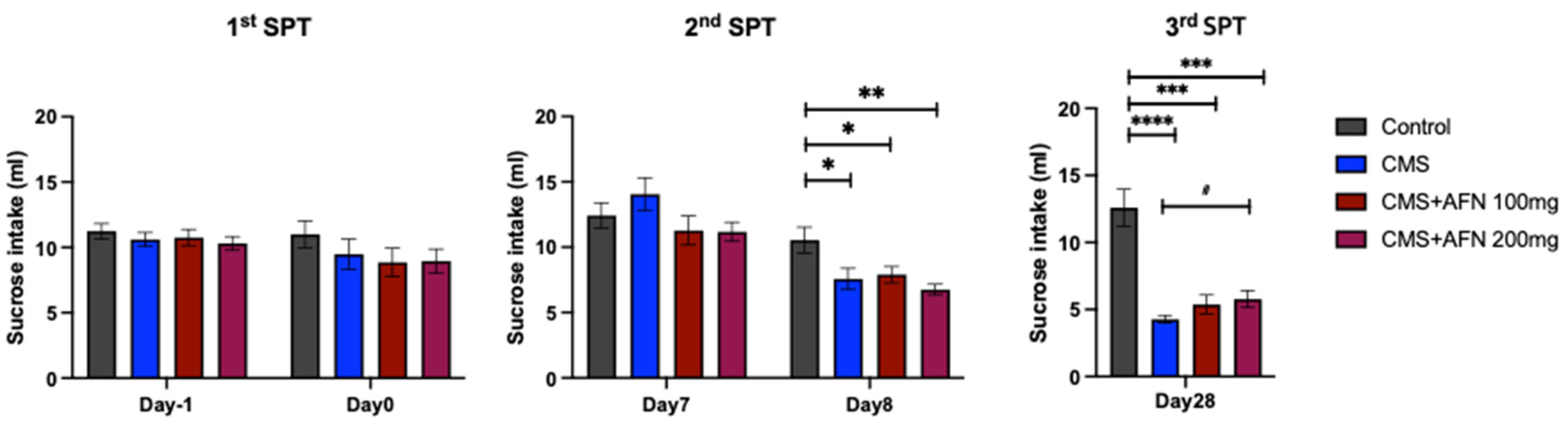
2.3.2. Sucrose Preference Test (SPT)
2.4. Tissue Processing and Histology
2.4.1. Nissl Staining
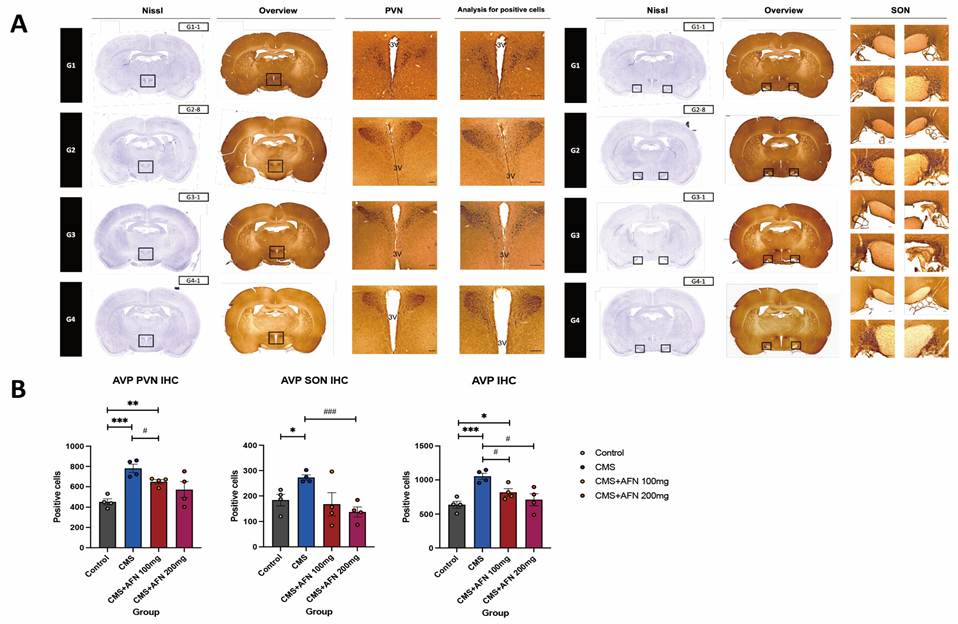
2.4.2. Immunohistochemistry (IHC) for AVP, c-fos, and GR
2.5. Quantification of BDNF and CRF Expression in Hypothalamic Tissues using Western Blot
2.6. Measurement of Plasma ACTH, CORT, and 5-HT Levels using ELISA Assay
2.7. Ethical Consideration
2.8. Image Analysis
3. Results
3.1. Changes in Body Weight, Food, and Water Intake
3.2. Sucrose Preference Test (SPT)
3.3. Immunohistochemistry for AVP, and GR in the Hypothalamic Area
3.3.1. Expression of AVP in the Hypothalamic PVN and SON Regions
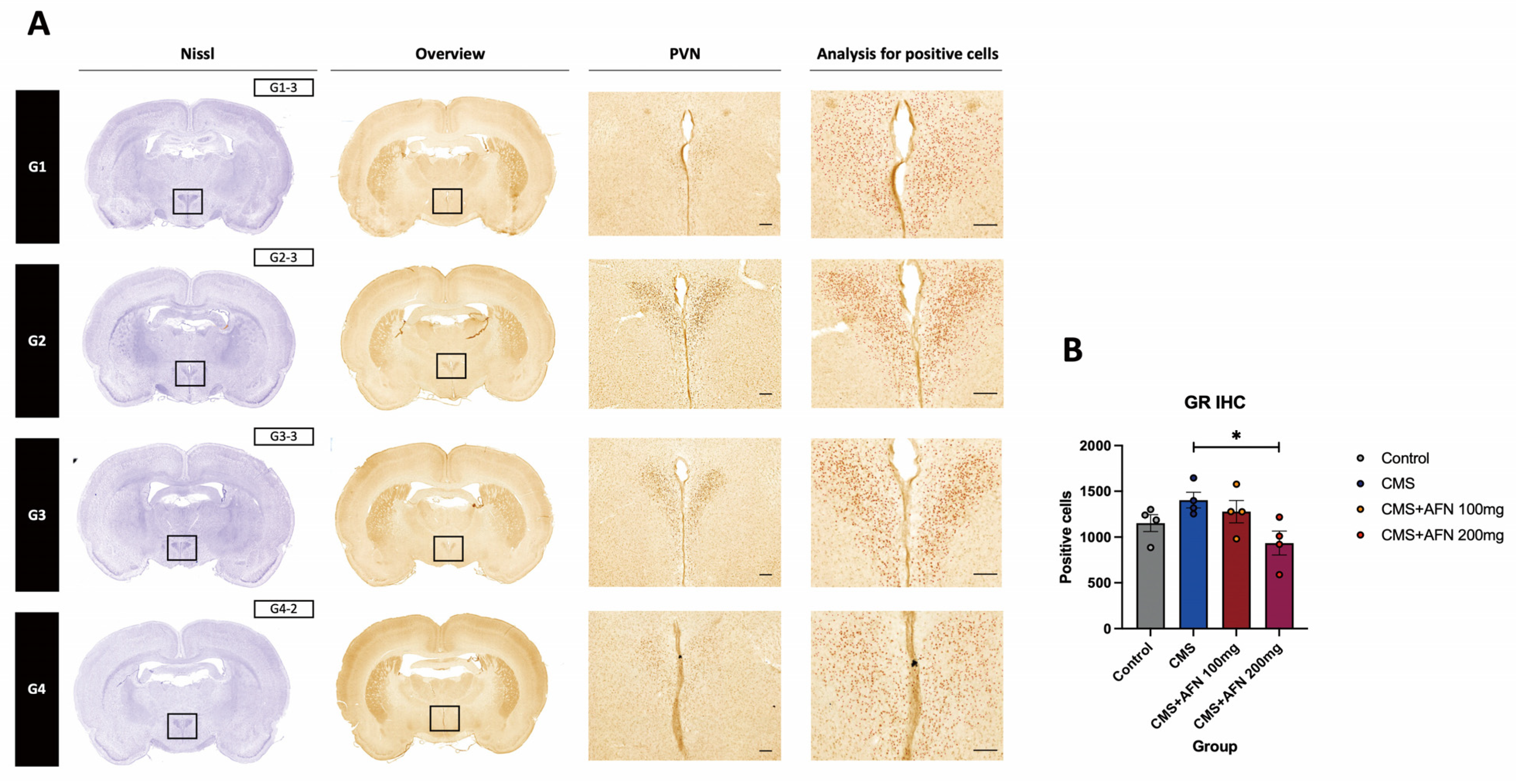
3.3.2. Expression of GR in the Hypothalamic PVN Region
3.3.3. c-fos Activition Patterns Post-AFN Administration in Stressed Animals
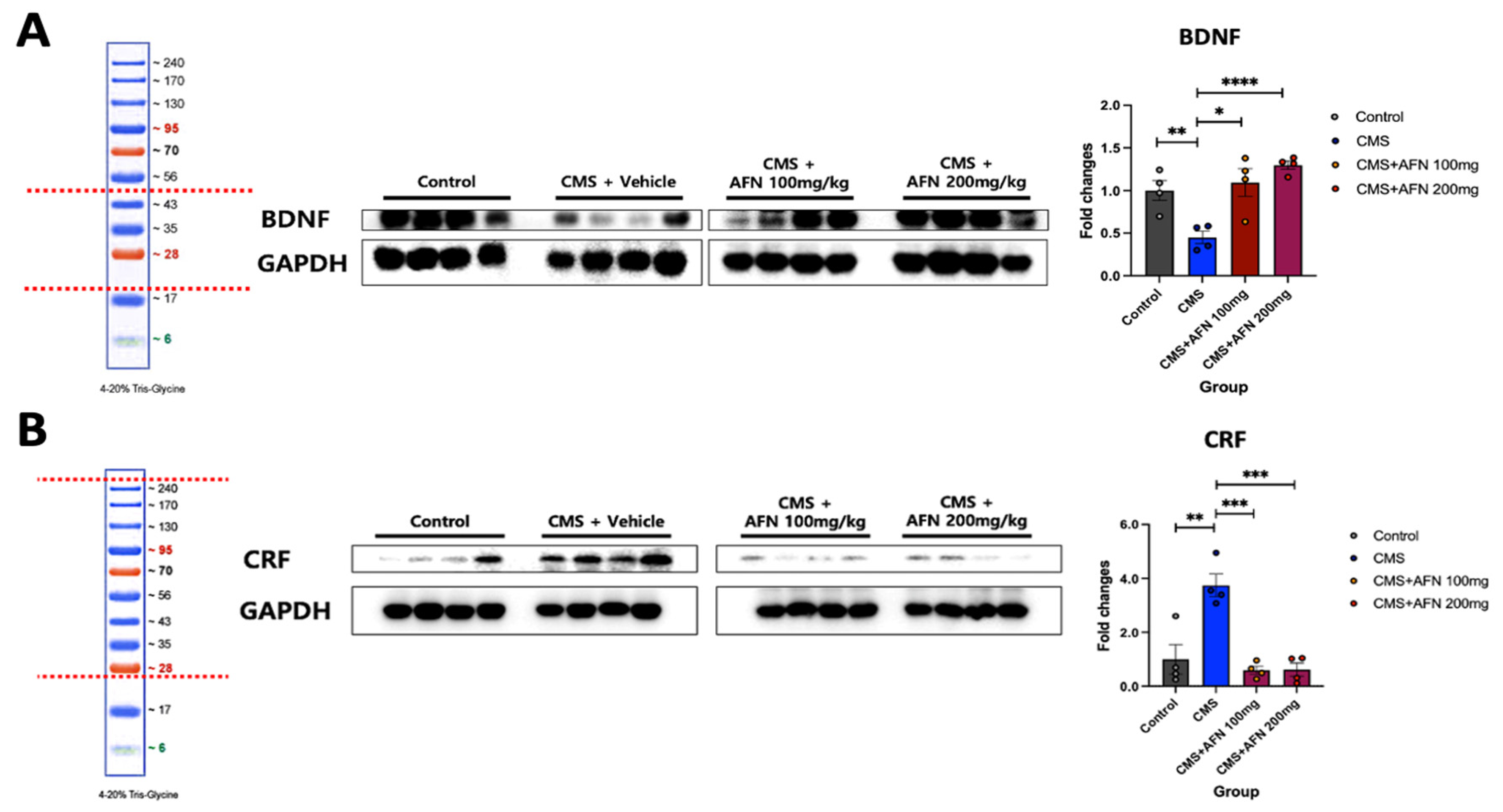
3.4. Quantitative Analysis of BDNF and CRF Expression in the Hypothalamus via Western Blot
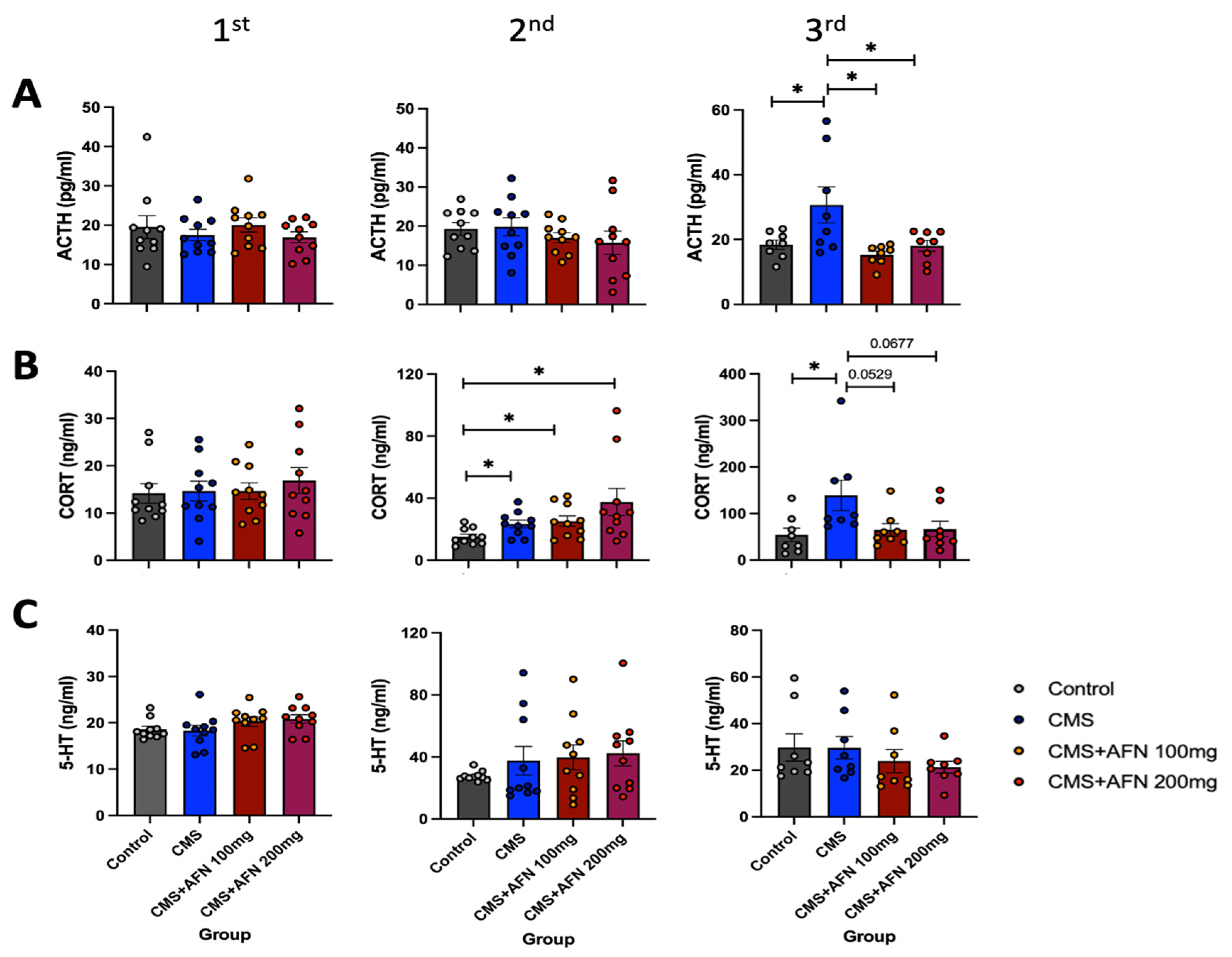
3.5. Plasma Levels of ACTH, CORT, and 5-HT Measured by ELISA Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brooks, S.K.; Webster, R.K.; Smith, L.E.; Woodland, L.; Wessely, S.; Greenberg, N.; Rubin, G.J. The psychological impact of quarantine and how to reduce it: Rapid review of the evidence. Lancet 2020, 395, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Bower, M.; Smout, S.; Donohoe-Bales, A.; O’Dean, S.; Teesson, L.; Boyle, J.; Lim, D.; Nguyen, A.; Calear, A.L.; Batterham, P.J. A hidden pandemic? An umbrella review of global evidence on mental health in the time of COVID-19. Front. Psychiatry 2023, 14, 1107560. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur. J. Pharmacol. 2008, 583, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Charney, D.S. Psychobiological mechanisms of resilience and vulnerability: Implications for successful adaptation to extreme stress. Am. J. Psychiatry 2004, 161, 195–216. [Google Scholar] [CrossRef] [PubMed]
- Mucci, N.; Giorgi, G.; Roncaioli, M.; Fiz Perez, J.; Arcangeli, G. The correlation between stress and economic crisis: A systematic review. Neuropsychiatr. Dis. Treat. 2016, 12, 983–993. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Akil, H. Revisiting the stress concept: Implications for affective disorders. J. Neurosci. 2020, 40, 12–21. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Morrison, J.H. The brain on stress: Vulnerability and plasticity of the prefrontal cortex over the life course. Neuron 2013, 79, 16–29. [Google Scholar] [CrossRef]
- McEwen, B.S. Protective and damaging effects of stress mediators. New Engl. J. Med. 1998, 338, 171–179. [Google Scholar] [CrossRef]
- McEwen, B.S. Allostasis and allostatic load: Implications for neuropsychopharmacology. Neuropsychopharmacology 2000, 22, 108–124. [Google Scholar] [CrossRef]
- Tsigos, C.; Chrousos, G.P. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 2002, 53, 865–871. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [PubMed]
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109, 69–75. [Google Scholar] [PubMed]
- Hosseinzadeh, H.; Noraei, N.B. Anxiolytic and hypnotic effect of Crocus sativus aqueous extract and its constituents, crocin and safranal, in mice. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. Phytother. Res. 2009, 23, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Moshiri, M.; Vahabzadeh, M.; Hosseinzadeh, H. Clinical applications of saffron (Crocus sativus) and its constituents: A review. Drug Res. 2015, 65, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, E.; Kadoglou, N.P.; Kostomitsopoulos, N.; Valsami, G. Saffron: A natural product with potential pharmaceutical applications. J. Pharm. Pharmacol. 2015, 67, 1634–1649. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Younesi, H.M. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002, 2, 7. [Google Scholar]
- Tamaddonfard, E.; Farshid, A.-A.; Eghdami, K.; Samadi, F.; Erfanparast, A. Comparison of the effects of crocin, safranal and diclofenac on local inflammation and inflammatory pain responses induced by carrageenan in rats. Pharmacol. Rep. 2013, 65, 1272–1280. [Google Scholar] [CrossRef]
- Nair, S.; Pannikar, B.; Panikkar, K. Antitumour activity of saffron (Crocus sativus). Cancer Lett. 1991, 57, 109–114. [Google Scholar] [CrossRef]
- Mohajeri, D.; Tabrizi, B.A.; Mousavi, G.; Mesgari, M. Anti-diabetic activity of Crocus sativus L. (Saffron) stigma ethanolic extract in alloxan-induced diabetic rats. Res. J. Biol. Sci. 2008, 3, 1102–1108. [Google Scholar]
- Kianbakht, S.; Hashem Dabaghian, F. Anti-obesity and anorectic effects of saffron and its constituent crocin in obese Wistar rat. J. Med. Plants 2015, 14, 25–33. [Google Scholar]
- Halataei, B.-a.-S.; Khosravi, M.; Arbabian, S.; Sahraei, H.; Golmanesh, L.; Zardooz, H.; Jalili, C.; Ghoshooni, H. Saffron (Crocus sativus) aqueous extract and its constituent crocin reduces stress-induced anorexia in mice. Phytother. Res. 2011, 25, 1833–1838. [Google Scholar] [CrossRef] [PubMed]
- Vahdati Hassani, F.; Naseri, V.; Razavi, B.M.; Mehri, S.; Abnous, K.; Hosseinzadeh, H. Antidepressant effects of crocin and its effects on transcript and protein levels of CREB, BDNF, and VGF in rat hippocampus. DARU J. Pharm. Sci. 2014, 22, 16. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Smith, S.J.; Hood, S.D.; Drummond, P.D. Efficacy of a standardised saffron extract (affron®) as an add-on to antidepressant medication for the treatment of persistent depressive symptoms in adults: A randomised, double-blind, placebo-controlled study. J. Psychopharmacol. 2019, 33, 1415–1427. [Google Scholar] [CrossRef] [PubMed]
- Kell, G.; Rao, A.; Beccaria, G.; Clayton, P.; Inarejos-García, A.M.; Prodanov, M. Affron® a novel saffron extract (Crocus sativus L.) improves mood in healthy adults over 4 weeks in a double-blind, parallel, randomized, placebo-controlled clinical trial. Complement. Ther. Med. 2017, 33, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Noorbala, A.; Akhondzadeh, S.; Tahmacebi-Pour, N.; Jamshidi, A. Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: A double-blind, randomized pilot trial. J. Ethnopharmacol. 2005, 97, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Drummond, P.D.; Inarejos-García, A.M.; Prodanov, M. Affron®, a standardised extract from saffron (Crocus sativus L.) for the treatment of youth anxiety and depressive symptoms: A randomised, double-blind, placebo-controlled study. J. Affect. Disord. 2018, 232, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Orio, L.; Alen, F.; Ballesta, A.; Martin, R.; Gomez de Heras, R. Antianhedonic and Antidepressant Effects of Affron®, a Standardized Saffron (Crocus sativus L.) Extract. Molecules 2020, 25, 3207. [Google Scholar] [CrossRef]
- Ishola, I.O.; Olubodun-Obadun, T.G.; Bakre, O.A.; Ojo, E.S.; Adeyemi, O.O. Kolaviron ameliorates chronic unpredictable mild stress-induced anxiety and depression: Involvement of the HPA axis, antioxidant defense system, cholinergic, and BDNF signaling. Drug Metab. Pers. Ther. 2022, 37, 277–287. [Google Scholar] [CrossRef]
- Yi, S.S.; Hwang, I.K.; Chun, M.S.; Kim, Y.N.; Kim, I.Y.; Lee, I.S.; Seong, J.K.; Yoon, Y.S. Glucocorticoid receptor changes associate with age in the paraventricular nucleus of type II diabetic rat model. Neurochem. Res. 2009, 34, 851–858. [Google Scholar] [CrossRef]
- Yi, S.S.; Hwang, I.K.; Shin, J.H.; Choi, J.H.; Lee, C.H.; Kim, I.Y.; Kim, Y.N.; Won, M.-H.; Park, I.-S.; Seong, J.K. Regulatory mechanism of hypothalamo-pituitary–adrenal (HPA) axis and neuronal changes after adrenalectomy in type 2 diabetes. J. Chem. Neuroanat. 2010, 40, 130–139. [Google Scholar] [CrossRef]
- Yi, S.S.; Kim, H.-J.; Do, S.-G.; Lee, Y.-B.; Ahn, H.J.; Hwang, I.K.; Yoon, Y.S. Arginine vasopressin (AVP) expressional changes in the hypothalamic paraventricular and supraoptic nuclei of stroke-prone spontaneously hypertensive rats. Anat. Cell Biol. 2012, 45, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Willner, P. The validity of animal models of depression. Psychopharmacology 1984, 83, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, B.H. Depression as a disease of modernity: Explanations for increasing prevalence. J. Affect. Disord. 2012, 140, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Hosseinian-Far, A.; Jalali, R.; Vaisi-Raygani, A.; Rasoulpoor, S.; Mohammadi, M.; Rasoulpoor, S.; Khaledi-Paveh, B. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: A systematic review and meta-analysis. Glob. Health 2020, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Schneiderman, N.; Ironson, G.; Siegel, S.D. Stress and health: Psychological, behavioral, and biological determinants. Annu. Rev. Clin. Psychol. 2005, 1, 607–628. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Drummond, P.D. Saffron (Crocus sativus) for depression: A systematic review of clinical studies and examination of underlying antidepressant mechanisms of action. Hum. Psychopharmacol. Clin. Exp. 2014, 29, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Ferri, F.; Deschenes, S.S.; Power, N.; Schmitz, N. Associations between cognitive function, metabolic factors and depression: A prospective study in Quebec, Canada. J. Affect. Disord. 2021, 283, 77–83. [Google Scholar] [CrossRef]
- Qiu, W.; Cai, X.; Zheng, C.; Qiu, S.; Ke, H.; Huang, Y. Update on the relationship between depression and neuroendocrine metabolism. Front. Neurosci. 2021, 15, 728810. [Google Scholar] [CrossRef]
- Novak, M.A.; Hamel, A.F.; Kelly, B.J.; Dettmer, A.M.; Meyer, J.S. Stress, the HPA axis, and nonhuman primate well-being: A review. Appl. Anim. Behav. Sci. 2013, 143, 135–149. [Google Scholar] [CrossRef]
- Pruessner, J.C.; Dedovic, K.; Pruessner, M.; Lord, C.; Buss, C.; Collins, L.; Dagher, A.; Lupien, S.J. Stress regulation in the central nervous system: Evidence from structural and functional neuroimaging studies in human populations—2008 Curt Richter Award Winner. Psychoneuroendocrinology 2010, 35, 179–191. [Google Scholar] [CrossRef]
- Koss, K.J.; Gunnar, M.R. Annual research review: Early adversity, the hypothalamic–pituitary–adrenocortical axis, and child psychopathology. J. Child Psychol. Psychiatry 2018, 59, 327–346. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Fentress, H.M.; Hoversten, M.T.; Zhang, L.; Hebda-Bauer, E.K.; Watson, S.J.; Seasholtz, A.F.; Akil, H. Early-life forebrain glucocorticoid receptor overexpression increases anxiety behavior and cocaine sensitization. Biol. Psychiatry 2012, 71, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Rozeboom, A.M.; Akil, H.; Seasholtz, A.F. Mineralocorticoid receptor overexpression in forebrain decreases anxiety-like behavior and alters the stress response in mice. Proc. Natl. Acad. Sci. USA 2007, 104, 4688–4693. [Google Scholar] [CrossRef] [PubMed]
- Joëls, M.; de Kloet, E.R. 30 YEARS OF THE MINERALOCORTICOID RECEPTOR: The brain mineralocorticoid receptor: A saga in three episodes. J. Endocrinol. 2017, 234, T49–T66. [Google Scholar] [CrossRef] [PubMed]
- Abuelezz, S.A.; Hendawy, N.; Magdy, Y. The potential benefit of combined versus monotherapy of coenzyme Q10 and fluoxetine on depressive-like behaviors and intermediates coupled to Gsk-3β in rats. Toxicol. Appl. Pharmacol. 2018, 340, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; Flak, J.; Jankord, R. Chronic stress plasticity in the hypothalamic paraventricular nucleus. Prog. Brain Res. 2008, 170, 353–364. [Google Scholar] [PubMed]
- Pacak, K.; Palkovits, M. Stressor specificity of central neuroendocrine responses: Implications for stress-related disorders. Endocr. Rev. 2001, 22, 502–548. [Google Scholar] [CrossRef]
- Wang, H.; Li, F.; Zheng, X.; Meng, L.; Chen, M.; Hui, Y.; Li, Y.; Xie, K.; Zhang, J.; Guo, G. Social defeat drives hyperexcitation of the piriform cortex to induce learning and memory impairment but not mood-related disorders in mice. Transl. Psychiatry 2022, 12, 380. [Google Scholar] [CrossRef]
- Lee, M.-J.; Ryu, J.-S.; Won, S.-K.; Namgung, U.; Jung, J.; Lee, S.-M.; Park, J.-Y. Effects of acupuncture on chronic stress-induced depression-like behavior and its central neural mechanism. Front. Psychol. 2019, 10, 1353. [Google Scholar] [CrossRef]
- Smith, D.M.; Barredo, J.; Mizumori, S.J. Complimentary roles of the hippocampus and retrosplenial cortex in behavioral context discrimination. Hippocampus 2012, 22, 1121–1133. [Google Scholar] [CrossRef]
- Smith, D.M.; Mizumori, S.J. Learning-related development of context-specific neuronal responses to places and events: The hippocampal role in context processing. J. Neurosci. 2006, 26, 3154–3163. [Google Scholar] [CrossRef]
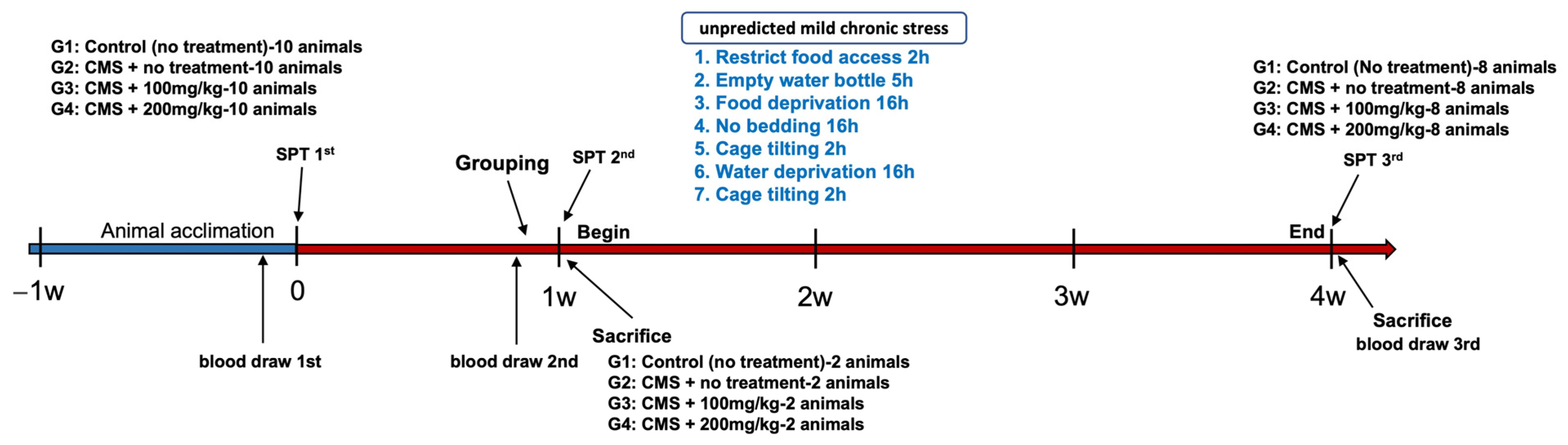


| Weeks | ||||
|---|---|---|---|---|
| 1st Week (No-Drug) | 2nd Week | 3rd Week | 4th Week | |
| Monday | RFA 1 2 h | RFA 2 h | RFA 2 h | RFA 2 h |
| Tuesday | EWB 2 5 h | EWB 5 h | EWB 5 h | EWB 5 h |
| Wednesday | FD 3 16 h | FD 16 h | FD 16 h | FD 16 h |
| Thursday | NB 4 16 h | NB 16 h | NB 16 h | NB 16 h |
| Friday | CT 5 2 h | CT 2 h | CT 2 h | CT 2 h |
| Saturday | WD 6 16 h | WD 16 h | WD 16 h | WD 16 h |
| Sunday | CT 7 2 h | CT 2 h | CT 2 h | CT 2 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.-Y.; Ko, K.; Choi, S.-H.; Jo, M.; Kim, J.; Yoon, S.; Yi, I.J.; Morán-Valero, M.I.; Kwon, M.-Y.; Sohn, J.; et al. Effects of Saffron Extract (Affron®) with 100 mg/kg and 200 mg/kg on Hypothalamic–Pituitary–Adrenal Axis and Stress Resilience in Chronic Mild Stress-Induced Depression in Wistar Rats. Nutrients 2023, 15, 4855. https://doi.org/10.3390/nu15234855
Kim C-Y, Ko K, Choi S-H, Jo M, Kim J, Yoon S, Yi IJ, Morán-Valero MI, Kwon M-Y, Sohn J, et al. Effects of Saffron Extract (Affron®) with 100 mg/kg and 200 mg/kg on Hypothalamic–Pituitary–Adrenal Axis and Stress Resilience in Chronic Mild Stress-Induced Depression in Wistar Rats. Nutrients. 2023; 15(23):4855. https://doi.org/10.3390/nu15234855
Chicago/Turabian StyleKim, Chae-Young, Kayoung Ko, Seo-Hee Choi, Miri Jo, Jinhye Kim, Sunmi Yoon, Isaac Jinwon Yi, María Inés Morán-Valero, Min-Young Kwon, Johann Sohn, and et al. 2023. "Effects of Saffron Extract (Affron®) with 100 mg/kg and 200 mg/kg on Hypothalamic–Pituitary–Adrenal Axis and Stress Resilience in Chronic Mild Stress-Induced Depression in Wistar Rats" Nutrients 15, no. 23: 4855. https://doi.org/10.3390/nu15234855
APA StyleKim, C.-Y., Ko, K., Choi, S.-H., Jo, M., Kim, J., Yoon, S., Yi, I. J., Morán-Valero, M. I., Kwon, M.-Y., Sohn, J., & Yi, S.-S. (2023). Effects of Saffron Extract (Affron®) with 100 mg/kg and 200 mg/kg on Hypothalamic–Pituitary–Adrenal Axis and Stress Resilience in Chronic Mild Stress-Induced Depression in Wistar Rats. Nutrients, 15(23), 4855. https://doi.org/10.3390/nu15234855