The Interplay between Gut Microbiota and Cognitive Functioning in the Healthy Aging Population: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Process and Risk of Bias
3. Results
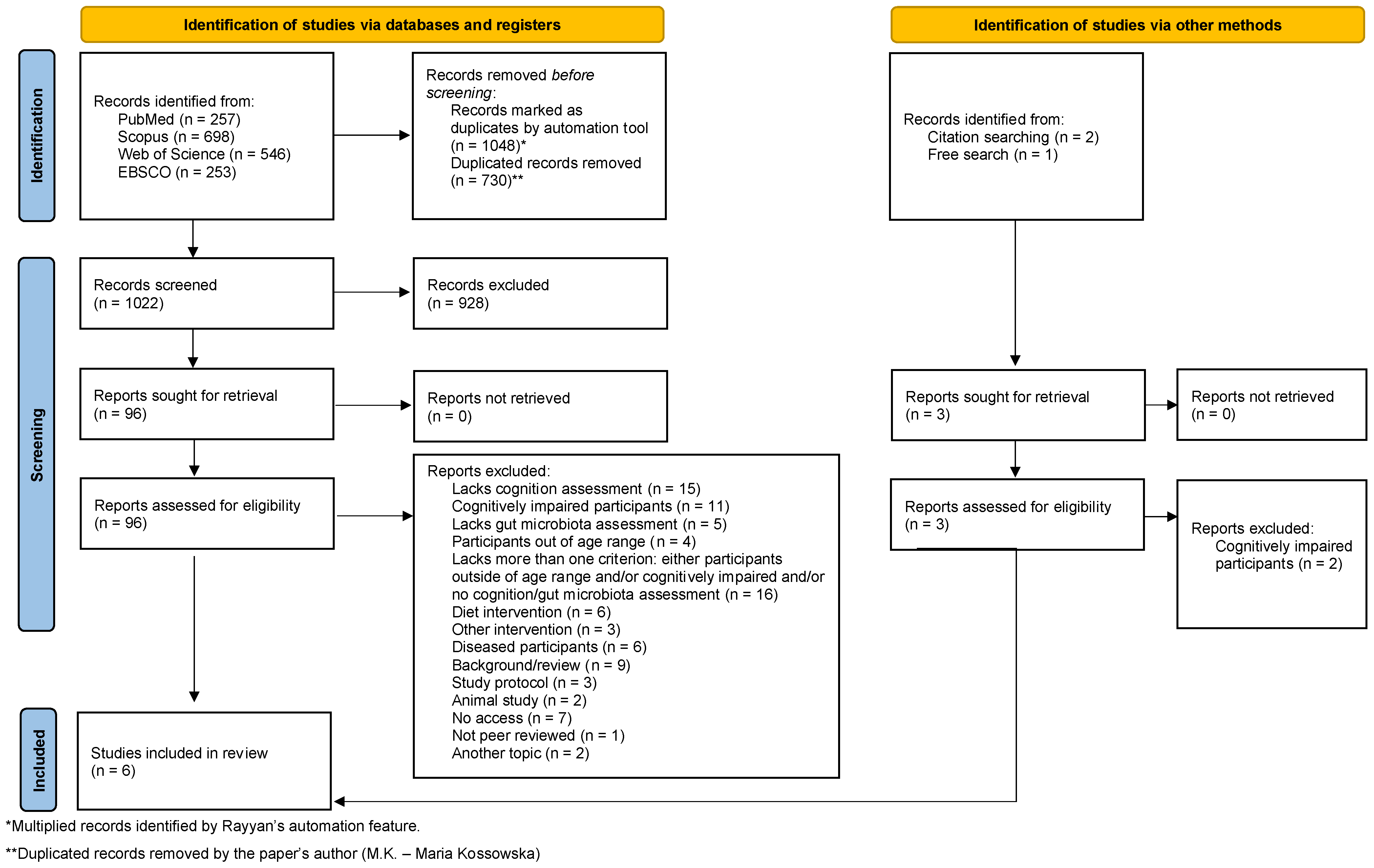
3.1. Study Selection
3.2. Study Characteristics
3.3. Heterogeneity
4. Findings
4.1. Microbiota Composition and Behavioral Tests
4.2. Alpha Diversity and Behavioural Tests
4.3. Alpha Diversity and Physiological Measurements
5. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, F.; Fei, M.; Hu, W.Z.; Wang, X.D.; Liu, S.; Zeng, Y.; Zhang, J.H.; Lv, Y.; Niu, J.P.; Meng, X.L.; et al. Prevalence of Constipation in Elderly and Its Association with Dementia and Mild Cognitive Impairment: A Cross-Sectional Study. Front. Neurosci. 2021, 15, 821654. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, L.; Cantoni, C.; Rotondo, E.; Galimberti, D. The Gut Microbiome–Brain Crosstalk in Neurodegenerative Diseases. Biomedicines 2022, 10, 1486. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.; Fonseca, S.; Carding, S.R. Gut Microbes and Metabolites as Modulators of Blood-Brain Barrier Integrity and Brain Health. Gut Microbes 2019, 11, 135–157. [Google Scholar] [CrossRef] [PubMed]
- Noble, E.E.; Hsu, T.M.; Kanoski, S.E. Gut to Brain Dysbiosis: Mechanisms Linking Western Diet Consumption, the Microbiome, and Cognitive Impairment. Front. Behav. Neurosci. 2017, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Knox, E.G.; Aburto, M.R.; Clarke, G.; Cryan, J.F.; O’Driscoll, C.M. The Blood-Brain Barrier in Aging and Neurodegeneration. Mol. Psychiatry 2022, 27, 2659–2673. [Google Scholar] [CrossRef]
- Li, H.; Ni, J.; Qing, H. Gut Microbiota: Critical Controller and Intervention Target in Brain Aging and Cognitive Impairment. Front. Aging Neurosci. 2021, 13, 671142. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. Human Gut Microbial Gene Catalogue Established by Metagenomic Sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean Diet Intervention Alters the Gut Microbiome in Older People Reducing Frailty and Improving Health Status: The NU-AGE 1-Year Dietary Intervention across Five European Countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef]
- Kendig, M.D.; Leigh, S.-J.; Morris, M.J. Unravelling the Impacts of Western-Style Diets on Brain, Gut Microbiota and Cognition. Neurosci. Biobehav. Rev. 2021, 128, 233–243. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Human Gut Microbiota/Microbiome in Health and Diseases: A Review. Antonie Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Dalton, A.; Mermier, C.; Zuhl, M. Exercise Influence on the Microbiome–Gut–Brain Axis. Gut Microbes 2019, 10, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Shandilya, S.; Kumar, S.; Kumar Jha, N.; Kumar Kesari, K.; Ruokolainen, J. Interplay of Gut Microbiota and Oxidative Stress: Perspective on Neurodegeneration and Neuroprotection. J. Adv. Res. 2021, 38, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.-Z.; Abe, F.; Osawa, R. Age-Related Changes in Gut Microbiota Composition from Newborn to Centenarian: A Cross-Sectional Study. BMC Microbiol. 2016, 16, 90. [Google Scholar] [CrossRef]
- Yu, H.J.; Jing, C.; Xiao, N.; Zang, X.M.; Zhang, C.Y.; Zhang, X.; Qu, Y.-N.; Li, Y.; Tan, Q.W. Structural Difference Analysis of Adult’s Intestinal Flora Basing on the 16S rDNA Gene Sequencing Technology. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 12983–12992. [Google Scholar] [CrossRef] [PubMed]
- Komanduri, M.; Gondalia, S.; Scholey, A.; Stough, C. The Microbiome and Cognitive Aging: A Review of Mechanisms. J. Psychopharmacol. 2019, 236, 1559–1571. [Google Scholar] [CrossRef]
- Alsegiani, A.S.; Shah, Z.A. The Influence of Gut Microbiota Alteration on Age-Related Neuroinflammation and Cognitive Decline. Neural Regen. Res. 2022, 17, 2407–2412. [Google Scholar] [CrossRef]
- Belblidia, H.; Leger, M.; Abdelmalek, A.; Quiedeville, A.; Calocer, F.; Boulouard, M.; Jozet-Alves, C.; Freret, T.; Schumann-Bard, P. Characterizing Age-Related Decline of Recognition Memory and Brain Activation Profile in Mice. Exp. Gerontol. 2018, 106, 222–231. [Google Scholar] [CrossRef]
- Morris, J.C.; Storandt, M.; Miller, J.P.; McKeel, D.W.; Price, J.L.; Rubin, E.H.; Berg, L. Mild Cognitive Impairment Represents Early-Stage Alzheimer Disease. Arch. Neurol. 2001, 58, 397–405. [Google Scholar] [CrossRef]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut Microbiome Alterations in Alzheimer’s Disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Stadlbauer, V.; Engertsberger, L.; Komarova, I.; Feldbacher, N.; Leber, B.; Pichler, G.; Fink, N.; Scarpatetti, M.; Schippinger, W.; Schmidt, R.; et al. Dysbiosis, Gut Barrier Dysfunction and Inflammation in Dementia: A Pilot Study. BMC Geriatr. 2020, 20, 248. [Google Scholar] [CrossRef]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C.; et al. Association of Brain Amyloidosis with Pro-Inflammatory Gut Bacterial Taxa and Peripheral Inflammation Markers in Cognitively Impaired Elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Christensen, H.; Kumar, R. Cognitive changes and the ageing. In The Ageing Brain; Sachdve, P.S., Ed.; Swets and Zeitlinger: Lisse, The Netherlands, 2003; Available online: https://jbi-global-wiki.refined.site/space/MANUAL/4689528/7.1+Introduction+to+etiological+evidence+and+systematic+reviews (accessed on 26 January 2024).
- Freedman, V.A.; Cornman, J.C.; Kasper, J.D. National Health and Aging Trends Study Trends Chart Book: Key Trends, Measures and Detailed Tables. 2021. Available online: https://micda.isr.umich.edu/wp-content/uploads/2022/03/NHATS-Companion-Chartbook-to-Trends-Dashboards-2020.pdf (accessed on 28 June 2023).
- Białecka-Dębek, A.; Granda, D.; Szmidt, M.K.; Zielińska, D. Gut Microbiota, Probiotic Interventions, and Cognitive Function in the Elderly: A Review of Current Knowledge. Nutrients 2021, 13, 2514. [Google Scholar] [CrossRef] [PubMed]
- Badal, V.D.; Vaccariello, E.D.; Murray, E.R.; Yu, K.E.; Knight, R.; Jeste, D.V.; Nguyen, T.T. The Gut Microbiome, Aging, and Longevity: A Systematic Review. Nutrients 2020, 12, 3759. [Google Scholar] [CrossRef]
- Ticinesi, A.; Tana, C.; Nouvenne, A. The Intestinal Microbiome and Its Relevance for Functionality in Older Persons. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Systematic Reviews of Etiology and Risk. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; Joanna Briggs Institute (JBI): Adelaide, Australia, 2020; pp. 217–269. [Google Scholar] [CrossRef]
- Anderson, J.R.; Carroll, I.; Azcarate-Peril, M.A.; Rochette, A.D.; Heinberg, L.J.; Peat, C.; Steffen, K.; Manderino, L.M.; Mitchell, J.; Gunstad, J. A Preliminary Examination of Gut Microbiota, Sleep, and Cognitive Flexibility in Healthy Older Adults. Sleep. Med. 2017, 38, 104–107. [Google Scholar] [CrossRef]
- Canipe, L.G.; Sioda, M.; Cheatham, C.L. Diversity of the Gut-Microbiome Related to Cognitive Behavioral Outcomes in Healthy Older Adults. Arch. Gerontol. Geriatr. 2021, 96, 104464. [Google Scholar] [CrossRef]
- Haimov, I.; Magzal, F.; Tamir, S.; Lalzar, M.; Asraf, K.; Milman, U.; Agmon, M.; Shochat, T. Variation in Gut Microbiota Composition Is Associated with Sleep Quality and Cognitive Performance in Older Adults with Insomnia. Nat. Sci. Sleep. 2022, 14, 1753–1767. [Google Scholar] [CrossRef]
- Komanduri, M.; Savage, K.; Lea, A.; McPhee, G.; Nolidin, K.; Deleuil, S.; Stough, C.; Gondalia, S. The relationship between gut microbiome and cognition in older Australians. Nutrients 2021, 14, 64. [Google Scholar] [CrossRef]
- Manderino, L.; Carroll, I.; Azcarate-Peril, M.A.; Rochette, A.; Heinberg, L.; Peat, C.; Steffen, K.; Mitchell, J.; Gunstad, J. Preliminary Evidence for an Association between the Composition of the Gut Microbiome and Cognitive Function in Neurologically Healthy Older Adults. J. Int. Neuropsychol. Soc. 2017, 23, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Verdi, S.; Jackson, M.A.; Beaumont, M.; Bowyer, R.C.E.; Bell, J.T.; Spector, T.D.; Steves, C.J. An Investigation into Physical Frailty as a Link between the Gut Microbiome and Cognitive Health. Front. Aging Neurosci. 2018, 10, 398. [Google Scholar] [CrossRef]
- Rosell-Díaz, M.; Santos-González, E.; Motger-Albertí, A.; Ramió-Torrentà, L.; Garre-Olmo, J.; Pérez-Brocal, V.; Moya, A.; Jové, M.; Pamplona, R.; Puig, J.; et al. Gut microbiota links to serum ferritin and cognition. Gut Microbes 2023, 5, 2290318. [Google Scholar] [CrossRef]
- Renson, A.; Kasselman, L.J.; Dowd, J.B.; Waldron, L.; Jones, H.E.; Herd, P. Gut bacterial taxonomic abundances vary with cognition, personality, and mood in the Wisconsin Longitudinal Study. BBI-Health 2020, 9, 100155. [Google Scholar] [CrossRef]
- Fernandez-Real, J.M.; Serino, M.; Blasco, G.; Puig, J.; Daunis-i-Estadella, J.; Ricart, W.; Burcelin, R.; Fernández-Aranda, F.; Portero-Otin, M. Gut microbiota interacts with brain microstructure and function. J. Clin. Endocrinol. Metab. 2015, 100, 4505–4513. [Google Scholar] [CrossRef] [PubMed]
- Orimo, H.; Ito, H.; Suzuki, T.; Araki, A.; Hosoi, T.; Sawabe, M. Reviewing the Definition of “Elderly”. Geriatr. Gerontol. Int. 2006, 6, 149–158. [Google Scholar] [CrossRef]
- Damian, A.M.; Jacobson, S.A.; Hentz, J.G.; Belden, C.M.; Shill, H.A.; Sabbagh, M.N.; Caviness, J.N.; Adler, C.H. The Montreal Cognitive Assessment and the Mini-mental State Examination as Screening Instruments for Cognitive Impairment: Item Analyses and Threshold Scores. Dement. Geriatr. Cogn. Disord. 2011, 31, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Luis, C.A.; Keegan, A.P.; Mullan, M. Cross Validation of the Montreal Cognitive Assessment in Community Dwelling Older Adults Residing in the Southeastern US. Int. J. Geriatr. Psychiatry 2009, 24, 197–201. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L., IV; Mazmanian, S.K. The gut microbiota–brain axis in behavior and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Bai, S.; Bai, H.; Li, D.; Zhong, Q.; Xie, J.; Chen, J.J. Gut microbiota-related inflammation factors as a potential biomarker for diagnosing major depressive disorder. Front. Cell. Infect. Microbiol. 2022, 12, 831186. [Google Scholar] [CrossRef]
- Molinero, N.; Antón, A.; Hernández, F.; Ávila, J.; Bartolomé, B.; Moreno-Arribas, M.V. Gut microbiota, an Additional Hallmark of Human Aging and Neurodegeneration. Neuroscience 2023, 518, 141–161. [Google Scholar] [CrossRef]
- Oren, A.; Garrity, G.M. Valid publication of the names of forty-two phyla of prokaryotes. IJSEM 2021, 71, 005056. [Google Scholar] [CrossRef]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef]
- Ling, Z.; Zhu, M.; Yan, X.; Cheng, Y.; Shao, L.; Liu, X.; Jiang, R.; Wu, S. Structural and Functional Dysbiosis of Fecal Microbiota in Chinese Patients with Alzheimer’s Disease. Front. Cell Dev. Biol. 2021, 8, 634069. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Yang, Y.; Xu, Q.; Wang, S.; Yu, J.; Zhang, B.; Wang, Z.; Zhang, Y.; Lu, W.; Hong, K. Gut Microbiota and Targeted Biomarkers Analysis in Patients with Cognitive Impairment. Front. Neurol. 2022, 13, 834403. [Google Scholar] [CrossRef]
- Saji, N.; Niida, S.; Murotani, K.; Hisada, T.; Tsuduki, T.; Sugimoto, T.; Kimura, A.; Toba, K.; Sakurai, T. Analysis of the Relationship between the Gut Microbiome and Dementia: A Cross-Sectional Study Conducted in Japan. Sci. Rep. 2019, 9, 1008. [Google Scholar] [CrossRef] [PubMed]
- Bairamian, D.; Sha, S.; Rolhion, N.; Sokol, H.; Dorothée, G.; Lemere, C.A.; Krantic, S. Microbiota in Neuroinflammation and Synaptic Dysfunction: A Focus on Alzheimer’s Disease. Mol. Neurodegener. 2022, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Arevalo-Rodriguez, I.; Smailagic, N.; Roqué i Figuls, M.; Ciapponi, A.; Sanchez-Perez, E.; Giannakou, A.; Pedraza, O.L.; Bonfill Cosp, X.; Cullum, S. Mini-Mental State Examination (MMSE) for the Detection of Alzheimer’s Disease and Other Dementias in People with Mild Cognitive Impairment (MCI). Cochrane Database Syst. Rev. 2015, 3, CD010783. [Google Scholar] [CrossRef]
- Hasan, N.; Yang, H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ 2019, 7, e7502. [Google Scholar] [CrossRef] [PubMed]
| Citation [Reference] | |||||||
|---|---|---|---|---|---|---|---|
| Item No | JBI Item | Anderson et al., 2017 [30] | Canipe et al., 2021 [31] | Haimov et al., 2022 [32] | Komanduri et al., 2021 [33] | Manderino et al., 2017 [34] | Verdi et al., 2018 [35] |
| 1. | Were the criteria for inclusion in the sample clearly defined? | Yes | Yes | Yes | Yes | Yes | Yes |
| 2. | Were the study subjects and the setting described in detail? | Unclear | Yes | Yes | Yes | Yes | Yes |
| 3. | Was the exposure measured in a valid and reliable way? | Not Applicable | Not Applicable | Not Applicable | Not Applicable | Not Applicable | Not Applicable |
| 4. | Were objective, standard criteria used for measurement of the condition? | Yes | Yes | Yes | Yes | Yes | Unclear |
| 5. | Were confounding factors identified? | Yes | Yes | Yes | Yes | Yes | Yes |
| 6. | Were strategies to deal with confounding factors stated? | Yes | Yes | Yes | Yes | Yes | Yes |
| 7. | Were the outcomes measured in a valid and reliable way? | Yes | Yes | Yes | Yes | Yes | Yes |
| 8. | Was appropriate statistical analysis used? | Yes | Yes | Yes | Yes | Yes | Yes |
| Overall Appraisal | Include | Include | Include | Include | Include | Include | |
| Country [Reference] | N, % Sex, Nationality | Age | Cognitive Function Assessment (Score) | Cognitive Test | Microbiome Assessment |
|---|---|---|---|---|---|
| USA [30] | 37, 73% female | 64.59 ± 7.54 | Stroop Word (48.51 ± 6.71) Stroop Color (48.30 ± 6.87) Stroop Color-Word subset (51.22 ± 10.22) | Stroop Word, Stroop Color, Stroop-Color-Word subset | Fecal samples, bacterial 16S rRNA |
| Southeastern US [31] | 63, 43.27% male | 74.63 ± 4.26 | MoCA (26.21 ± 4.16) | ERP active discrimination, ERP passive oddball, CANTAB | Fecal samples, bacterial 16S rRNA |
| Israel [32] | 72, 77.77% female | 73.19 ± 5.73 | MMSE (>26) | CANTAB | Fecal samples, bacterial 16S rRNA |
| Australia [33] | 69, 49% male | 65.06 ± 4.01 | MMSE (28.78 ± 1.29) | QESM, QWM, PoC, CoA, SoM | Fecal samples, bacterial 16S rRNA |
| USA [34] | 43, Intact 32% female, Impaired 33.3% female | Intact 64.08 ± 6.49, Impaired 64.06 ± 9.37 | MMSE (Intact 29.28 ± 0.98, Impaired 28.00 ± 1.85) | FAB, TMT-A, TMT-B, SCWT, HVLT-R, ROCF, verbal fluency, animal naming | Fecal samples, bacterial 16S rRNA |
| UK [35] | 1551, 90% female | 63 (40–89) | MMSE (mean 29) | verbal fluency, DLRT, CANTAB-PAL | Fecal samples, bacterial 16S rRNA |
| Country [Reference] | Taxonomic Composition/Diversity Pattern | Cognitive Functions/Psychophysiological Measures |
|---|---|---|
| Microbiota composition/Alpha diversity and behavioral tests | ||
| USA Anderson et al., 2017 [30] | ↑ Verrucomicrobia ↑ Verrucomicrobia | ↑ Stroop Word |
| ↑ Stroop Color | ||
| ↑ Lentisphaerae | ↑ Stroop Color-Word subset | |
| Israel Haimov et al., 2022 [32] | ↑ Lachnospiraceae (Firmicutes) | ↑ SWM (less SWMBE—Spatial Working Memory Between Errors) |
| ↓ Ruminococcus gauvreauii group (Firmicutes) | ||
| ↓ Propionibacteriaceae (Actinobacteria) | ||
| ↓ Tannerellaceae (Bacteroidetes) | ||
| ↓ Blautia (Firmicutes) | ↑ MTTLMD (Median Reaction Latency) | |
| ↓ Lachnospiraceae (Firmicutes) | ||
| Australia Komanduri et al., 2021 [33] | ↑ Carnobacteriaceae (Firmicutes) | ↑ QESM |
| ↑ Clostridiaceae (Firmicutes) | ↑ QWM | |
| ↑ Alcaligenacea (Proteobacteria) | ↓ QWM | |
| ↑ Bacteroidaceae, (Bacteroidetes) | ↑ PoC | |
| ↑ Barnesiellaceae (Bacteroidetes) | ||
| ↑ Gemellaceae (Firmicutes) | ||
| ↑ Rikenellaceae (Bacteroidetes) | ||
| ↑ Clostridiaceae (Firmicutes) | ↑ CoA | |
| ↑ Rikenellaceae (Bacteroidetes) | ||
| ↑ Verrucomicrobia | ↓ CoA | |
| ↑ Bacteroidaceae (Bacteroidetes) | ↑ SoM | |
| ↑ Barnesiellaceae (Bacteroidetes) | ||
| ↑ Gemellaceae (Firmicutes) | ||
| ↑ Micrococcaceae (Actinobacteria) | ||
| USA Manderino et al., 2017 [34] | ↑ Verrucomicrobia | ↑ TMT-A |
| ↑ TMT-B | ||
| ↑ SCWT Word | ||
| ↑ SCWT Color | ||
| ↑ HVLT-R Total Learning | ||
| ↑ Proterobacteria | ↓ FAB | |
| ↓ HVLT-R Recognition/Discrimination | ||
| ↓ FAS | ||
| ↑ Firmicutes | ↑ CFT Immediate and delayed recall | |
| ↑ Baceroidetes | ↓ CFT Immediate and delayed recall | |
| UK Verdi et al., 2018 [35] | ↑ alpha diversity | ↑ verbal fluency |
| ↓ alpha diversity | ↑ DLRT | |
| ↓ order: Burkholderiales, class: Betaproteobacteria (Proteobacteria) | ||
| Alpha diversity and physiological measurements | ||
| Southeastern US Canipe et al., 2021 [31] | ↑ alpha diversity | ↑ N1 minimum amplitude and mean amplitude (50–190 ms; frontal clusters; target condition) |
| ↓ alpha diversity | ↑ N2 latency to peak amplitude (200–350 ms frontal cluster; familiar condition) | |
| ↑ P3 maximum amplitude (350–1500 ms; temporal left cluster; familiar condition) | ||
| ↑ P3 mean amplitude (350–1500 ms; temporal left cluster; familiar condition | ||
| ↑ total errors PAL | ||
| ↑ mean time success SWM | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kossowska, M.; Olejniczak, S.; Karbowiak, M.; Mosiej, W.; Zielińska, D.; Brzezicka, A. The Interplay between Gut Microbiota and Cognitive Functioning in the Healthy Aging Population: A Systematic Review. Nutrients 2024, 16, 852. https://doi.org/10.3390/nu16060852
Kossowska M, Olejniczak S, Karbowiak M, Mosiej W, Zielińska D, Brzezicka A. The Interplay between Gut Microbiota and Cognitive Functioning in the Healthy Aging Population: A Systematic Review. Nutrients. 2024; 16(6):852. https://doi.org/10.3390/nu16060852
Chicago/Turabian StyleKossowska, Maria, Sylwia Olejniczak, Marcelina Karbowiak, Wioletta Mosiej, Dorota Zielińska, and Aneta Brzezicka. 2024. "The Interplay between Gut Microbiota and Cognitive Functioning in the Healthy Aging Population: A Systematic Review" Nutrients 16, no. 6: 852. https://doi.org/10.3390/nu16060852
APA StyleKossowska, M., Olejniczak, S., Karbowiak, M., Mosiej, W., Zielińska, D., & Brzezicka, A. (2024). The Interplay between Gut Microbiota and Cognitive Functioning in the Healthy Aging Population: A Systematic Review. Nutrients, 16(6), 852. https://doi.org/10.3390/nu16060852






