Course of Vitamin D Levels in Newly Diagnosed Non-Metastatic Breast Cancer Patients over One Year with Quarterly Controls and Substitution
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Statistics
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 25(OH)D | Serum 25-hydroxyvitamin D |
| BMI | Body mass index |
| HER2 | Human epidermal growth factor 2 |
| GEE | Generalized estimating equations analysis |
| CTC | Common toxicity criteria |
References
- World Health Organization. Obesity Report. Available online: https://Www.Who.Int/Data/Gho/Indicator-Metadata-Registry/Imr-Details/3420#:~:Text=The%20relationship%20between%20poor%20healthCaused%20by%20overweight%20or%20obesity (accessed on 15 June 2023).
- Gröber, U.; Holzhauer, P.; Kisters, K.; Holick, M.F.; Adamietz, I.A. Micronutrients in Oncological Intervention. Nutrients 2016, 8, 163. [Google Scholar] [CrossRef] [PubMed]
- Hintzpeter, B.; Mensink, G.B.M.; Thierfelder, W.; Müller, M.J.; Scheidt-Nave, C. Vitamin D Status and Health Correlates among German Adults. Eur. J. Clin. Nutr. 2008, 62, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Linseisen, J.; Bechthold, A.; Bischoff-Ferrari, H.; Hinzpeter, B.; Leschik-Bonnet, E.; Reichrath, J.; Stehle, P.; Volkert, D.; Wolfram, G.; Zittermann, A. Vitamin D und Prävention Ausgewählter Chronischer Krankheiten; Deutsche Gesellschaft für Ernährung eV: Bonn, Germany, 2011. [Google Scholar]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D Deficiency 2.0: An Update on the Current Status Worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Hoang, T.; Kim, J. Dietary Factors and Breast Cancer Prognosis among Breast Cancer Survivors: A Systematic Review and Meta-Analysis of Cohort Studies. Cancers 2021, 13, 5329. [Google Scholar] [CrossRef] [PubMed]
- Shao, T.; Klein, P.; Grossbard, M.L. Vitamin D and Breast Cancer. Oncologist 2012, 17, 36–45. [Google Scholar] [CrossRef]
- Vanhevel, J.; Verlinden, L.; Doms, S.; Wildiers, H.; Verstuyf, A. The Role of Vitamin D in Breast Cancer Risk and Progression. Endocr. Relat. Cancer 2022, 29, R33–R55. [Google Scholar] [CrossRef]
- de La Puente-Yagüe, M.; Cuadrado-Cenzual, M.A.; Ciudad-Cabañas, M.J.; Hernández-Cabria, M.; Collado-Yurrita, L. Vitamin D: And Its Role in Breast Cancer. Kaohsiung J. Med. Sci. 2018, 34, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Zemlin, C.; Altmayer, L.; Stuhlert, C.; Schleicher, J.T.; Wörmann, C.; Lang, M.; Scherer, L.-S.; Thul, I.C.; Spenner, L.S.; Simon, J.A.; et al. Prevalence and Relevance of Vitamin D Deficiency in Newly Diagnosed Breast Cancer Patients: A Pilot Study. Nutrients 2023, 15, 1450. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Cancer Stat Facts: Female Breast Cancer Subtypes. Available online: https://seer.cancer.gov/statfacts/html/breast-subtypes.html (accessed on 1 February 2024).
- Xu, J.; Cao, B.; Li, C.; Li, G. The Recent Progress of Endocrine Therapy-Induced Osteoporosis in Estrogen-Positive Breast Cancer Therapy. Front. Oncol. 2023, 13, 1218206. [Google Scholar] [CrossRef]
- Deroo, B.J. Estrogen Receptors and Human Disease. J. Clin. Investig. 2006, 116, 561–570. [Google Scholar] [CrossRef]
- Nisha, Y.; Dubashi, B.; Bobby, Z.; Sahoo, J.P.; Kayal, S.; Ananthakrishnan, R.; Ganesan, P. Cytotoxic Chemotherapy Is Associated with Decreased Bone Mineral Density in Postmenopausal Women with Early and Locally Advanced Breast Cancer. Arch. Osteoporos. 2023, 18, 41. [Google Scholar] [CrossRef]
- Jennaro, T.S.; Fang, F.; Kidwell, K.M.; Smith, E.M.L.; Vangipuram, K.; Burness, M.L.; Griggs, J.J.; Van Poznak, C.; Hayes, D.F.; Henry, N.L.; et al. Vitamin D Deficiency Increases Severity of Paclitaxel-Induced Peripheral Neuropathy. Breast Cancer Res. Treat. 2020, 180, 707–714. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Kok, D.E.; van den Berg, M.M.G.A.; Posthuma, L.; van ’t Erve, I.; van Duijnhoven, F.J.B.; de Roos, W.K.; Grosfeld, S.; Los, M.; Sommeijer, D.W.; van Laarhoven, H.W.M.; et al. Changes in Circulating Levels of 25-Hydroxyvitamin D3 in Breast Cancer Patients Receiving Chemotherapy. Nutr. Cancer 2019, 71, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Koh, B.S.; Yu, J.H.; Lee, J.W.; Son, B.H.; Kim, S.B.; Ahn, S.H. Changes in Serum Hydroxyvitamin D Levels of Breast Cancer Patients during Tamoxifen Treatment or Chemotherapy in Premenopausal Breast Cancer Patients. Eur. J. Cancer 2014, 50, 1403–1411. [Google Scholar] [CrossRef]
- Bleizgys, A. Vitamin D Dosing: Basic Principles and a Brief Algorithm (2021 Update). Nutrients 2021, 13, 4415. [Google Scholar] [CrossRef] [PubMed]
- Zemlin, C.; Stuhlert, C.; Schleicher, J.T.; Wörmann, C.; Altmayer, L.; Lang, M.; Scherer, L.S.; Thul, I.C.; Müller, C.; Kaiser, E.; et al. Longitudinal Assessment of Physical Activity, Fitness, Body Composition, Immunological Biomarkers, and Psychological Parameters during the First Year after Diagnosis in Women with Non-Metastatic Breast Cancer: The BEGYN Study Protocol. Front. Oncol. 2021, 11, 762709. [Google Scholar] [CrossRef] [PubMed]
- Hardin, J.W.; Hilbe, J.M. Generalized Estimating Equations; Chapman and Hall/CRC: Boca Raton, FL, USA, 2002; ISBN 9780429140976. [Google Scholar]
- National Cancer Institute, Division of Cancer Treatment & Diagnosis, Common Terminology Criteria for Adverse Events (CTCAE). Available online: https://ctep.cancer.gov/ (accessed on 13 February 2024).
- Souci, S.W.; Fachmann, W.; Kraut, H.; Andersen, G. Souci/Fachmann/Kraut Food Composition and Nutrition, 8th ed.; MedPharm: Stuttgart, Germany, 2008. [Google Scholar]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D Deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef]
- Schweizer, A.-M.; Leiderer, A.; Mitterwallner, V.; Walentowitz, A.; Mathes, G.H.; Steinbauer, M.J. Outdoor Cycling Activity Affected by COVID-19 Related Epidemic-Control-Decisions. PLoS ONE 2021, 16, e0249268. [Google Scholar] [CrossRef] [PubMed]
- Park, A.H.; Zhong, S.; Yang, H.; Jeong, J.; Lee, C. Impact of COVID-19 on Physical Activity: A Rapid Review. J. Glob. Health 2022, 12, 05003. [Google Scholar] [CrossRef]
- AGO Mamma Guidelines Breast Version 2023.1E Adjuvant Endocrine-Based Therapy in Pre- and Postmenopausal Patients. 2023. Available online: www.ago-online.de (accessed on 31 January 2024).
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early Breast Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Takacs, I.; Boyanov, M.; Belaya, Z.; Diaconu, C.C.; Mokhort, T.; Zherdova, N.; Rasa, I.; Payer, J.; Pilz, S. Clinical Practice in the Prevention, Diagnosis and Treatment of Vitamin D Deficiency: A Central and Eastern European Expert Consensus Statement. Nutrients 2022, 14, 1483. [Google Scholar] [CrossRef]
- Maeda, S.S.; Kunii, I.S.; Hayashi, L.; Lazaretti-Castro, M. The Effect of Sun Exposure on 25-Hydroxyvitamin D Concentrations in Young Healthy Subjects Living in the City of São Paulo, Brazil. Braz. J. Med. Biol. Res. 2007, 40, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased Bioavailability of Vitamin D in Obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Kweder, H.; Eidi, H. Vitamin D Deficiency in Elderly: Risk Factors and Drugs Impact on Vitamin D Status. Avicenna J. Med. 2018, 8, 139–146. [Google Scholar] [CrossRef] [PubMed]
- MacLaughlin, J.; Holick, M.F. Aging Decreases the Capacity of Human Skin to Produce Vitamin D3. J. Clin. Investig. 1985, 76, 1536–1538. [Google Scholar] [CrossRef]
- Levin, G.P.; Robinson-Cohen, C.; de Boer, I.H.; Houston, D.K.; Lohman, K.; Liu, Y.; Kritchevsky, S.B.; Cauley, J.A.; Tanaka, T.; Ferrucci, L.; et al. Genetic Variants and Associations of 25-Hydroxyvitamin D Concentrations With Major Clinical Outcomes. JAMA 2012, 308, 1898. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.S. Vitamin D Update. Curr. Dermatol. Rep. 2020, 9, 323–330. [Google Scholar] [CrossRef]
- Poskitt, E.M.E.; Cole, T.J.; Lawson, D.E.M. Diet, Sunlight, and 25-Hydroxy Vitamin D in Healthy Children and Adults. Br. Med. J. 1979, 1, 221–223. [Google Scholar] [CrossRef]
- Heidari, B.; Mirghassemi, M.B.H. Seasonal Variations in Serum Vitamin D According to Age and Sex. Casp. J. Intern. Med. 2012, 3, 535–540. [Google Scholar]
- Franca Gois, P.; Wolley, M.; Ranganathan, D.; Seguro, A. Vitamin D Deficiency in Chronic Kidney Disease: Recent Evidence and Controversies. Int. J. Environ. Res. Public Health 2018, 15, 1773. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.; Jabczyk, M.; Jagielski, P.; Hudzik, B.; Brukało, K.; Borszcz, J.; Zubelewicz-Szkodzińska, B. Could Vitamin D Concentration Be a Marker of a Long Hospital Stay in Older Adults Patients? Front. Nutr. 2023, 10, 1277350. [Google Scholar] [CrossRef]
- Harris, S.S.; Dawson-Hughes, B. Plasma Vitamin D and 25OHD Responses of Young and Old Men to Supplementation with Vitamin D. J. Am. Coll. Nutr. 2002, 21, 357–362. [Google Scholar] [CrossRef]
- Clemens, T.L.; Zhouf, X.-Y.; Myles, M.; Endres, D.; Linsay, R. Serum Vitamin D2 and Vitamin D3 Metabolite Concentrations and Absorption of Vitamin D2 in Elderly Subjects. J. Clin. Endocrinol. Metab. 1986, 63, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.S.; Swanson, B.J.; Larson-Meyer, D.E. Vitamin D Knowledge, Awareness, and Attitudes of Adolescents and Adults: A Systematic Review. J. Nutr. Educ. Behav. 2023, 55, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Borecka, O.; Farrar, M.D.; Osman, J.E.; Rhodes, L.E.; Webb, A.R. Older Adults Who Spend More Time Outdoors in Summer and Have Higher Dietary Vitamin D than Younger Adults Can Present at Least as High Vitamin D Status: A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 3364. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.C.; Yalamanchili, V.; Smith, L.M. The Effect of Vitamin D Supplementation on Serum 25OHD in Thin and Obese Women. J. Steroid Biochem. Mol. Biol. 2013, 136, 195–200. [Google Scholar] [CrossRef]
- Kim, M.R.; Jeong, S.J. Relationship between Vitamin D Level and Lipid Profile in Non-Obese Children. Metabolites 2019, 9, 125. [Google Scholar] [CrossRef]
- Uwitonze, A.M.; Razzaque, M.S. Role of Magnesium in Vitamin D Activation and Function. J. Am. Osteopath. Assoc. 2018, 118, 181. [Google Scholar] [CrossRef]
- Cheung, M.M.; Dall, R.D.; Shewokis, P.A.; Altasan, A.; Volpe, S.L.; Amori, R.; Singh, H.; Sukumar, D. The Effect of Combined Magnesium and Vitamin D Supplementation on Vitamin D Status, Systemic Inflammation, and Blood Pressure: A Randomized Double-Blinded Controlled Trial. Nutrition 2022, 99–100, 111674. [Google Scholar] [CrossRef]
- Dai, Q.; Zhu, X.; Manson, J.E.; Song, Y.; Li, X.; Franke, A.A.; Costello, R.B.; Rosanoff, A.; Nian, H.; Fan, L.; et al. Magnesium Status and Supplementation Influence Vitamin D Status and Metabolism: Results from a Randomized Trial. Am. J. Clin. Nutr. 2018, 108, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Goldvaser, H.; Barnes, T.A.; Šeruga, B.; Cescon, D.W.; Ocaña, A.; Ribnikar, D.; Amir, E. Toxicity of Extended Adjuvant Therapy With Aromatase Inhibitors in Early Breast Cancer: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2018, 110, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.V.; Swami, S.; Peng, L.; Wang, J.; Moreno, J.; Feldman, D. Tissue-Selective Regulation of Aromatase Expression by Calcitriol: Implications for Breast Cancer Therapy. Endocrinology 2010, 151, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Al Khalifah, R.; Alsheikh, R.; Alnasser, Y.; Alsheikh, R.; Alhelali, N.; Naji, A.; Al Backer, N. The Impact of Vitamin D Food Fortification and Health Outcomes in Children: A Systematic Review and Meta-Regression. Syst. Rev. 2020, 9, 144. [Google Scholar] [CrossRef]
- Nikooyeh, B.; Neyestani, T.R. The Effects of Vitamin D-Fortified Foods on Circulating 25(OH)D Concentrations in Adults: A Systematic Review and Meta-Analysis. Br. J. Nutr. 2022, 127, 1821–1838. [Google Scholar] [CrossRef] [PubMed]
- Niedermaier, T.; Gredner, T.; Kuznia, S.; Schöttker, B.; Mons, U.; Lakerveld, J.; Ahrens, W.; Brenner, H. Vitamin D Food Fortification in European Countries: The Underused Potential to Prevent Cancer Deaths. Eur. J. Epidemiol. 2022, 37, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Xiao, P.; Ma, Y.; Fan, Z.; Zhou, F.; Zheng, J.; Zhang, L. Prevalence, Trend, and Predictor Analyses of Vitamin D Deficiency in the US Population, 2001–2018. Front. Nutr. 2022, 9, 965376. [Google Scholar] [CrossRef] [PubMed]
- Marcinowska-Suchowierska, E.; Kupisz-Urbańska, M.; Łukaszkiewicz, J.; Płudowski, P.; Jones, G. Vitamin D Toxicity—A Clinical Perspective. Front. Endocrinol. 2018, 9, 550. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, G.; Ascenti, V.; Desiderio, E.; Carrafiello, G. Vitamin D Intoxication: Myth or Reality. Minerva Med. 2023, 114, S0026–S4806. [Google Scholar] [CrossRef] [PubMed]
- Šimoliūnas, E.; Rinkūnaitė, I.; Bukelskienė, Ž.; Bukelskienė, V. Bioavailability of Different Vitamin D Oral Supplements in Laboratory Animal Model. Medicina 2019, 55, 265. [Google Scholar] [CrossRef] [PubMed]
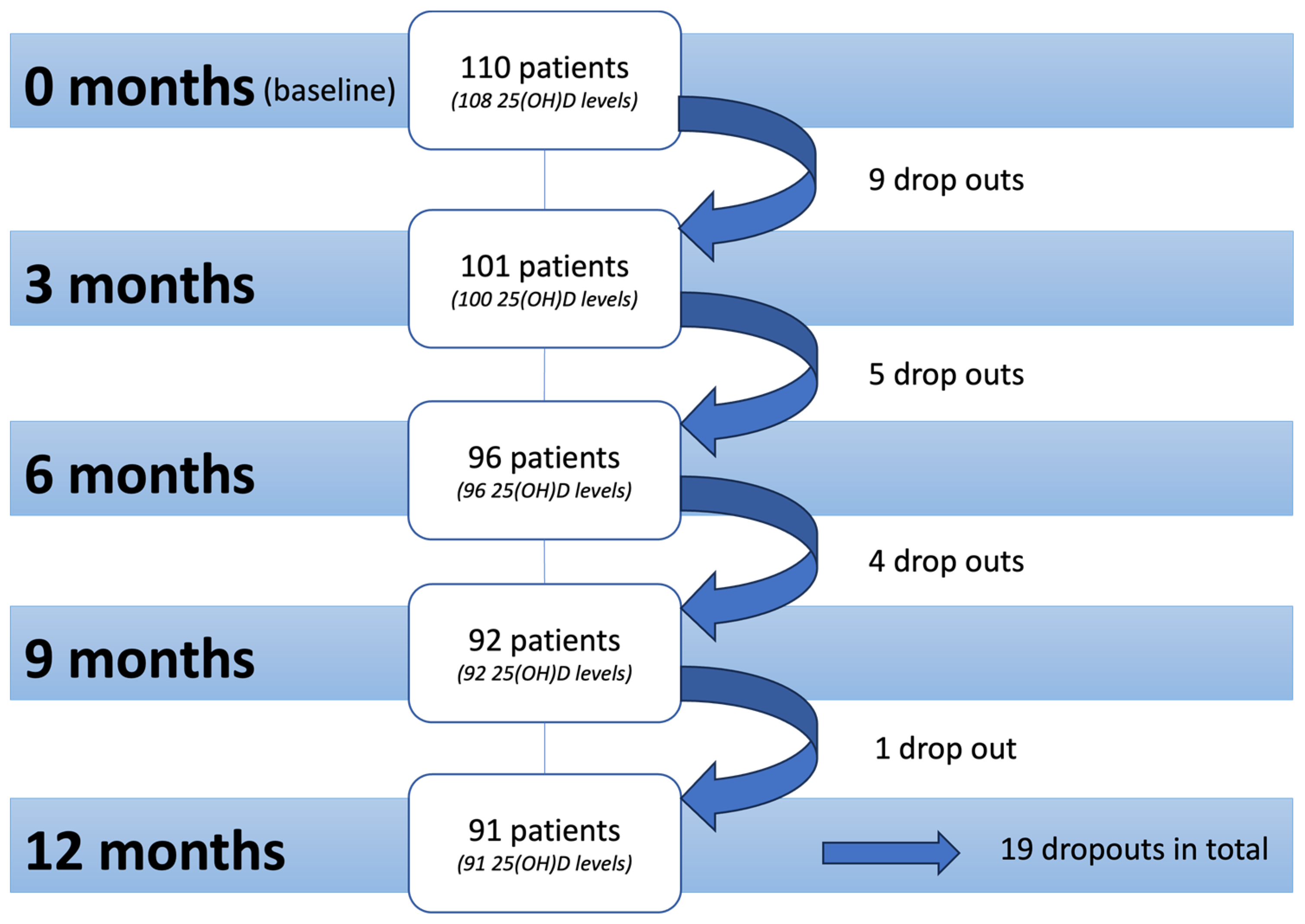
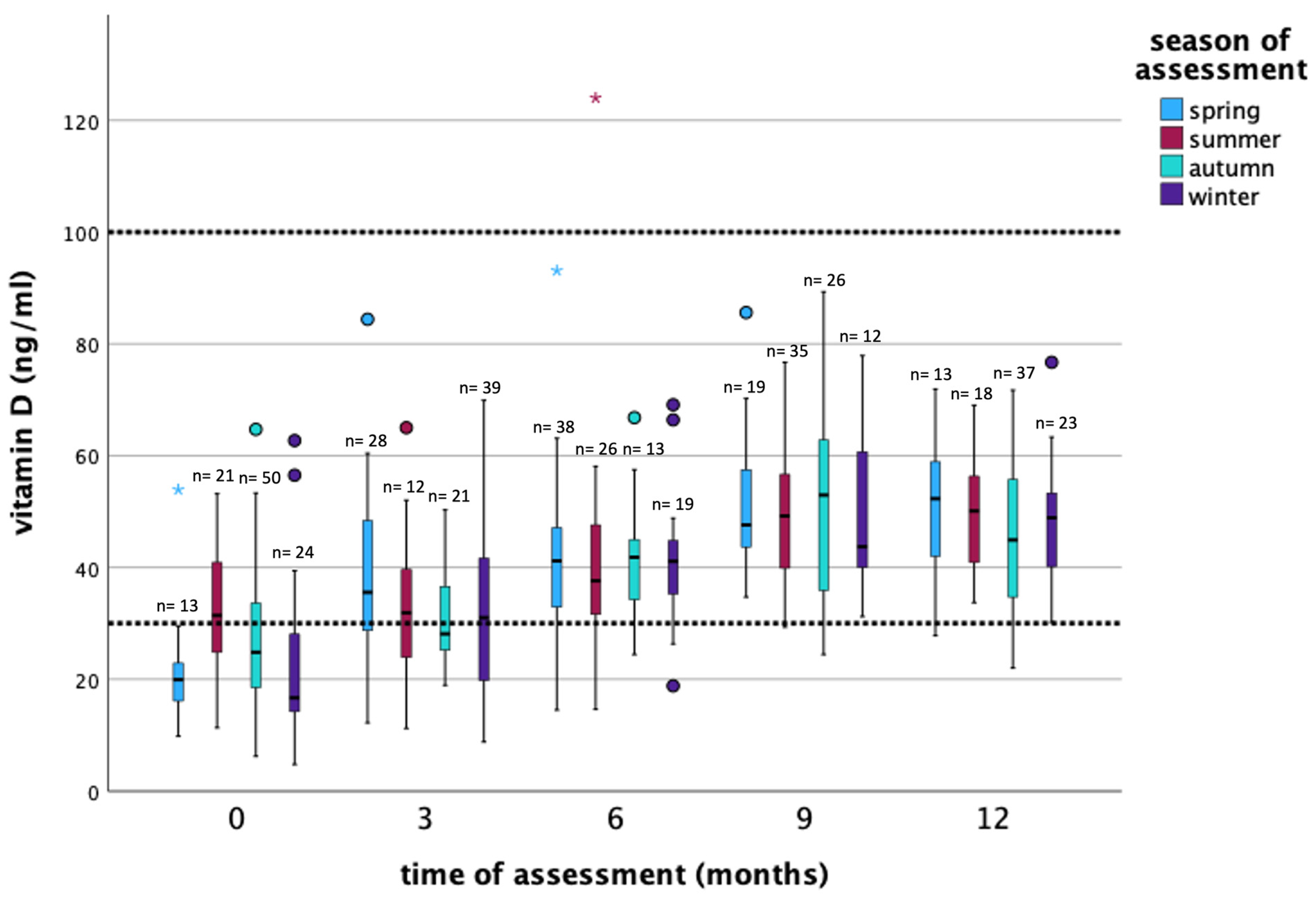
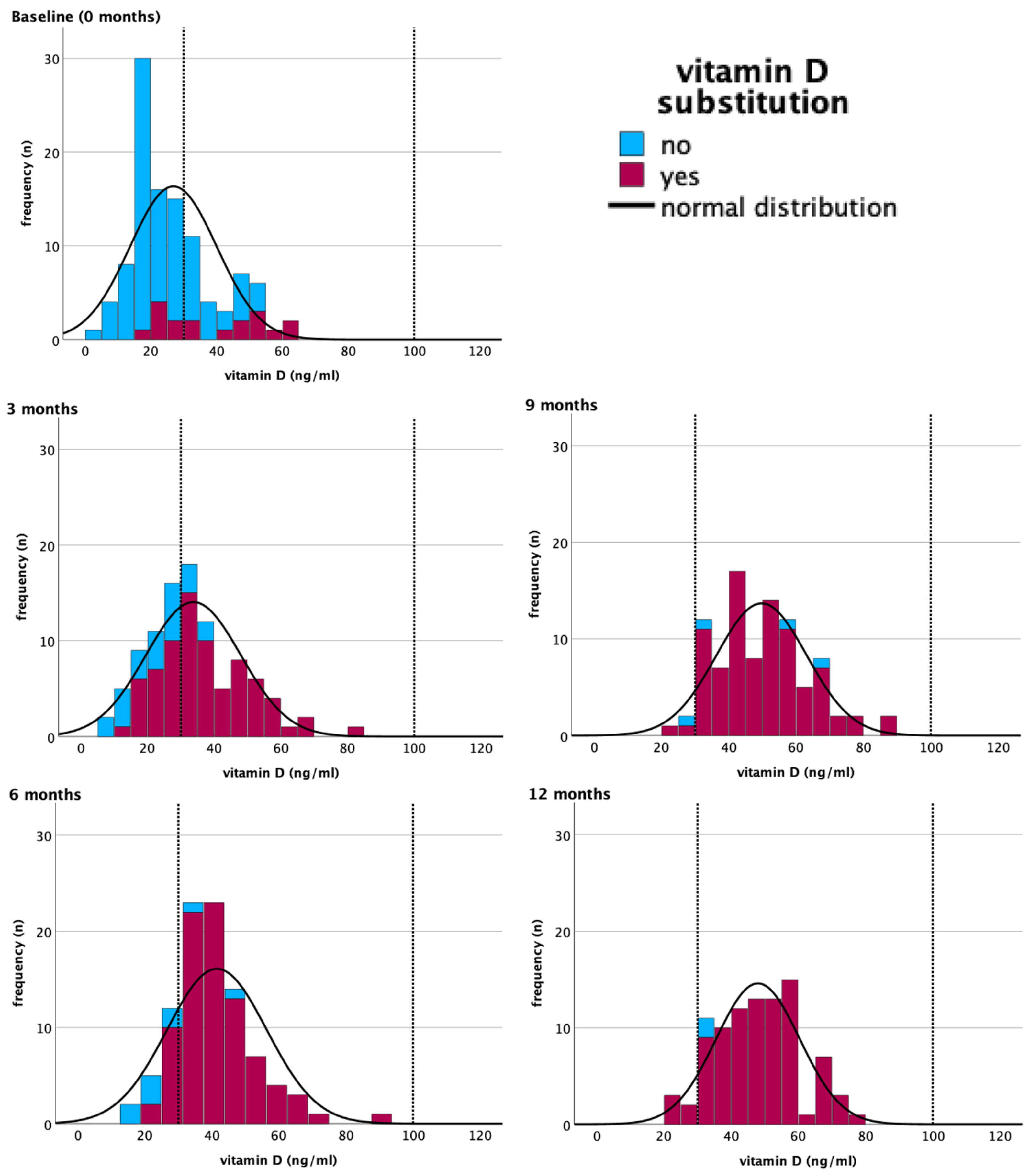
| Time of Assessment | Substitution | Frequency n (%) | Mean ng/mL (±SD *) | Median ng/mL (Min–Max **) | Season of Assessment *** n (%) |
|---|---|---|---|---|---|
| 0 months (baseline) | no | 90 (83.3%) | 24.3 (±11.1) | 21.5 (4.8–53.2) | SP = 11 (12.2%) SU = 19 (21.1%) AU = 41 (45.6%) WI = 19 (21.1%) |
| yes | 18 (16.7%) | 39.4 (±15.7) | 38.1 (15.7–64.7) | SP = 2 (11.1%) SU = 2 (11.1%) AU = 9 (50%) WI = 5 (27.8%) | |
| all patients | 108 (100%) | 26.8 (±13.2) | 24.2 (4.8–64.7) | SP = 13 (12.0%) SU = 21 (19.4%) AU = 50 (46.3%) WI = 24 (22.2%) | |
| 3 months | no | 24 (24%) | 22.7 (±9.1) | 22.7 (8.8–39.4) | SP = 4 (16.7%) SU = 3 (12.5%) AU = 5 (20.8%) WI = 12 (50%) |
| yes | 76 (76%) | 37.3 (±13.8) | 34.4 (11.9–84.4) | SP = 24 (31.6%) SU = 9 (11.8%) AU = 16 (21.1%) WI = 27 (35.5%) | |
| all patients | 100 (100%) | 33.8 (±14.2) | 32.7 (8.8–84.4) | SP = 28 (28%) SU = 12 (12%) AU = 21 (21%) WI = 39 (39%) | |
| 6 months | no | 9 (9.4%) | 25.9 (±11.0) | 23.2 (14.5–47.6) | SP = 4 (44.5%) SU = 3 (33.3%) AU = 0 (0%) WI = 2 (22.2%) |
| yes | 87 (90.6%) | 43.1 (±14.3) | 41.3 (20.9–124.0) | SP = 34 (39.1%) SU = 23 (26.4%) AU =13 (14.9%) WI = 17 (19.6%) | |
| all patients | 96 (100%) | 41.5 (±14.9) | 40.8 (14.5–124.0) | SP = 38 (39.6%) SU = 26 (27.1%) AU = 13 (13.5%) WI = 19 (19.8%) | |
| 9 months | no | 4 (4.3%) | 46.4 (±18.3) | 44.9 (29.3–66.5) | SP = 1 (25.0%) SU = 1 (25.0%) AU = 2 (50.0%) WI = 0 (0%) |
| yes | 88 (95.7%) | 49.8 (±13.3) | 49.0 (24.4–89.3) | SP = 18 (20.5%) SU = 34 (38.6%) AU = 24 (27.3%) WI = 12 (13.6%) | |
| all patients | 92 (100%) | 49.7 (±13.4) | 49.0 (24.4–89.3) | SP = 19 (20.7%) SU = 35 (38.0%) AU = 26 (28.3%) WI = 12 (13.0%) | |
| 12 months | No | 2 (2.2%) | 32.2 (±2.2) | 32.2 (30.6–33.7) | SP = 0 (0%) SU = 1 (50.0%) AU = 1 (50.0%) WI = 0 (0%) |
| yes | 89 (97.8%) | 48.3 (±12.3) | 48.7 (22.0–76.7) | SP = 13 (14.6%) SU = 17 (19.1%) AU = 36 (40.5%) WI = 23 (25.8%) | |
| all patients | 91 (100%) | 47.9 (±12.4) | 48.1 (22.0–76.7) | SP = 13 (14.2%) SU = 18 (19.8%) AU = 37 (40.7%) WI = 23 (25.3%) |
| Time of Assessment | 0 Months (Baseline) | 3 Months | 6 Months | 9 Months | 12 Months |
|---|---|---|---|---|---|
| Vitamin D serum levels | |||||
| Total Vitamin D deficiency (<30 ng/mL) | 74 (68.5%) | 43 (43%) | 14 (14.6%) | 3 (3.3%) | 5 (5.5%) |
| Vitamin D normal range (30–100 ng/mL) | 34 (31.5%) | 57 (57%) | 81 (84.4%) | 89 (96.7%) | 86 (94.5%) |
| Vitamin D overdosage (>100 ng/mL) | 0 | 0 | 1 (1%) | 0 | 0 |
| Total | 108 (100%) | 100 (100%) | 96 (100%) | 92 (100%) | 91 (100%) |
| Vitamin D deficiency | |||||
| Severe vitamin D deficiency (<10 ng/mL) | 5 (4.6%) | 2 (2%) | 0 | 0 | 0 |
| Moderate vitamin D deficiency (10–19.9 ng/mL) | 38 (35.2%) | 14 (14%) | 4 (4.2%) | 0 | 0 |
| Mild vitamin D deficiency (20–29.9 ng/mL) | 31 (28.7%) | 27 (27%) | 10 (10.4%) | 3 (3.3%) | 5 (5.5%) |
| Vitamin D normal range | |||||
| Vitamin D normal range (30–100 ng/mL) | 34 (100%) | 57 (100%) | 81 (100%) | 89 (100%) | 86 (94.5%) |
| How many patients substituted to have normal range | 11 (32.4%) | 52 (91.2%) | 78 (96.3%) | 86 (96.6%) | 84 (97.7%) |
| Vitamin D substitution in patients with normal range (IU/week) * | 20,000 IU (2000–40,000 IU) | 20,000 IU (2000–140,000 IU) | 20,000 IU (2000–60,000 IU) | 20,000 IU (2000–140,000 IU) | 20,000 IU (5600–140,000 IU) |
| Time of Assessment | 0 Months (Baseline) | 3 Months | 6 Months | 9 Months | 12 Months |
|---|---|---|---|---|---|
| Weight (kg) | 69.6 (45.6–107.4) | 68.5 (43.9–103.4) | 68.6 (44.5–106.1) | 68.7 (43.6–107.0) | 69.2 (45.4–108.4) |
| BMI (kg/m2) | 25.7 (19.2–39.0) | 25.7 (18.8–38.0) | 25.7 (18.9–37.5) | 25.8 (17.9–37.5) | 25.7 (18.6–38.0) |
| Body fat (%) | 34.9 (16.9–48.5) | 33.1 (17.0–48.2) | 32.0 (17.1–46.1) | 33.8 (19.1–47.1) | 34.2 (17.3–46.4) |
| Muscle mass (kg) | 43.6 (34.4–55.8) | 43.5 (33.8–63.2) | 43.9 (32.4–59.4) | 43.7 (30.9–56.0) | 43.2 (31.6–59.4) |
| Bone mass (kg) | 2.3 (1.9–3.0) | 2.3 (1.8–4.6) | 2.4 (1.8–3.2) | 2.3 (1.7–3.0) | 2.3 (1.7–3.2) |
| Visceral fat (kg) | 7.0 (2.0–15.0) | 7.0 (2.0–17.0) | 7.0 (2.0–13.0) | 7.0 (2.0–14.0) | 7.0 (3.0–14.0) |
| Time of Assessment | Reference Values | 0 Months (Baseline) | 3 Months | 6 Months | 9 Months | 12 Months |
|---|---|---|---|---|---|---|
| Vitamin D (ng/mL) | 30–100 | 24.15 (4.8–64.7) | 32.75 (8.8–84.4) | 40.8 (14.5–124.0) | 49.0 (24.4–89.3) | 48.1 (22.0–76.7) |
| Cortisol (µg/dL) | 4.82–19.5 | 14.1 (4.0–39.0) | 12.1 (3.0–33.0) | 11.9 (3.0–26.0) | 13.4 (5.0–25.0) | 14.0 (5.0–31.0) |
| TSH (µIU/mL) | 0.51–4.3 | 1.6 (0.2–8.1) | 1.6 (0.05–5.9) | 1.5 (0.2–4.2) | 1.5 (0.02–11.0) | 1.7 (0.04–4.8) |
| T3 (pg/mL) | 2.6–5 | 3.2 (2.2–4.4) | 3.0 (2.0–4.5) | 3.2 (2.4–5.9) | 3.1 (1.9–5.3) | 3.2 (2.3–4.6) |
| T4 (ng/dL) | 0.93–1.7 | 1.3 (1.0–8.0) | 1.2 (0.9–2.1) | 1.2 (0.9–2.0) | 1.2 (0.7–2.0) | 1.2 (0.8–1.9) |
| HbA1c (%) | 4–6 | 5.65 (4.7–8.0) | 5.6 (4.2–7.9) | 5.4 (3.8–7.9) | 5.5 (4.7–8.3) | 5.5 (4.2–8.5) |
| Calcium (mmol/L) | 2.1–2.6 | 2.4 (2.0–2.6) | 2.4 (2.1–2.6) | 2.4 (1.9–2.7) | 2.4 (2.2–2.8) | 2.4 (2.1–2.7) |
| Magnesium (mmol/L) | 0.66–1.07 | 0.83 (0.6–1.0) | 0.82 (0.6–2.3) | 0.82 (0.3–0.9) | 0.82 (0.5–0.9) | 0.82 (0.51–0.94) |
| Selenium (µg/L) | 50–120 | 81.45 (44.4–123.2) | 78.7 (41.9–139.3) | 84.5 (47.2–143.1) | 82.4 (40.7–210.5) | 84.3 (38.4–138.7) |
| Iron (µg/dL) | 33–193 | 97.5 (19.0–212.0) | 83.0 (26.0–264.0) | 80.5 (14.0–185.0) | 89.5 (32.0–197.0) | 87.0 (7.2–255.0) |
| LDH (U/L) | 0–289 | 214.0 (147.0–440.0) | 221.0 (163.0–779.0) | 222.5 (154.0–477.0) | 216.0 (160.0–521.0) | 217.0 (149.0–336.0) |
| Lipase (U/L) | 13–60 | 35.0 (14.0–144.0) | 30.5 (11.0–74.0) | 32.5 (15.0–77.0) | 33.0 (14.0–81.0) | 34.0 (15.0–101.0) |
| LDL-Cholesterol (mg/dL) | <116 | 127.0 (61.0–251.0) | 128.0 (68.0–251.0) | 134.5 (61.0–246.0) | 125.5 (47.0–265.0) | 126.0 (45.0–269.0) |
| HDL-Cholesterol (mg/dL) | >45 | 64.0 (32.0–103.0) | 56.5 (32.0–103.0) | 61.0 (30.0–136.0) | 64.0 (34.0–145.0) | 63.0 (34.0–105.0) |
| VLDL-Cholesterol (mg/dL) | <30 | 10.0 (0–77.0) | 12.0 (0–110.0) | 12.0 (0–62.0) | 13.0 (1.0–58.0) | 13.0 (0–66.0) |
| Triglyceride (mg/dL) | <150 | 103.0 (38.0–525.0) | 112.5 (45.0–531.0) | 117.5 (44.0–441.0) | 112.5 (43.0–387.0) | 115.0 (47.0–424.0) |
| CA 15-3 (U/mL) | <26.2 | 18.6 (6.2–79.9) | 22.3 (6.1–58.2) | 19.9 (5.2–46.1) | 16.3 (6.4–37.5) | 17.3 (6.5–38.2) |
| Food | Median (Min–Max) | Vitamin D Content (μg per 100 g) * |
|---|---|---|
| Herring/trout/salmon | 3.0 (0–12) | 7.80–25.00 |
| Mackerel/tuna | 1.0 (0–8) | 4.00 |
| Eggs/margarine | 12.0 (0–28) | 2.50–7.50 |
| Cream/gouda/butter | 20.0 (0–28) | 1.20–1.30 |
| Whole milk/quark/yogurt | 20.0 (0–28) | 0.09 |
| Chanterelles/champignons/porcini mushrooms | 3.0 (0–28) | 1.90–2.10 |
| Beef/veal liver | 0 (0–5) | 0.33–1.70 |
| Lifestyle Variables | |||
| Regression Coefficient | 95% Confidence Interval | p-Value | |
| Amount of vitamin D substitution (IU/week) | 0.001 | (0; 0.001) | <0.001 |
| Season of assessment | <0.001 (all seasons) | ||
| Spring | 3.19 | (0.64, 5.73) | 0.01 |
| Summer | 6.66 | (3.70, 9.62) | <0.001 |
| Autumn | 4.74 | (2.11, 7.37) | <0.001 |
| (Winter was used as reference) | |||
| Age | 0.15 | (0.01, 0.28) | 0.03 |
| Weight | 0.35 | (−0.63, 1.33) | 0.49 |
| BMI | −0.71 | (−1.57, 0.15) | 0.11 |
| Body composition: | |||
| Body fat | −0.27 | (−1.28, 0.75) | 0.61 |
| Muscle mass | −0.52 | (−1.98, 0.94) | 0.49 |
| Bone mass | −3.90 | (−9.24, 1.44) | 0.15 |
| Visceral fat | −0.48 | (−1.07, 0.12) | 0.12 |
| Cortisol | 0.06 | (−0.24, 0.35) | 0.71 |
| LDL | −0.04 | (−0.08, −0.01) | 0.09 |
| HDL | 0.11 | (−0.03, 0.24) | 0.11 |
| VLDL | −0.15 | (−0.22, −0.08) | <0.001 |
| HbA1c | −1.43 | (−4.97, 2.11) | 0.43 |
| TSH | 0.16 | (−0.88, 1.20) | 0.77 |
| T3 | 1.25 | (−2.78, 5.28) | 0.54 |
| T4 | 0.55 | (−6.12, 7.21) | 0.87 |
| Minerals | |||
| Calcium | 8.62 | (−1.63, 18.88) | 0.10 |
| Magnesium | −9.75 | (−16.65, −2.84) | 0.006 |
| Trace elements | |||
| Iron | −0.02 | (−0.06, 0.02) | 0.35 |
| Selenium | −0.01 | (−0.12, 0.11) | 0.91 |
| Antineoplastic Therapy Related Factors | |||
| Regression Coefficient | 95% Confidence Interval | p-Value | |
| Chemotherapy | 1.41 | (−1.42, 4.24) | 0.33 |
| Radiotherapy | −0.33 | (−2.78; 2.11) | 0.79 |
| Endocrine therapy | <0.001 | ||
| Tamoxifen | 3.35 | (−1.10; 7.79) | 0.14 |
| Aromatase inhibitor | 8.58 | (5.17; 11.99) | <0.001 |
| (no endocrine therapy served as reference) | |||
| Her2 targeted therapy | −2.05 | (−5.49; 1.40) | 0.24 |
| Cancer and Side Effect Related Factors | |||
| Regression Coefficient | 95% Confidence Interval | p-Value | |
| Tumor marker (CA 15−3) | −0.08 | (−0.21, 0.06) | 0.25 |
| Polyneuropathy | |||
| CTC grade 0 | −4.24 | (−11.07, 2.58) | 0.22 |
| CTC grade 1 | −2.35 | (−8.98, 4.27) | 0.49 |
| CTC grade 2 | 0.24 | (−5.87, 6.35) | 0.94 |
| (CTC grade 3 served as reference) | |||
| Lifestyle Variables * | |||
| Dietary Habits | Regression Coefficient | 95% Confidence Interval | p-Value |
| Herring/trout/salmon | 0.26 | (−0.74, 1.26) | 0.61 |
| Mackerel/tuna | −0.46 | (−1.75, 0.83) | 0.48 |
| Eggs/margarine | 0.11 | (−0.25, 0.47) | 0.55 |
| Cream/gouda/butter | −0.38 | (−0.62, −0.14) | 0.003 |
| Whole milk/quark/yoghurt | 0.004 | (−0.29, 0.29) | 0.98 |
| Chanterelles/champignons/porcini mushrooms | 0.06 | (−0.55, 0.66) | 0.85 |
| Beef/veal liver | −1.06 | (−3.75, 1.64) | 0.44 |
| Cancer/Antineoplastic Therapy Related Factors | |||
| Regression Coefficient | 95% Confidence Interval | p-Value | |
| Genetic mutation | 2.63 | (−6.5, 11.8) | 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zemlin, C.; Altmayer, L.; Lang, M.; Schleicher, J.T.; Stuhlert, C.; Wörmann, C.; Scherer, L.-S.; Thul, I.C.; Spenner, L.S.; Simon, J.A.; et al. Course of Vitamin D Levels in Newly Diagnosed Non-Metastatic Breast Cancer Patients over One Year with Quarterly Controls and Substitution. Nutrients 2024, 16, 854. https://doi.org/10.3390/nu16060854
Zemlin C, Altmayer L, Lang M, Schleicher JT, Stuhlert C, Wörmann C, Scherer L-S, Thul IC, Spenner LS, Simon JA, et al. Course of Vitamin D Levels in Newly Diagnosed Non-Metastatic Breast Cancer Patients over One Year with Quarterly Controls and Substitution. Nutrients. 2024; 16(6):854. https://doi.org/10.3390/nu16060854
Chicago/Turabian StyleZemlin, Cosima, Laura Altmayer, Marina Lang, Julia Theresa Schleicher, Caroline Stuhlert, Carolin Wörmann, Laura-Sophie Scherer, Ida Clara Thul, Lisanne Sophie Spenner, Jana Alisa Simon, and et al. 2024. "Course of Vitamin D Levels in Newly Diagnosed Non-Metastatic Breast Cancer Patients over One Year with Quarterly Controls and Substitution" Nutrients 16, no. 6: 854. https://doi.org/10.3390/nu16060854
APA StyleZemlin, C., Altmayer, L., Lang, M., Schleicher, J. T., Stuhlert, C., Wörmann, C., Scherer, L.-S., Thul, I. C., Spenner, L. S., Simon, J. A., Wind, A., Kaiser, E., Weber, R., Goedicke-Fritz, S., Wagenpfeil, G., Zemlin, M., Solomayer, E.-F., Reichrath, J., & Müller, C. (2024). Course of Vitamin D Levels in Newly Diagnosed Non-Metastatic Breast Cancer Patients over One Year with Quarterly Controls and Substitution. Nutrients, 16(6), 854. https://doi.org/10.3390/nu16060854







