Effect of Probiotic Lacticaseibacillus rhamnosus LB1.5 on Anxiety-like Behavior, Neuroprotection and Neuroinflammation Markers of Male Mice Fed a High-Fat Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Treatments
2.3. Light–Dark Box Test
2.4. Tissue and Blood Collection
2.5. Cytokines Assay
2.6. Histological Analysis and Quantification of Cell
2.7. Molecular Analysis
2.7.1. Total RNA Extraction
2.7.2. RT-PCR
2.7.3. Analysis of Gene Expression by qPCR
2.8. Statistical Analyses
3. Results
3.1. Weight Gain, Food Intake, BMI, and Biochemical Parameters
3.2. Inflammatory Cytokines
3.3. Anxious-like Behavior
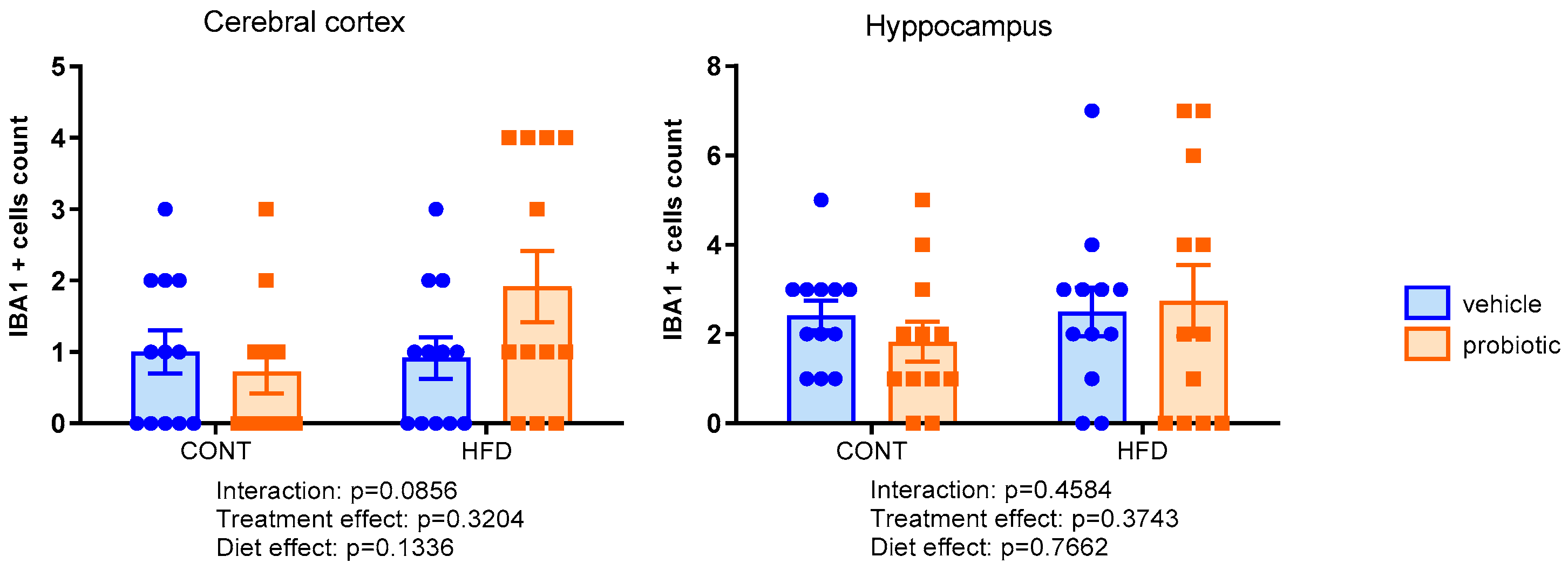
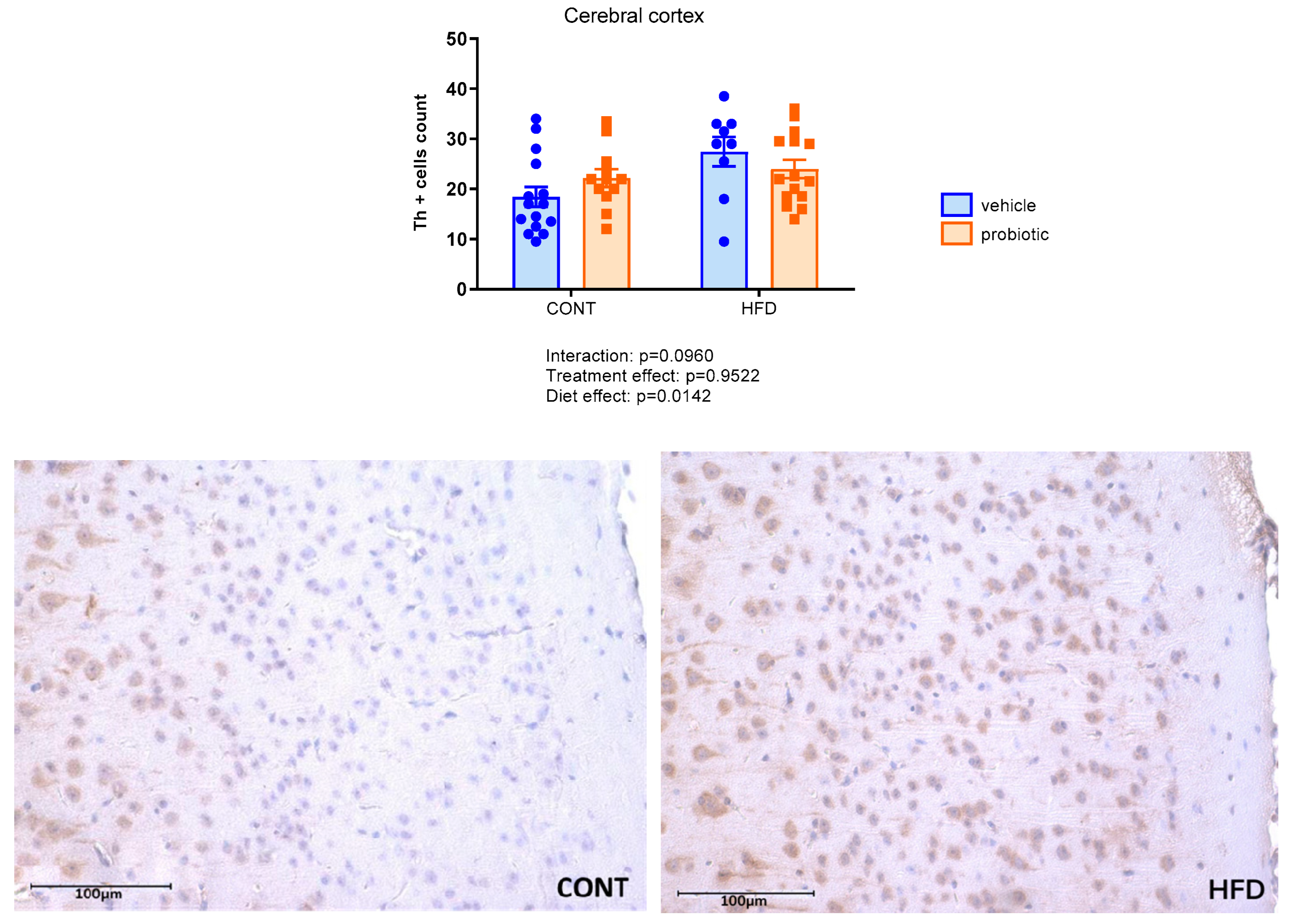
3.4. Neuroinflammation Markers in the Cerebral Cortex and Hippocampus
3.5. Gene Expression of Sirt1, Nrf2 and Bdnf in the Prefrontal Cortex
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beilharz, J.E.; Maniam, J.; Morris, M.J. Short-term exposure to a diet high in fat and sugar, or liquid sugar, selectively impairs hippocampal-dependent memory, with differential impacts on inflammation. Behav. Brain. Res. 2016, 306, 1–7. [Google Scholar] [CrossRef]
- Takase, K.; Tsuneoka, Y.; Oda, S.; Kuroda, M.; Funato, H. High-fat diet feeding alters olfactory-, social-, and reward-related behaviors of mice independent of obesity. Obesity 2016, 24, 886–894. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Effect of High-Fat Diets on Oxidative Stress, Cellular Inflammatory Response and Cognitive Function. Nutrients 2019, 11, 2579. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, H.; Liu, Y.; Yang, L. High fat diet induced obesity model using four strains of mice: Kunming, C57BL/6, BALB/c and ICR. Exp. Anim. 2020, 69, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Popkin, B.M. Dietary fat intake does affect obesity! Am. J. Clin. Nutr. 1998, 68, 1157–1173. [Google Scholar] [CrossRef] [PubMed]
- Mathers, J.C. Early nutrition: Impact on epigenetics. Forum Nutr. 2007, 60, 42–48. [Google Scholar] [CrossRef]
- Silveira, P.P.; Portella, A.K.; Goldani, M.Z.; Barbieri, M.A. Origens desenvolvimentistas da saúde e da doença (DOHaD). Pediatry 2007, 83, 494–504. [Google Scholar] [CrossRef]
- Mazloom, K.; Siddiqi, I.; Covasa, M. Probiotics: How effective are they in the fight against obesity? Nutrients 2019, 11, 258. [Google Scholar] [CrossRef]
- Song, W.; Song, C.; Li, L.; Wang, T.; Hu, J.; Zhu, L.; Yue, T. Lactobacillus alleviated obesity induced by high-fat diet in mice. J. Food Sci. 2021, 86, 5439–5451. [Google Scholar] [CrossRef]
- Morelli, L.; Capurso, L. FAO/WHO Guidelines on Probiotics. J. Clin. Gastroenterol. 2012, 46, S1–S2. [Google Scholar] [CrossRef] [PubMed]
- Divyashri, G.; Krishna, G.; Muralidhara; Prapulla, S.G. Probiotic attributes, antioxidant, anti-inflammatory and neuromodulatory effects of Enterococcus faecium CFR 3003: In vitro and in vivo evidence. J. Med. Microbiol. 2015, 64, 1527–1540. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Chiou, S.Y.; Hsu, A.H.; Lin, Y.C.; Lin, J.S. Lactobacillus rhamnosus Strain LRH05 Intervention Ameliorated Body Weight Gain and Adipose Inflammation via Modulating the Gut Microbiota in High-Fat Diet-Induced Obese Mice. Mol. Nutr. Food Res. 2022, 66, e2100348. [Google Scholar] [CrossRef] [PubMed]
- Núñez, I.N.; Galdeano, C.M.; de LeBlanc, A.D.; Perdigón, G. Lactobacillus casei CRL 431 administration decreases inflammatory cytokines in a diet-induced obese mouse model. Nutrition 2015, 31, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Wang, Y.; Tian, Y.; Chen, Y.Y.; Guan, W.Y.; Piao, C.H.; Wang, Y.H. Lactobacillus plantarum LP104 ameliorates hyperlipidemia induced by AMPK pathways in C57BL/6N mice fed high-fat diet. J. Funct. Foods 2020, 64, 103665. [Google Scholar] [CrossRef]
- Romo-Araiza, A.; Ibarra, A. Prebiotics and probiotics as potential therapy for cognitive impairment. Med. Hypotheses 2020, 134, 109410. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Naik, B.; Kumar, A.; Khanduri, N.; Rustagi, S.; Kumar, S. Probiotics media: Significance, challenges, and future perspective—A mini review. Food Prod. Process. Nutr. 2022, 4, 17. [Google Scholar] [CrossRef]
- Lalonde, R.; Strazielle, C. Probiotic effects on anxiety-like behavior in animal models. Rev. Neurosci. 2022, 33, 691–701. [Google Scholar] [CrossRef]
- Choi, J.; Kim, Y.K.; Han, P.L. Extracellular vesicles derived from Lactobacillus plantarum increase BDNF expression in cultured hippocampal neurons and produce antidepressant-like effects in mice. Exp. Neurobiol. 2019, 28, 158. [Google Scholar] [CrossRef]
- Breyer, G.M.; Arechavaleta, N.N.; Siqueira, F.M.; da Motta, A.S. Characterization of Lactic Acid Bacteria in Raw Buffalo Milk: A Screening for Novel Probiotic Candidates and Their Transcriptional Response to Acid Stress. Probiotics Antimicrob. Proteins 2020, 13, 468–483. [Google Scholar] [CrossRef]
- Bondarczuk, N.H.; Schmidt, N.P.; Breyer, G.M.; de Moura, A.C.; Molz, P.; Barshack, A.G.; da Motta, A.S.; Guedes, R.P.; Giovenardi, M. A high-fat diet changes placental morphology but does not change biochemical parameters, placental oxidative stress or cytokine levels. Placenta 2023, 135, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Novelli, E.L.; Diniz, Y.S.; Galhardi, C.M.; Ebaid, G.M.; Rodrigues, H.G.; Mani, F.; Fernandes, A.A.; Cicogna, A.C.; Novelli Filho, J.L. Anthropometrical parameters and markers of obesity in rats. Lab. Anim. 2007, 41, 111–119. [Google Scholar] [CrossRef]
- Crawley, J.; Goodwin, F.K. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol. Biochem. Behav. 1980, 13, 167–170. [Google Scholar] [CrossRef]
- Rodgers, R.J.; Shepherd, J.K. Influence of prior maze experience on behaviour and response to diazepam in the elevated plus-maze and light/dark tests of anxiety in mice. Psychopharmacology 1993, 113, 237–242. [Google Scholar] [CrossRef]
- Sztainberg, Y.; Kuperman, Y.; Tsoory, M.; Lebow, M.; Chen, A. The anxiolytic effect of environmental enrichment is mediated via amygdalar CRF receptor type 1. Mol. Psychiatry 2010, 15, 905–917. [Google Scholar] [CrossRef]
- Teixeira, D.; Cecconello, A.L.; Partata, W.A.; de Fraga, L.S.; Ribeiro, M.F.M.; Guedes, R.P. The metabolic and neuroinflammatory changes induced by consuming a cafeteria diet are age-dependent. Nutr. Neurosci. 2019, 22, 284–294. [Google Scholar] [CrossRef]
- Moura, A.C.D.; Lazzari, V.M.; Agnes, G.; Almeida, S.; Giovenardi, M.; Veiga, A.B.G.D. Transcriptional expression study in the central nervous system of rats: What gene should be used as internal control? Einstein 2014, 12, 336–341. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Liu, T.W.; Cephas, K.D.; Holscher, H.D.; Kerr, K.R.; Mangian, H.F.; Tappenden, K.A.; Swanson, K.S. Nondigestible Fructans Alter Gastrointestinal Barrier Function, Gene Expression, Histomorphology, and the Microbiota Profiles of Diet-Induced Obese C57BL/6J Mice. J. Nutr. 2016, 146, 949–956. [Google Scholar] [CrossRef]
- Totten, M.S.; Pierce, D.M.; Erikson, K.M. Diet-Induced Obesity Disrupts Trace Element Homeostasis and Gene Expression in the Olfactory Bulb. Nutrients 2020, 12, 3909. [Google Scholar] [CrossRef]
- Wang, Z.; Ge, Q.; Wu, Y.; Zhang, J.; Gu, Q.; Han, J. Impairment of Long-term Memory by a Short-term High-fat Diet via Hippocampal Oxidative Stress and Alterations in Synaptic Plasticity. Neuroscience 2020, 424, 24–33. [Google Scholar] [CrossRef]
- Lee, C.S.; Park, M.H.; Kim, B.K.; Kim, S.H. Antiobesity Effect of Novel Probiotic Strains in a Mouse Model of High-Fat Diet–Induced Obesity. Probiotics Antimicro. Prot. 2021, 13, 1054–1067. [Google Scholar] [CrossRef]
- Cortez-Pinto, H.; Borralho, P.; Machado, J.; Lopes, M.T.; Gato, I.V.; Santos, A.M.; Guerreiro, A.S. Microbiota Modulation with Synbiotic Decreases Liver Fibrosis in a High Fat Choline Deficient Diet Mice Model of Non-Alcoholic Steatohepatitis (NASH). J. Gastroenterol. 2016, 23, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Buford, T.W.; Sun, Y.; Roberts, L.M.; Banerjee, A.; Peramsetty, S.; Knighton, A.; Verma, A.; Morgan, D.; Torres, G.E.; Li, Q.; et al. Angiotensin (1–7) delivered orally via probiotic, but not subcutaneously, benefits the gut-brain axis in older rats. GeroScience 2020, 42, 1307–1321. [Google Scholar] [CrossRef]
- Carter, C.S.; Morgan, D.; Verma, A.; Lobaton, G.; Aquino, V.; Sumners, E.; Raizada, M.; Li, Q.; Buford, T.W. Therapeutic Delivery of Ang(1–7) via Genetically Modified Probiotic: A Dosing Study. J. Gerontol. 2020, 75, 1299–1303. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Zhu, P.; Xu, K.; Du, T.; Liao, S.; Liang, Z.; Raizada, M.K.; Li, Q. Angiotensin-(1–7) Expressed from Lactobacillus Bacteria Protect Diabetic Retina in Mice. Trans. Vis. Sci. Tech. 2020, 9, 20. [Google Scholar] [CrossRef]
- Hernandez, A.; Sun, Y.; Banerjee, A.; Yang, Y.; Verma, A.; Li, Q.; Baptista, L.; Buford, T.W.; Carter, C.S. Angiotensin (1–7) delivered orally via probiotic in combination with exercise: Sex-dependent influence on health span. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 223–226. [Google Scholar] [CrossRef]
- Duan, Y.; Zeng, L.; Zheng, C.; Song, B.; Li, F.; Kong, X.; Xu, K. Inflammatory Links Between High Fat Diets and Diseases. Front. Immunol. 2018, 9, 2649. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.A.; Essa, M.E.A.; Mollica, A.; Stefanucci, A.; Zengin, G.; Ahmed, H. Gum Arabic modifies anti-inflammatory cytokine in mice fed with high fat diet induced obesity. Bioact. Carbohydr. Diet. Fibre 2021, 25, 100258. [Google Scholar] [CrossRef]
- Kiran, S.; Rakib, A.; Kodidela, S.; Kumar, S.; Singh, U.P. High-Fat Diet-Induced Dysregulation of Immune Cells Correlates with Macrophage Phenotypes and Chronic Inflammation in Adipose Tissue. Cells 2022, 11, 1327. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Guillemot-Legris, O.; Muccioli, G.G. Obesity-Induced Neuroinflammation: Beyond the Hypothalamus. Trends Neurosci. 2017, 40, 237–253. [Google Scholar] [CrossRef]
- Buie, J.J.; Watson, L.S.; Smith, C.J.; Sims-Robinson, C. Obesity-related cognitive impairment: The role of endothelial dysfunction. Neurobiol. Dis. 2019, 132, 104580. [Google Scholar] [CrossRef] [PubMed]
- Salas-Venegas, V.; Flores-Torres, R.P.; Rodríguez-Cortés, Y.M.; Rodríguez-Retana, D.; Ramírez-Carreto, R.J.; Concepción-Carrillo, L.E.; Pérez-Flores, L.J.; Alarcón-Aguilar, A.; López-Díazguerrero, N.E.; Gómez-González, B.; et al. The Obese Brain: Mechanisms of Systemic and Local Inflammation, and Interventions to Reverse the Cognitive Deficit. Front. Integr. Neurosci. 2022, 16, 798995. [Google Scholar] [CrossRef] [PubMed]
- Neto, J.; Jantsch, J.; de Oliveira, S.; Braga, M.F.; Castro, L.F.D.S.; Diniz, B.F.; Moreira, J.C.F.; Giovenardi, M.; Porawski, M.; Guedes, R.P. DHA/EPA supplementation decreases anxiety-like behaviour, but it does not ameliorate metabolic profile in obese male rats. Br. J. Nutr. 2022, 128, 964–974. [Google Scholar] [CrossRef] [PubMed]
- González, L.P.F.; Rodrigues, F.S.; Jantsch, J.; Fraga, G.F.; Squizani, S.; Castro, L.F.S.; Correia, L.L.; Neto, J.P.; Giovenardi, M.; Porawski, M.; et al. Effects of omega-3 supplementation on anxiety-like behaviors and neuroinflammation in Wistar rats following cafeteria diet-induced obesity. Nutr. Neurosci. 2023, 1, 12. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, Q.; Kalavagunta, P.K.; Huang, Q.; Lv, W.; An, X.; Chen, H.; Wang, T.; Heriniaina, R.M.; Qiao, T.; et al. Normal diet Vs High fat diet-A comparative study: Behavioral and neuroimmunological changes in adolescent male mice. Metab. Brain Dis. 2018, 33, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Giuliani, F. The Role of Inflammation in Depression and Fatigue. Front. Immunol. 2019, 19, 1696. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Nepal, P.; Odelade, A.; Freely, F.D.; Belton, D.M.; Graves, J.L., Jr.; Maldonado-Devincci, A.M. High-Fat Diet-Induced Weight Gain, Behavioral Deficits, and Dopamine Changes in Young C57BL/6J Mice. Front. Nutr. 2021, 20, 591161. [Google Scholar] [CrossRef]
- Cai, D.; Khor, S. “Hypothalamic microinflammation” paradigm in aging and metabolic diseases. Cell. Metab. 2019, 30, 19–35. [Google Scholar] [CrossRef]
- Lee, C.H.; Suk, K.; Yu, R.; Kim, M.S. Cellular contributors to hypothalamic inflammation in obesity. Mol. Cells 2020, 43, 431–437. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Gómez-Apo, E.; Mondragón-Maya, A.; Ferrari-Díaz, M.; Silva-Pereyra, J. Structural Brain Changes Associated with Overweight and Obesity. J. Obes. 2021, 16, 6613385. [Google Scholar] [CrossRef]
- Gunstad, J.; Paul, R.H.; Cohen, R.A.; Tate, D.F.; Spitznagel, M.B.; Grieve, S.; Gordon, E. Relationship between body mass index and brain volume in healthy adults. Int. J. Neurosci. 2008, 118, 1582–1593. [Google Scholar] [CrossRef]
- Raji, C.A.; Ho, A.J.; Parikshak, N.N.; Becker, J.T.; Lopez, O.L.; Kuller, L.H.; Hua, X.; Leow, A.D.; Toga, A.W.; Thompson, P.M. Brain structure and obesity. Hum. Brain Mapp. 2010, 31, 353–364. [Google Scholar] [CrossRef]
- Nguyen, J.C.; Killcross, A.S.; Jenkins, T.A. Obesity and cognitive decline: Role of inflammation and vascular changes. Front. Neurosci. 2014, 8, 375. [Google Scholar] [CrossRef]
- Calcia, M.A.; Bonsall, D.R.; Bloomfield, P.S.; Selvaraj, S.; Barichello, T.; Howes, O.D. Stress and neuroinflammation: A systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology 2016, 233, 1637–1650. [Google Scholar] [CrossRef] [PubMed]
- Lituma, P.J.; Woo, E.; O’Hara, B.F.; Castillo, P.E.; Sibinga, N.E.S.; Nandi, S. Altered synaptic connectivity and brain function in mice lacking microglial adapter protein Iba1. Proc. Natl. Acad. Sci. USA 2021, 118, e2115539118. [Google Scholar] [CrossRef] [PubMed]
- Saiyasit, N.; Chunchai, T.; Prus, D.; Suparan, K.; Pittayapong, P.; Apaijai, N.; Pratchayasakul, W.; Sripetchwandee, J.; Chattipakorn, N.; Chattipakorn, S.C. Gut dysbiosis develops before metabolic disturbance and cognitive decline in high-fat diet-induced obese condition. Nutrition 2020, 69, 110576. [Google Scholar] [CrossRef] [PubMed]
- Chunchai, T.; Thunapong, W.; Yasom, S.; Wanchai, K.; Eaimworawuthikul, S.; Metzler, G.; Lungkaphin, A.; Pongchaidecha, A.; Sirilun, S.; Chaiyasut, C.; et al. Decreased microglial activation through gut-brain axis by prebiotics, probiotics, or synbiotics effectively restored cognitive function in obese-insulin resistant rats. J. Neuroinflamm. 2018, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Channer, B.; Matt, S.M.; Nickoloff-Bybel, E.A.; Pappa, V.; Agarwal, Y.; Wickman, J.; Gaskill, P.J. Dopamine, Immunity, and Disease. Pharmacol. Rev. 2023, 75, 62–158. [Google Scholar] [CrossRef] [PubMed]
- Baik, J.H. Dopaminergic Control of the Feeding Circuit. Endocrinol. Metab. 2021, 36, 229–239. [Google Scholar] [CrossRef]
- Alonso-Alonso, M.; Woods, S.C.; Pelchat, M.; Grigson, P.S.; Stice, E.; Farooqi, S.; Khoo, C.S.; Mattes, R.D.; Beauchamp, G.K. Food reward system: Current perspectives and future research needs. Nutr. Rev. 2015, 73, 296–307. [Google Scholar] [CrossRef]
- Li, Y.; South, T.; Han, M.; Chen, J.; Wang, R.; Huang, X.F. High-fat diet decreases tyrosine hydroxylase mRNA expression irrespective of obesity susceptibility in mice. Brain Res. 2009, 1268, 181–189. [Google Scholar] [CrossRef]
- Zaydi, A.I.; Lew, L.C.; Hor, Y.Y.; Jaafar, M.H.; Chuah, L.O.; Yap, K.P.; Azlan, A.; Azzam, G.; Liong, M.T. Lactobacillus plantarum DR7 improved brain health in aging rats via the serotonin, inflammatory and apoptosis pathways. Benef. Microbes 2020, 11, 753–766. [Google Scholar] [CrossRef]
- Adedeji, T.G.; Jeje, S.O.; Omayone, T.P.; Agbonifo, W.O. Oxidative stress and inflammatory response to high dietary fat and carbonated soda intake in male and female Wistar rats. Nutrition 2022, 1, 111800. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Reifenberg, G.; Schirra, C.; Li, H. The involvement of sirtuin 1 dysfunction in high-fat diet-induced vascular dysfunction in mice. Antioxidants 2022, 11, 541. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, G.; Trinchese, G.; Penna, E.; Cimmino, F.; Pirozzi, C.; Lama, A.; Annunziata, C.; Catapano, A.; Mattace Raso, G.; Meli, R.; et al. High-fat diet induces neuroinflammation and mitochondrial impairment in mice cerebral cortex and synaptic fraction. Front. Cell Neurosci. 2019, 13, 509. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, B.; Dong, F.; Zhu, X.; Liu, B.; Liu, Y. Effects of inflammatory responses, apoptosis, and STAT3/NF-κB- and Nrf2-mediated oxidative stress on benign prostatic hyperplasia induced by a high-fat diet. Aging 2019, 11, 5570–5578. [Google Scholar] [CrossRef] [PubMed]
- Mullins, C.A.; Gannaban, R.B.; Khan, M.S.; Shah, H.; Siddik, M.A.B.; Hegde, V.K.; Reddy, P.H.; Shin, A.C. Neural Underpinnings of Obesity: The Role of Oxidative Stress and Inflammation in the Brain. Antioxidants 2020, 9, 1018. [Google Scholar] [CrossRef]
- Li, X. SIRT1 and energy metabolism. Acta Biochim. Biophys. Sin. 2013, 45, 51–60. [Google Scholar] [CrossRef]
- Pfluger, P.T.; Herranz, D.; Velasco-Miguel, S.; Serrano, M.; Tschöp, M.H. Sirt1 protects against high-fat diet-induced metabolic damage. Proc. Natl. Acad. Sci. USA 2008, 105, 9793–9798. [Google Scholar] [CrossRef]
- Vasconcelos, A.R.; Dos Santos, N.B.; Scavone, C.; Munhoz, C.D. Nrf2/ARE pathway modulation by dietary energy regulation in neurological disorders. Front. Pharmacol. 2019, 10, 428667. [Google Scholar] [CrossRef]
- Kanoski, S.E.; Meisel, R.L.; Mullins, A.J.; Davidson, T.L. The effects of energy-rich diets on discrimination reversal learning and on BDNF in the hippocampus and prefrontal cortex of the rat. Behav. Brain Res. 2007, 182, 57–66. [Google Scholar] [CrossRef]
- Miranda, M.; Morici, J.F.; Zanoni, M.B. Brain-derived neurotrophic factor: A key molecule for memory in the healthy and the pathological brain. Front. Cell Neurosci. 2019, 3, 363. [Google Scholar] [CrossRef] [PubMed]
- Babaei, P.; Azari, H.B. Exercise training improves memory performance in older adults: A narrative review of evidence and possible mechanisms. Front. Hum. Neurosci. 2022, 15, 771553. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.Y.; Shen, C.L.; Tsai, C.Y.; Tarn, W.Y. Activation of TrkB signaling mitigates cerebellar anomalies caused by Rbm4-Bdnf deficiency. Commun. Biol. 2023, 6, 910. [Google Scholar] [CrossRef] [PubMed]
- Bastías-Pérez, M.; Serra, D.; Herrero, L. Dietary options for rodents in the study of obesity. Nutrients 2020, 12, 3234. [Google Scholar] [CrossRef]



| Parameters | CONT (n = 10) | CONT + PROBIOTIC (n = 10) | HFD (n = 10) | HFD + PROBIOTIC (n = 10) | Two-Way ANOVA | ||
|---|---|---|---|---|---|---|---|
| Interaction | Diet Effect | Treatment Effect | |||||
| Glucose mg/dL) | 179.34 ± 15.06 | 173.52 ± 10.61 | 193.84 ± 14.68 | 194.01 ± 9.02 | 0.8114 | 0.1695 | 0.8220 |
| Triglycerides (mg/dL) | 84.75 ± 12.84 | 107.02 ± 14.00 | 76.72 ± 7.50 | 91.56 ± 10.81 | 0.7557 | 0.3286 | 0.1269 |
| Total cholesterol (mg/dL) | 58.34 ± 5.41 | 55.34 ± 5.708 | 105.85 ± 7.83 | 89.77 ± 8.68 | 0.3596 | <0.0001 | 0.1845 |
| HDL a (mg/dL) | 24.43 ± 2.87 | 30.16 ± 3.14 | 46.26 ± 7.88 | 34.89 ± 4.96 | 0.0962 | 0.0125 | 0.5736 |
| LDL b (mg/dL) | 27.36 ± 9.04 | 23.83 ± 8.83 | 63.83 ± 13.83 | 45.13 ± 9.26 | 0.5008 | 0.0149 | 0.3263 |
| Body Mass Index (g/cm2) | 0.52 ± 0.02 | 0.52 ± 0.02 | 0.63 ± 0.04 | 0.59 ± 0.04 | 0.5182 | 0.0081 | 0.5908 |
| Food intake per week (g) | 11.83 ± 0.99 | 10.39 ± 1.04 | 13.57 ± 0.76 | 14.64 ± 1.10 | 0.2203 | 0.0055 | 0.8504 |
| Behaviors | CONT (n = 10) | CONT + PROBIOTIC (n = 10) | HFD (n = 10) | HFD + PROBIOTIC (n = 10) | Two-Way ANOVA | ||
|---|---|---|---|---|---|---|---|
| Interaction | Diet Effect | Treatment Effect | |||||
| Latency to the first transition (s) | 17.49 ± 4.75 | 29.63 ± 5.28 | 8.19 ± 4.60 | 26.48 ± 6.60 | 0.5696 | 0.2527 | 0.0079 |
| Time spent in the light compartment (s) | 147.40 ± 21.27 | 184.78 ± 12.24 | 149.34 ± 20.75 | 192.11 ± 20.46 | 0.8911 | 0.8141 | 0.0489 |
| Total distance traveled in the light compartment (m) | 4.38 ± 0.63 | 5.27 ± 0.56 | 4.01 ± 0.66 | 5.39 ± 0.20 | 0.6812 | 0.8510 | 0.0466 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, N.P.; Molz, P.; Fraga, B.S.; Bondarczuk, N.H.; Silveira, P.D.; Ferri, M.H.; Crestani, T.B.; Breyer, G.M.; Guimarães, G.R.; Motta, A.d.S.d.; et al. Effect of Probiotic Lacticaseibacillus rhamnosus LB1.5 on Anxiety-like Behavior, Neuroprotection and Neuroinflammation Markers of Male Mice Fed a High-Fat Diet. Nutrients 2024, 16, 879. https://doi.org/10.3390/nu16060879
Schmidt NP, Molz P, Fraga BS, Bondarczuk NH, Silveira PD, Ferri MH, Crestani TB, Breyer GM, Guimarães GR, Motta AdSd, et al. Effect of Probiotic Lacticaseibacillus rhamnosus LB1.5 on Anxiety-like Behavior, Neuroprotection and Neuroinflammation Markers of Male Mice Fed a High-Fat Diet. Nutrients. 2024; 16(6):879. https://doi.org/10.3390/nu16060879
Chicago/Turabian StyleSchmidt, Natália Perin, Patrícia Molz, Brenda Santos Fraga, Nicole Hiller Bondarczuk, Priscila Dutra Silveira, Milena Henrique Ferri, Thais Busatto Crestani, Gabriela Merker Breyer, Giuliano Rizzoto Guimarães, Amanda de Souza da Motta, and et al. 2024. "Effect of Probiotic Lacticaseibacillus rhamnosus LB1.5 on Anxiety-like Behavior, Neuroprotection and Neuroinflammation Markers of Male Mice Fed a High-Fat Diet" Nutrients 16, no. 6: 879. https://doi.org/10.3390/nu16060879
APA StyleSchmidt, N. P., Molz, P., Fraga, B. S., Bondarczuk, N. H., Silveira, P. D., Ferri, M. H., Crestani, T. B., Breyer, G. M., Guimarães, G. R., Motta, A. d. S. d., Guedes, R. P., & Giovenardi, M. (2024). Effect of Probiotic Lacticaseibacillus rhamnosus LB1.5 on Anxiety-like Behavior, Neuroprotection and Neuroinflammation Markers of Male Mice Fed a High-Fat Diet. Nutrients, 16(6), 879. https://doi.org/10.3390/nu16060879







