Hypophosphatemia after Start of Medical Nutrition Therapy Indicates Early Refeeding Syndrome and Increased Electrolyte Requirements in Critically Ill Patients but Has No Impact on Short-Term Survival
Abstract
1. Introduction
2. Methods
2.1. Study Design and Cohort
2.2. Nutrition-Related Data and Refeeding Syndrome
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Basic Characteristics
3.2. Incidence of RFS
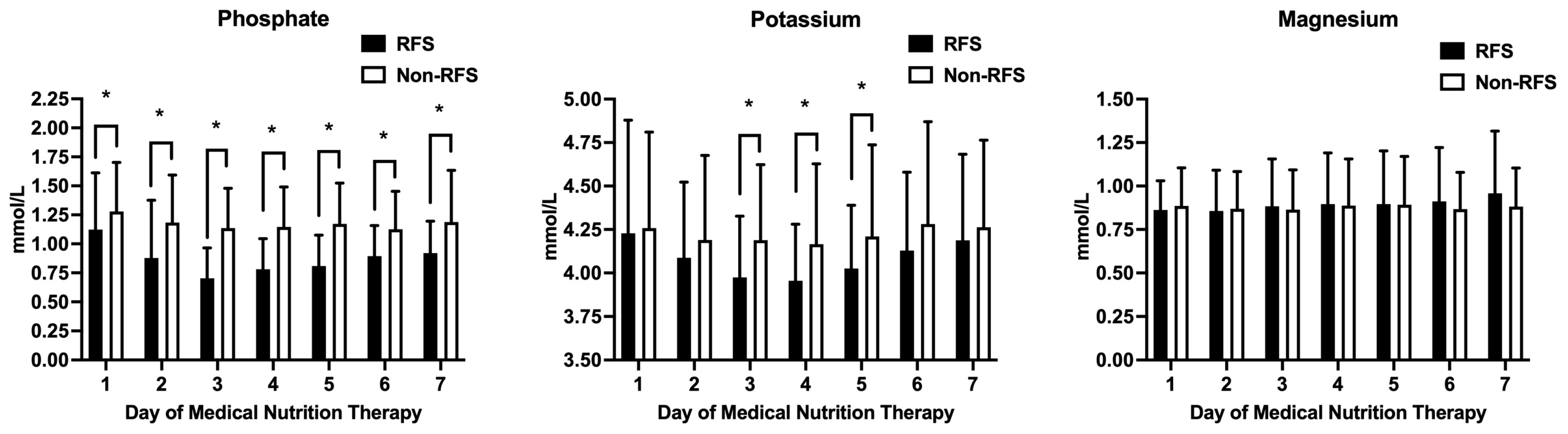
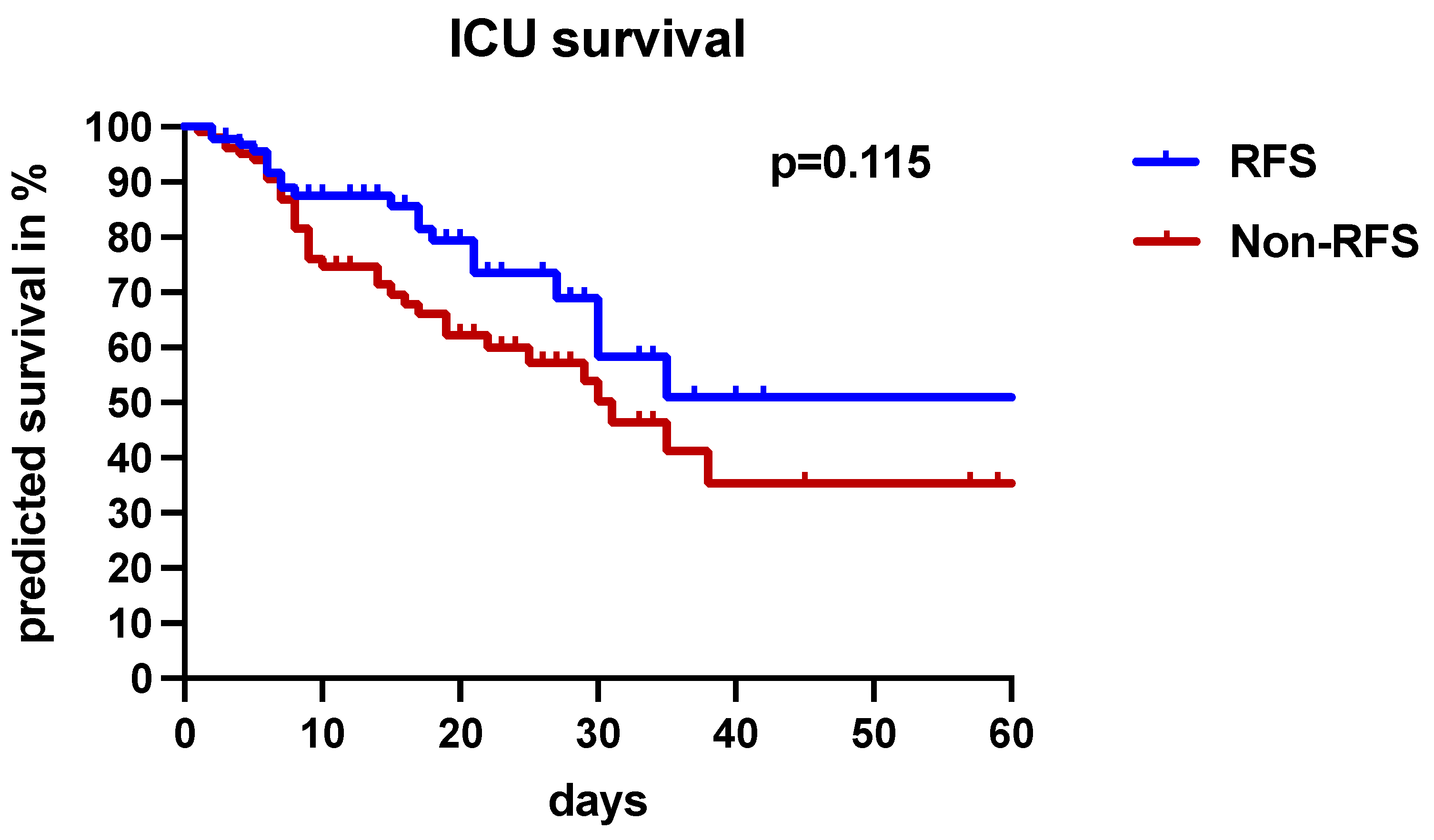
3.3. The Clinical Significance of RFS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASPEN | American Society for Parenteral and Enteral Nutrition |
| BMI | Body mass index |
| CPR | Cardiopulmonary resuscitation |
| DM | Diabetes mellitus |
| EN | Enteral nutrition |
| ICU | Intensive care unit |
| IQR | Interquartile range |
| I.U. | International unit |
| LOS | Length of stay |
| IMV | Invasive mechanical ventilation |
| MNT | Medical nutrition therapy |
| PN | Parenteral nutrition |
| RFS | Refeeding syndrome |
| SAPS II | Simplified Acute Physiology Score |
| SOFA | Sequential Organ Failure Assessment Score |
| sPN | Supplemental parenteral nutrition |
| tEN | Total enteral nutrition |
| tPN | Total parenteral nutrition |
References
- White, J.V.; Guenter, P.; Jensen, G.; Malone, A.; Schofield, M.; Academy Malnutrition Work Group; A.S.P.E.N. Malnutrition Task Force; A.S.P.E.N. Board of Directors. Consensus statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition. JPEN J. Parenter. Enter. Nutr. 2012, 36, 275–283. [Google Scholar] [CrossRef]
- Freijer, K.; Tan, S.S.; Koopmanschap, M.A.; Meijers, J.M.; Halfens, R.J.; Nuijten, M.J. The economic costs of disease related malnutrition. Clin. Nutr. 2013, 32, 136–141. [Google Scholar] [CrossRef]
- Felder, S.; Lechtenboehmer, C.; Bally, M.; Fehr, R.; Deiss, M.; Faessler, L.; Kutz, A.; Steiner, D.; Rast, A.C.; Laukemann, S.; et al. Association of nutritional risk and adverse medical outcomes across different medical inpatient populations. Nutrition 2015, 31, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Pirlich, M.; Schutz, T.; Norman, K.; Gastell, S.; Lubke, H.J.; Bischoff, S.C.; Bolder, U.; Frieling, T.; Guldenzoph, H.; Hahn, K.; et al. The german hospital malnutrition study. Clin. Nutr. 2006, 25, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, J.; Kondrup, J.; Prokopowicz, J.; Schiesser, M.; Krahenbuhl, L.; Meier, R.; Liberda, M. EuroOOPS: An international, multicentre study to implement nutritional risk screening and evaluate clinical outcome. Clin. Nutr. 2008, 27, 340–349. [Google Scholar] [CrossRef]
- Lew, C.C.H.; Yandell, R.; Fraser, R.J.L.; Chua, A.P.; Chong, M.F.F.; Miller, M. Association between malnutrition and clinical outcomes in the intensive care unit: A systematic review. JPEN J. Parenter. Enter. Nutr. 2017, 41, 744–758. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef] [PubMed]
- Wirth, R.; Diekmann, R.; Janssen, G.; Fleiter, O.; Fricke, L.; Kreilkamp, A.; Modreker, M.K.; Marburger, C.; Nels, S.; Pourhassan, M.; et al. Refeeding syndrome: Pathophysiology, risk factors, prevention, and treatment. Der Internist 2018, 59, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Elia, M. Defining, recognizing, and reporting malnutrition. Int. J. Low Extrem. Wounds 2017, 16, 230–237. [Google Scholar] [CrossRef]
- Marik, P.E.; Bedigian, M.K. Refeeding hypophosphatemia in critically ill patients in an intensive care unit. A prospective study. Arch. Surg. 1996, 131, 1043–1047. [Google Scholar] [CrossRef]
- Rio, A.; Whelan, K.; Goff, L.; Reidlinger, D.P.; Smeeton, N. Occurrence of refeeding syndrome in adults started on artificial nutrition support: Prospective cohort study. BMJ Open 2013, 3, e002173. [Google Scholar] [CrossRef]
- Friedli, N.; Baumann, J.; Hummel, R.; Kloter, M.; Odermatt, J.; Fehr, R.; Felder, S.; Baechli, V.; Geiser, M.; Deiss, M.; et al. Refeeding syndrome is associated with increased mortality in malnourished medical inpatients: Secondary analysis of a randomized trial. Medicine 2020, 99, e18506. [Google Scholar] [CrossRef]
- da Silva, J.S.V.; Seres, D.S.; Sabino, K.; Adams, S.C.; Berdahl, G.J.; Citty, S.W.; Cober, M.P.; Evans, D.C.; Greaves, J.R.; Gura, K.M.; et al. ASPEN consensus recommendations for refeeding syndrome. Nutr. Clin. Pract. 2020, 35, 178–195. [Google Scholar] [CrossRef]
- Cioffi, I.; Ponzo, V.; Pellegrini, M.; Evangelista, A.; Bioletto, F.; Ciccone, G.; Pasanisi, F.; Ghigo, E.; Bo, S. The incidence of the refeeding syndrome. a systematic review and meta-analyses of literature. Clin. Nutr. 2021, 40, 3688–3701. [Google Scholar] [CrossRef]
- Janssen, G.; Pourhassan, M.; Lenzen-Grossimlinghaus, R.; Jager, M.; Schafer, R.; Spamer, C.; Cuvelier, I.; Volkert, D.; Wirth, R. The refeeding syndrome revisited: You can only diagnose what you know. Eur. J. Clin. Nutr. 2019, 73, 1458–1463. [Google Scholar] [CrossRef]
- Crook, M.A. Refeeding syndrome: Problems with definition and management. Nutrition 2014, 30, 1448–1455. [Google Scholar] [CrossRef]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonca, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Le Gall, J.R.; Lemeshow, S.; Saulnier, F. A new simplified acute physiology score (SAPS II) based on a european/north american multicenter study. JAMA 1993, 270, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Coskun, R.; Gundogan, K.; Baldane, S.; Guven, M.; Sungur, M. Refeeding hypophosphatemia: A potentially fatal danger in the intensive care unit. Turk. J. Med. Sci. 2014, 44, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Olthof, L.E.; Koekkoek, W.; van Setten, C.; Kars, J.C.N.; van Blokland, D.; van Zanten, A.R.H. Impact of caloric intake in critically ill patients with, and without, refeeding syndrome: A retrospective study. Clin. Nutr. 2018, 37, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Huang, H.; Wu, Y.; Wang, S.; Wang, D.; Ji, Z.; Lin, Z.; Zang, N.; Pan, S.; Huang, K. Incidence and outcome of refeeding syndrome in neurocritically ill patients. Clin. Nutr. 2021, 40, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Meira, A.P.C.; Santos, C.O.D.; Lucho, C.L.C.; Kasmirscki, C.; Silva, F.M. Refeeding syndrome in patients receiving parenteral nutrition is not associated to mortality or length of hospital stay: A retrospective observational study. Nutr. Clin. Pract. 2021, 36, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Reber, E.; Friedli, N.; Vasiloglou, M.F.; Schuetz, P.; Stanga, Z. Management of refeeding syndrome in medical inpatients. J. Clin. Med. 2019, 8, 2202. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.; Yeh, D.D.; Quraishi, S.A.; Johnson, E.A.; Kaafarani, H.; Lee, J.; King, D.R.; DeMoya, M.; Fagenholz, P.; Butler, K.; et al. Hypophosphatemia in enterally fed patients in the surgical intensive care unit: Common but unrelated to timing of initiation or aggressiveness of nutrition delivery. Nutr. Clin. Pract. 2017, 32, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W. Fluid and electrolyte disturbances in critically ill patients. Electrolyte Blood Press. 2010, 8, 72–81. [Google Scholar] [CrossRef]
- De Vries, M.C.; Koekkoek, W.K.; Opdam, M.H.; van Blokland, D.; van Zanten, A.R. Nutritional assessment of critically ill patients: Validation of the modified NUTRIC score. Eur. J. Clin. Nutr. 2018, 72, 428–435. [Google Scholar] [CrossRef]
- Heyland, D.K.; Dhaliwal, R.; Jiang, X.; Day, A.G. Identifying critically ill patients who benefit the most from nutrition therapy: The development and initial validation of a novel risk assessment tool. Crit. Care 2011, 15, R268. [Google Scholar] [CrossRef]



| Basic Characteristics | Total Population (n = 195) | RFS Group (n = 92) | Non-RFS Group (n = 103) | p-Value |
|---|---|---|---|---|
| Age, median (IQR) | 62 (50–71) | 58 (49–70) | 65 (52–73) | 0.059 |
| Male, n (%) | 126 (64.6) | 61 (66.3) | 65 (63.1) | 0.656 |
| Female, n (%) | 69 (35.4) | 31 (33.7) | 38 (36.9) | 0.656 |
| Weight (kg), median (IQR) | 80.0 (68.8–94.1) | 82 (70–95) | 80 (64–93) | 0.083 |
| Height (cm), median (IQR) | 172.5 (165–180) | 175 (165.8–180) | 170 (165–179.3) | 0.026 |
| BMI (kg/m2), median (IQR) | 26.3 (23.3–30.3) | 26.5 (23.9–30.2) | 26.2 (22.0–30.9) | 0.325 |
| ICU mortality, n (%) | 57 (29.2) | 21 (22.8) | 36 (35) | 0.083 |
| ICU-LOS (days), n (%) | 14 (6–22) | 15 (6.3–21.8) | 11 (6–23) | 0.674 |
| SAPS II, median (IQR) | 60.0 (48.8–72.0) | 60 (51–71) | 59.5 (45.0–73.3) | 0.688 |
| SOFA, median (IQR) | 10 (8–13) | 10 (8–12) | 10 (7–13) | 0.313 |
| IMV, n (%) | 181 (92.8) | 83 (90.2) | 98 (95.2) | 0.267 |
| IMV duration, median (IQR) | 9 (5.9–18) | 18 (4–19) | 10 (6–17) | 0.190 |
| tEN, n (%) | 153 (78.5) | 69 (75) | 84 (81.6) | 0.298 |
| tPN, n (%) | 9 (4.6) | 6 (6.5) | 3 (2.9) | 0.311 |
| sPN, n (%) | 32 (16.4) | 16 (17.4) | 16 (15.5) | 0.847 |
| Reason for ICU admission | ||||
| Cardiopulmonary resuscitation, n (%) | 73 (37.4) | 31 (33.7) | 42 (40.8) | 0.374 |
| Cardiovascular event, n (%) | 13 (6.7) | 4 (4.4) | 9 (8.7) | 0.261 |
| Respiratory failure, n (%) | 38 (19.5) | 19 (20.7) | 19 (18.5) | 0.721 |
| Sepsis, n (%) | 38 (19.5) | 17 (18.5) | 21 (20.4) | 0.857 |
| Gastrointestinal bleeding, n (%) | 7 (3.6) | 4 (4.4) | 3 (2.9) | 0.709 |
| Neurological failure, n (%) | 17 (8.7) | 12 (13) | 5 (4.9) | 0.073 |
| Acute liver failure, n (%) | 3 (1.5) | 2 (2.2) | 1 (1) | 0.603 |
| Other, n (%) | 6 (3.1) | 3 (3.3) | 3 (2.9) | >0.999 |
| Comorbidities | Total Population (n = 195) | RFS Group (n = 92) | Non-RFS Group (n = 103) | p-Value |
|---|---|---|---|---|
| Cardiovascular disease, n (%) | 106 (54.4) | 43 (46.7) | 63 (61.2) | 0.046 |
| DM type I or II, n (%) | 28 (14.4) | 10 (10.9) | 18 (17.5) | 0.223 |
| Nicotine abuse, n (%) | 10 (5.1) | 5 (5.4) | 5 (4.9) | >0.999 |
| Alcohol and/or drug abuse, n (%) | 12 (6.2) | 11 (12) | 1 (1) | 0.002 |
| Advanced chronic liver disease, n (%) | 31 (15.9) | 21 (22.8) | 10 (9.7) | 0.018 |
| COPD, n (%) | 26 (13.5) | 14 (15.2) | 12 (11.7) | 0.530 |
| Malignant disease, n (%) | 17 (8.7) | 5 (5.4) | 12 (11.7) | 0.137 |
| Neurological disease, n (%) | 20 (10.3) | 9 (9.8) | 11 (10.7) | >0.999 |
| Organ transplantation, n (%) | 9 (4.6) | 3 (3.3) | 6 (5.8) | 0.504 |
| Chronic kidney disease, n (%) | 14 (7.2) | 4 (4.4) | 10 (9.7) | 0.174 |
| Psychiatric disease, n (%) | 3 (1.5) | 1 (1.1) | 2 (1.9) | >0.999 |
| Immunological disease, n (%) | 5 (2.6) | 2 (2.2) | 3 (2.9) | >0.999 |
| Gastrointestinal disease, n (%) | 5 (2.6) | 2 (2.2) | 3 (2.9) | >0.999 |
| Pre-Existing Low Electrolyte Levels Prior to Nutrition Support | RFS Group | Non-RFS Group | p-Value |
|---|---|---|---|
| PO4 levels ≤ 0.8 mmol/L, n (%) | 24 (26.1) | 14 (13.6) | 0.031 |
| Mg2+ levels ≤ 0.65 mmol/L, n (%) | 7 (7.6) | 9 (8.7) | 0.801 |
| K+ levels ≤ 3.5 mmol/L, n (%) | 9 (9.8) | 8 (7.8) | 0.312 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schneeweiss-Gleixner, M.; Haselwanter, P.; Schneeweiss, B.; Zauner, C.; Riedl-Wewalka, M. Hypophosphatemia after Start of Medical Nutrition Therapy Indicates Early Refeeding Syndrome and Increased Electrolyte Requirements in Critically Ill Patients but Has No Impact on Short-Term Survival. Nutrients 2024, 16, 922. https://doi.org/10.3390/nu16070922
Schneeweiss-Gleixner M, Haselwanter P, Schneeweiss B, Zauner C, Riedl-Wewalka M. Hypophosphatemia after Start of Medical Nutrition Therapy Indicates Early Refeeding Syndrome and Increased Electrolyte Requirements in Critically Ill Patients but Has No Impact on Short-Term Survival. Nutrients. 2024; 16(7):922. https://doi.org/10.3390/nu16070922
Chicago/Turabian StyleSchneeweiss-Gleixner, Mathias, Patrick Haselwanter, Bruno Schneeweiss, Christian Zauner, and Marlene Riedl-Wewalka. 2024. "Hypophosphatemia after Start of Medical Nutrition Therapy Indicates Early Refeeding Syndrome and Increased Electrolyte Requirements in Critically Ill Patients but Has No Impact on Short-Term Survival" Nutrients 16, no. 7: 922. https://doi.org/10.3390/nu16070922
APA StyleSchneeweiss-Gleixner, M., Haselwanter, P., Schneeweiss, B., Zauner, C., & Riedl-Wewalka, M. (2024). Hypophosphatemia after Start of Medical Nutrition Therapy Indicates Early Refeeding Syndrome and Increased Electrolyte Requirements in Critically Ill Patients but Has No Impact on Short-Term Survival. Nutrients, 16(7), 922. https://doi.org/10.3390/nu16070922





