Efficacy and Tolerability of a Scutellaria lateriflora L. and Cistus × incanus L.-Based Chewing Gum on the Symptoms of Gingivitis: A Monocentric, Randomized, Double-Blind, Placebo-Controlled Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Food Supplement and Placebo
2.2. Volunteers
2.3. Validated Questionnaires
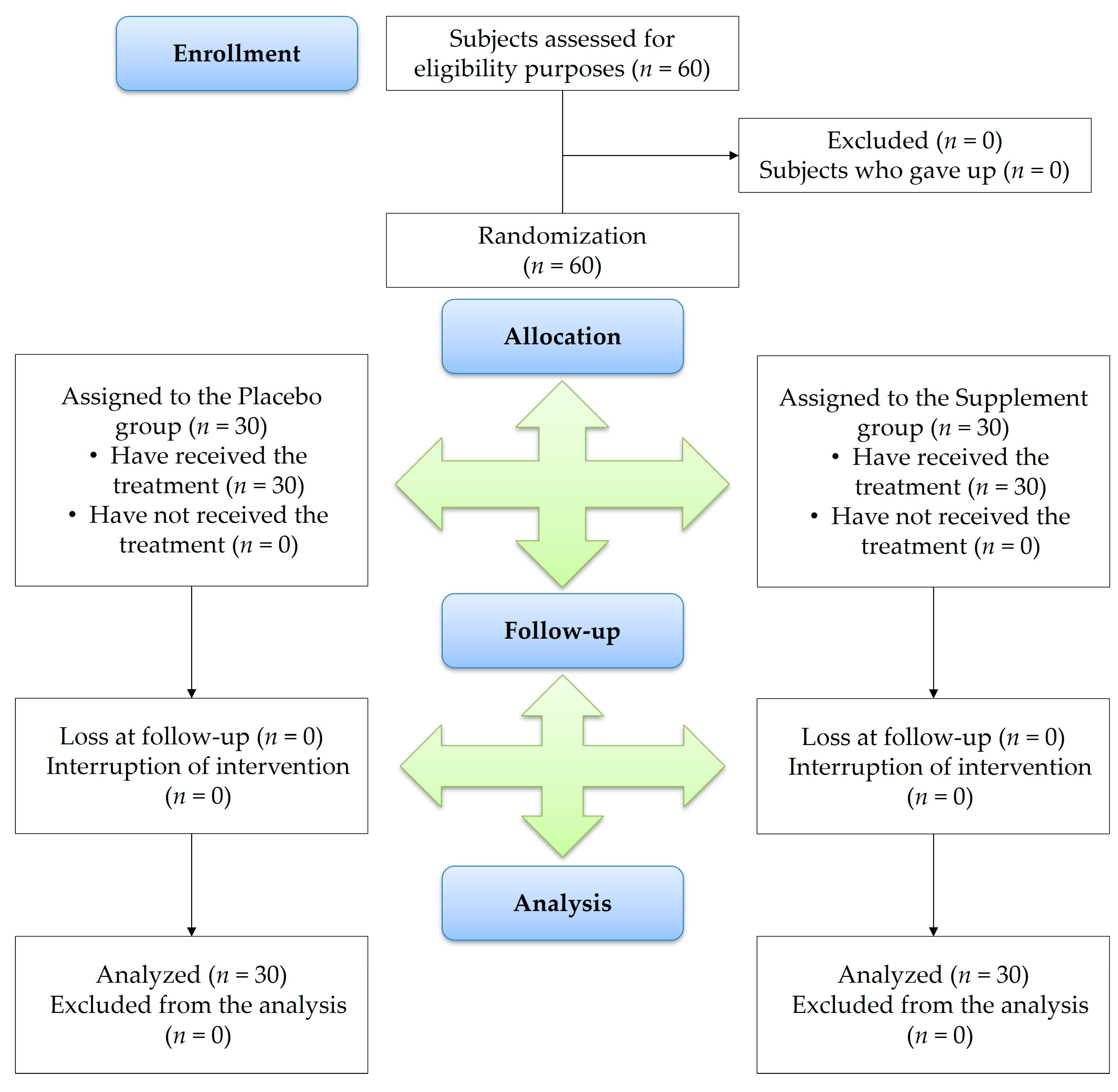
2.4. Trial Design
2.4.1. Outcomes of Study
2.4.2. Data Collection
2.5. Safety and Tolerability
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holmstrup, P.; Plemons, J.; Meyle, J. Non-plaque-induced gingival diseases. J. Clin. Periodontol. 2018, 45, S28–S43. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Lamster, I.B.; Levin, L. Current concepts in the management of periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Armitage, G.C. Periodontal diagnoses and classification of periodontal diseases. Periodontology 2000 2004, 34, 9–21. [Google Scholar] [CrossRef]
- Ullah, H.; Minno, A.D.; Filippis, A.; Sommella, E.; Buccato, D.G.; Lellis, L.F.; El-Seedi, H.R.; Khalifa, S.A.M.; Piccinocchi, R.; Galdiero, M.; et al. In Vitro antimicrobial and antibiofilm properties and bioaccessibility after oral digestion of chemically characterized extracts obtained from Cistus × incanus L., Scutellaria lateriflora L., and their combination. Foods 2023, 12, 1826. [Google Scholar] [CrossRef] [PubMed]
- Herrero, E.R.; Fernandes, S.; Verspecht, T.; Ugarte-Berzal, E.; Boon, N.; Proost, P.; Bernaerts, K.; Quirynen, M.; Teughels, W. Dysbiotic biofilms deregulate the periodontal inflammatory response. J. Dent. Res. 2018, 97, 547–555. [Google Scholar] [CrossRef]
- Rathee, M.; Jain, P. Gingivitis. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557422/ (accessed on 27 November 2023).
- Heitz-Mayfield, L.J.; Trombelli, L.; Heitz, F.; Needleman, I.; Moles, D. A systematic review of the effect of surgical debridement vs non-surgical debridement for the treatment of chronic periodontitis. J. Clin. Periodontol. 2002, 29, 92–102. [Google Scholar] [CrossRef]
- Ming, J.; Zhuoneng, L.; Guangxun, Z. Protective role of flavonoid baicalin from Scutellaria baicalensis in periodontal disease pathogenesis: A literature review. Complement. Ther. Med. 2018, 38, 11–18. [Google Scholar] [CrossRef]
- Rajendiran, M.; Trivedi, H.M.; Chen, D.; Gajendrareddy, P.; Chen, L. Recent development of active ingredients in mouthwashes and toothpastes for periodontal diseases. Molecules 2021, 26, 2001. [Google Scholar] [CrossRef]
- Garg, S.; Kapoor, K.K. The quantitative gingival bleeding index. J. Indian Dent. Assoc. 1985, 57, 112–113. [Google Scholar]
- Trombelli, L.; Tatakis, D.N.; Scapoli, C.; Bottega, S.; Orlandini, E.; Tosi, M. Modulation of clinical expression of plaque-induced gingivitis. II. Identification of “high-responder” and “low-responder” subjects. J. Clin. Periodontol. 2004, 31, 239–252. [Google Scholar] [CrossRef]
- Reissmann, D.R.; Aarabi, G.; Harter, M.; Heydecke, G.; Kriston, L. Measuring oral health: The Physical Oral Health Index. J. Dent. 2022, 118, 103946. [Google Scholar] [CrossRef]
- Banakar, M.; Moayedi, S.; Shamsoddin, E.; Vahedi, Z.; Banakar, M.H.; Mousavi, S.M.; Rokaya, D.; Bagheri Lankarani, K. Chewing Gums as a Drug Delivery Approach for Oral Health. Int. J. Dent. 2022, 2022, 9430988. [Google Scholar] [CrossRef] [PubMed]
- Marcal, F.F.; Mota de Paulo, J.P.; Barreto, L.G.; de Carvalho Guerra, L.M.; Silva, P.G.B. Effectiveness of orthodontic toothbrush versus conventional toothbrush on plaque and gingival index reduction: A systematic review and meta-analysis. Int. J. Dent. Hyg. 2022, 20, 87–99. [Google Scholar] [CrossRef]
- Veynachter, T.; Orti, V.; Moulis, E.; Rousseau, H.; Thilly, N.; Anagnostou, F.; Jeanne, S.; Bisson, C. Prevalence and associated factors of self-reported gingival bleeding: A multicenter study in France. Int. J. Environ. Res. Public Health 2020, 17, 8563. [Google Scholar] [CrossRef]
- Chiarotto, A.; Maxwell, L.J.; Ostelo, R.W.; Boers, M.; Tugwell, P.; Terwee, C.B. Measurement properties of Visual Analogue Scale, Numeric Rating Scale, and Pain Severity Subscale of the Brief Pain Inventory in patients with low back pain: A systematic review. J. Pain 2019, 20, 245–263. [Google Scholar] [CrossRef]
- Sivanandy, P.; Leey, T.C.; Xiang, T.C.; Ling, T.C.; Wey Han, S.A.; Semilan, S.L.A.; Hong, P.K. Systematic review on Parkinson’s disease medications, emphasizing on three recently approved drugs to control Parkinson’s symptoms. Int. J. Environ. Res. Public Health 2021, 19, 364. [Google Scholar] [CrossRef] [PubMed]
- Istituto Superiore di Sanità. VigiErbe. Available online: https://www.vigierbe.it (accessed on 1 December 2023).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. arXiv 2014, arXiv:1406.5823. [Google Scholar]
- R Core Team. A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2022. Available online: https://www.r-project.org/ (accessed on 9 December 2023).
- Calvert, M.; Blazeby, J.; Altman, D.G.; Revicki, D.A.; Moher, D.; Brundage, M.D.; CONSORT PRO Group. Reporting of patient-reported outcomes in randomized trials: The CONSORT PRO extension. JAMA 2013, 309, 814–822. [Google Scholar] [CrossRef]
- Bamashmous, S.; Kotsakis, G.A.; Kerns, K.A.; Leroux, B.G.; Zenobia, C.; Chen, D.; Trivedi, H.M.; McLean, J.S.; Darveau, R.P. Human variation in gingival inflammation. Proc. Natl. Acad. Sci. USA 2021, 118, e2012578118. [Google Scholar] [CrossRef]
- Trombelli, L.; Farina, R. A review of factors influencing the incidence and severity of plaque-induced gingivitis. Minerva Stomatol. 2013, 62, 207–234. [Google Scholar]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yu, Y.; Lin, X.L.; Zhang, T.; Huang, J.L.; Xiao, L.; Liang, M.; Wang, Y.F.; Qi, J. Efficacy confirmation of Scutellaria baicalensis Georgi in the treatment of periodontitis via topical administration and active ingredients screening. J. Ethnopharmacol. 2023, 300, 115699. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, S.; Sun, J.; Shi, J.; Li, Y.; Gou, J.; Li, A.; He, L. Screening of osteoanagenesis-active compounds from Scutellaria baicalensis Georgi by hPDLC/CMC-online-HPLC/MS. Fitoterapia 2014, 93, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Qasim, S.S.B.; Al-Otaibi, D.; Al-Jasser, R.; Gul, S.S.; Zafar, M.S. An evidence-based update on the molecular mechanisms underlying periodontal diseases. Int. J. Mol. Sci. 2020, 21, 3829. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.R.; Nam, S.H. The effects of Scutellariae radix extract gargling solution on the prevention of periodontal disease. Biomed. Res. 2017, 28, 557–562. [Google Scholar]
- Ren, M.; Zhao, Y.; He, Z.; Lin, J.; Xu, C.; Liu, F.; Hu, R.; Deng, H.; Wang, Y. Baicalein inhibits inflammatory response and promotes osteogenic activity in periodontal ligament cells challenged with lipopolysaccharides. BMC Complement. Med. Ther. 2021, 21, 43. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Huang, Q.; Huang, B.; Lu, F. The effect of Scutellaria baicalensis Georgi on immune response in mouse model of experimental periodontitis. J. Dent. Sci. 2013, 8, 405–411. [Google Scholar] [CrossRef][Green Version]
- Eastcott, J.W.; Yamashita, K.; Taubman, M.A.; Harada, Y.; Smith, D.J. Adoptive transfer of cloned T helper cells ameliorates periodontal disease in nude rats. Oral Microbiol. Immunol. 1994, 9, 284–289. [Google Scholar] [CrossRef]
- Guzmán, B.; Vargas, P. Systematics, character evolution, and biogeography of Cistus L. (Cistaceae) based on ITS, trnL-trnF, and matK sequences. Mol. Phylogenet. Evol. 2005, 37, 644–660. [Google Scholar] [CrossRef]
- Zalegh, I.; Akssira, M.; Bourhia, M.; Mellouki, F.; Rhallabi, N.; Salamatullah, A.M.; Alkaltham, M.S.; Khalil Alyahya, H.; Mhand, R.A. A review on Cistus sp.: Phytochemical and antimicrobial activities. Plants 2021, 10, 1214. [Google Scholar] [CrossRef]
- Móricz, Á.; Szeremeta, D.; Knas, M.; Długosz, E.; Ott, P.; Kowalska, T.; Sajewicz, M. Antibacterial potential of the Cistus incanus L. phenolics as studied with use of thin-layer chromatography combined with direct bioautography and in situ hydrolysis. J. Chromatogr. A 2017, 1534, 170–178. [Google Scholar] [CrossRef]
- Hannig, C.; Spitzmuller, B.; Al-Ahmad, A.; Hannig, M. Effects of Cistus-tea on bacterial colonization and enzyme activities of the in situ pellicle. J. Dent. 2008, 36, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Ma, Q.; Li, Y.; Zou, J. Molecular and regulatory mechanisms of oxidative stress adaptation in Streptococcus mutans. Mol. Oral Microbiol. 2023, 38, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hannig, C.; Sorg, J.; Spitzmuller, B.; Hannig, M.; Al-Ahmad, A. Polyphenolic beverages reduce initial bacterial adherence to enamel in situ. J. Dent. 2009, 37, 560–566. [Google Scholar] [CrossRef]
- Petracca, F.; Tempre, R.; Cucciniello, M.; Ciani, O.; Pompeo, E.; Sannino, L.; Lovato, V.; Castaman, G.; Ghirardini, A.; Tarricone, R. An electronic patient-reported outcome mobile app for data collection in type A hemophilia: Design and usability study. JMIR Form. Res. 2021, 5, e25071. [Google Scholar] [CrossRef]
- Tosetto, A.; Rodeghiero, F.; Castaman, G.; Goodeve, A.; Federici, A.B.; Batlle, J.; Meyer, D.; Fressinaud, E.; Mazurier, C.; Goudemand, J.; et al. A quantitative analysis of bleeding symptoms in type 1 von Willebrand disease: Results from a multicenter European study (MCMDM-1 VWD). J. Thromb. Haemost. 2006, 4, 766–773. [Google Scholar] [CrossRef] [PubMed]

| Characteristics of Enrolled Subjects | Group 1, Placebo (n = 30) | Group 2, Supplement (n = 30) |
|---|---|---|
| Ethnicity | ||
| Caucasian | 30 | 30 |
| Gender | ||
| Males (n) | 13 | 17 |
| Females (n) | 17 | 13 |
| Age (years) | ||
| Males | 35.8 ± 12.0 | 51.5 ± 12.1 |
| Females | 37.5 ± 11.8 | 47.3 ± 16.5 |
| Median age (years, range) | 38.5 ± 14.1 (20–68) | 48.5 ± 14.4 (22–68) |
| Variable | Placebo Group (n = 30) | Treatment Group (n = 30) | ||||||
|---|---|---|---|---|---|---|---|---|
| t0 | t1 | t2 | t3 | t0 | t1 | t2 | t3 | |
| QGBI | 1.9 ± 0.8 | 1.7 ± 0.8 | 1.7 ± 0.7 | 2.0 ± 0.8 | 2.3 ± 0.9 | 1.9 ± 0.7 | 1.7 ± 0.7 | 1.1 ± 0.7 |
| 0–3 | 0–3 | 0–3 | 0–3 | 0–3 | 0–3 | 0–3 | 0–3 | |
| MGI | 2.0 ± 0.7 | 1.9 ± 0.7 | 1.9 ± 0.7 | 2.0 ± 0.7 | 1.8 ± 1.0 | 1.6 ± 0.9 | 1.2 ± 0.7 | 1.1 ± 0.7 |
| 1–3 | 1–3 | 1–3 | 1–3 | 0–3 | 0–3 | 0–2 | 0–3 | |
| 0H-15 | 36.1 ± 10.6 | 36.8 ± 10.1 | 36.8 ± 10.1 | 38.5 ± 10.2 | 34.8 ± 12.2 | 33.4 ± 11.6 | 31.8 ± 10.9 | 30.5 ± 11.3 |
| 16–50 | 18–51 | 17–49 | 18–51 | 17–54 | 17–52 | 16–50 | 16–53 | |
| VAS | 4.1 ± 2.0 | 4.8 ± 1.8 | 5.2 ± 1.9 | 6.0 ± 2.0 | 4.1 ± 2.3 | 3.4 ± 1.9 | 3.0 ± 1.7 | 2.5 ± 1.7 |
| 1–8 | 1–8 | 2–8 | 2–8 | 0–8 | 0–7 | 0–7 | 0–7 | |
| CGI-S | 3.2 ± 1.3 | 3.0 ± 0.9 | 3.3 ± 1.0 | 3.7 ± 1.2 | 3.3 ± 1.4 | 3.0 ± 1.2 | 2.5 ± 1.1 | 2.4 ± 1.1 |
| 1–6 | 1–5 | 1–5 | 1–6 | 1–6 | 1–5 | 1–6 | 1–5 | |
| Model | GDL NUM | GDL ON | F | p Value |
|---|---|---|---|---|
| QGBI | ||||
| Measure | 3 | 174 | 10.93 | <0.001 |
| Group | 1 | 56 | 0.179 | 0.67 |
| Sex | 1 | 56 | 0.222 | 0.64 |
| Age | 1 | 56 | 0.156 | 0.69 |
| Size x Group | 3 | 174 | 18.48 | <0.001 |
| MGI | ||||
| Measure | 3 | 174 | 4.663 | 0.0036 |
| Group | 1 | 56 | 7.889 | 0.0068 |
| Sex | 1 | 56 | 0.120 | 0.73 |
| Age | 1 | 56 | 0.970 | 0.33 |
| Size x Group | 3 | 174 | 6.054 | <0.001 |
| OH-15 (relative) | ||||
| Measure | 2 | 172 | 0.113 | 0.89 |
| Group | 1 | 172 | 5.412 | 0.021 |
| Sex | 1 | 172 | 3.876 | 0.051 |
| Age | 1 | 172 | 5.278 | 0.023 |
| Size x Group | 2 | 172 | 1.063 | 0.35 |
| Model | GDL NUM | GDL ON | F | p |
|---|---|---|---|---|
| VAS | ||||
| Measure | 3 | 174 | 0.413 | 0.74 |
| Group | 1 | 56 | 9.769 | 0.0028 |
| Sex | 1 | 56 | 2.957 | 0.091 |
| Age | 1 | 56 | 3.668 | 0.061 |
| Size x Group | 3 | 174 | 31.72 | <0.001 |
| CGI-S | ||||
| Measure | 3 | 174 | 3.028 | 0.031 |
| Group | 1 | 56 | 1.854 | 0.18 |
| Sex | 1 | 56 | 0.107 | 0.74 |
| Age | 1 | 56 | 0.692 | 0.41 |
| Size x Group | 3 | 174 | 14.49 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Minno, A.; Ullah, H.; De Lellis, L.F.; Buccato, D.G.; Baldi, A.; Cuomo, P.; El-Seedi, H.R.; Khalifa, S.A.M.; Xiao, X.; Piccinocchi, R.; et al. Efficacy and Tolerability of a Scutellaria lateriflora L. and Cistus × incanus L.-Based Chewing Gum on the Symptoms of Gingivitis: A Monocentric, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2024, 16, 862. https://doi.org/10.3390/nu16060862
Di Minno A, Ullah H, De Lellis LF, Buccato DG, Baldi A, Cuomo P, El-Seedi HR, Khalifa SAM, Xiao X, Piccinocchi R, et al. Efficacy and Tolerability of a Scutellaria lateriflora L. and Cistus × incanus L.-Based Chewing Gum on the Symptoms of Gingivitis: A Monocentric, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients. 2024; 16(6):862. https://doi.org/10.3390/nu16060862
Chicago/Turabian StyleDi Minno, Alessandro, Hammad Ullah, Lorenza Francesca De Lellis, Daniele Giuseppe Buccato, Alessandra Baldi, Paola Cuomo, Hesham R. El-Seedi, Shaden A. M. Khalifa, Xiang Xiao, Roberto Piccinocchi, and et al. 2024. "Efficacy and Tolerability of a Scutellaria lateriflora L. and Cistus × incanus L.-Based Chewing Gum on the Symptoms of Gingivitis: A Monocentric, Randomized, Double-Blind, Placebo-Controlled Clinical Trial" Nutrients 16, no. 6: 862. https://doi.org/10.3390/nu16060862
APA StyleDi Minno, A., Ullah, H., De Lellis, L. F., Buccato, D. G., Baldi, A., Cuomo, P., El-Seedi, H. R., Khalifa, S. A. M., Xiao, X., Piccinocchi, R., Piccinocchi, G., Sacchi, R., & Daglia, M. (2024). Efficacy and Tolerability of a Scutellaria lateriflora L. and Cistus × incanus L.-Based Chewing Gum on the Symptoms of Gingivitis: A Monocentric, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients, 16(6), 862. https://doi.org/10.3390/nu16060862








