The Mechanism of the Anti-Obesity Effects of a Standardized Brassica juncea Extract in 3T3-L1 Preadipocytes and High-Fat Diet-Induced Obese C57BL/6J Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Standards
2.2. Standardized BJE Preparation
2.3. Cell Culture and XTT Assay
2.4. Oil Red O Staining
2.5. Animal Experiment
2.6. Biochemical Analysis
2.7. Histological Analysis
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. Sinigrin Content in Standardized BJE
3.2. Effects of BJE on the Viability and Lipid Accumulation of 3T3-L1 Adipocytes
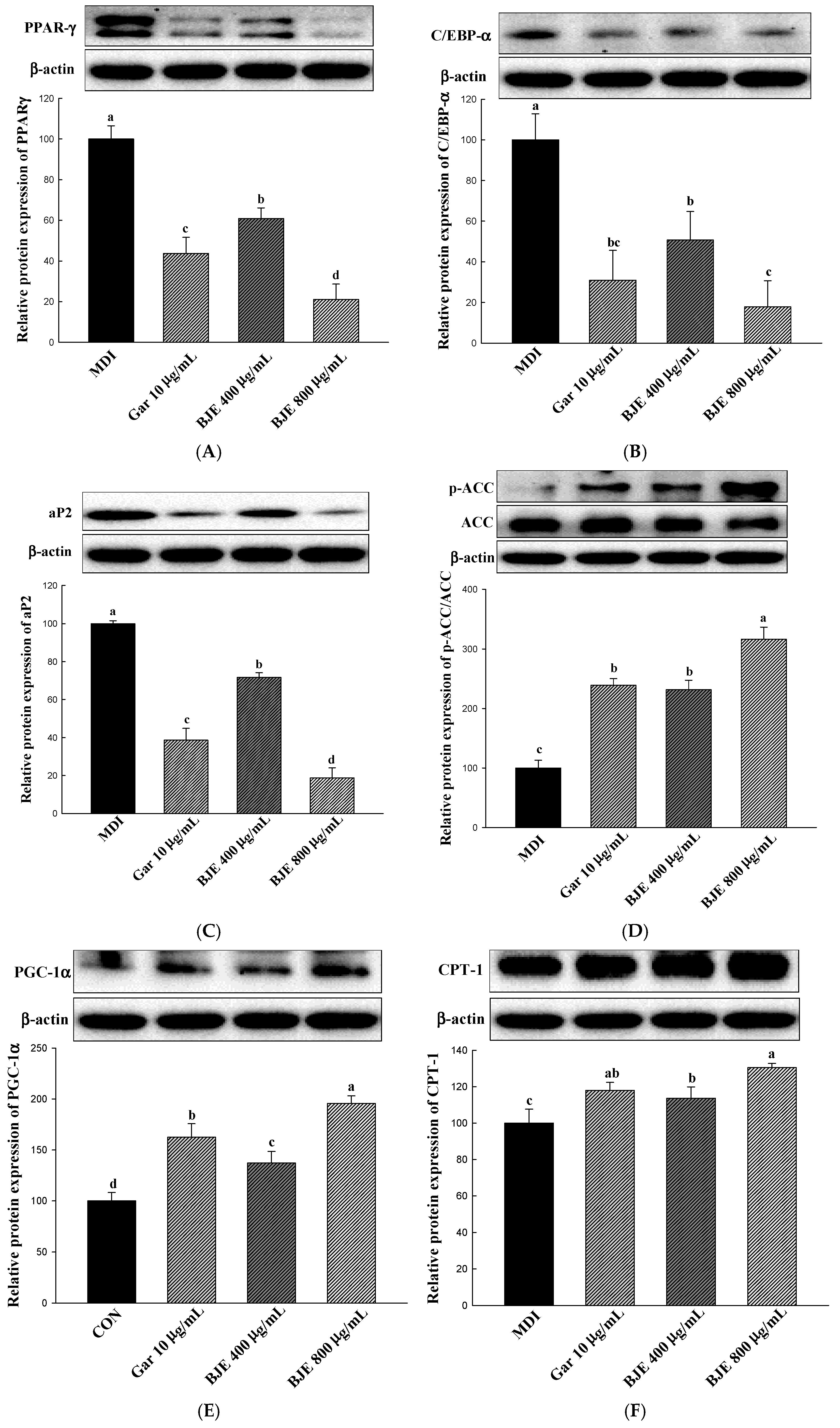
3.3. Effect of BJE on the Regulation of Protein Expression Associated with Adipogenesis, Lipid Synthesis, Heat Generation, and Fatty Acid Oxidation in 3T3-L1 Adipocytes
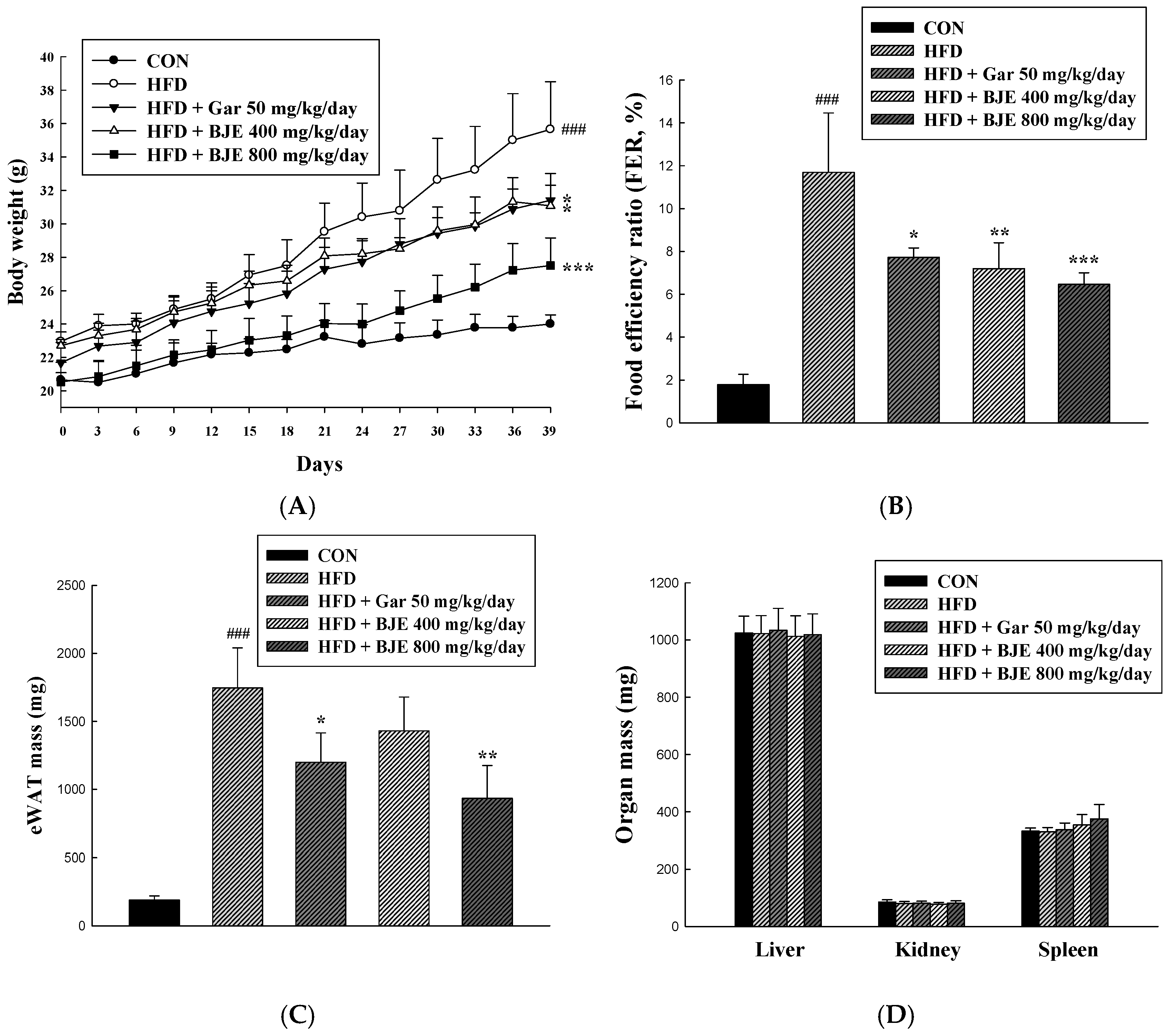
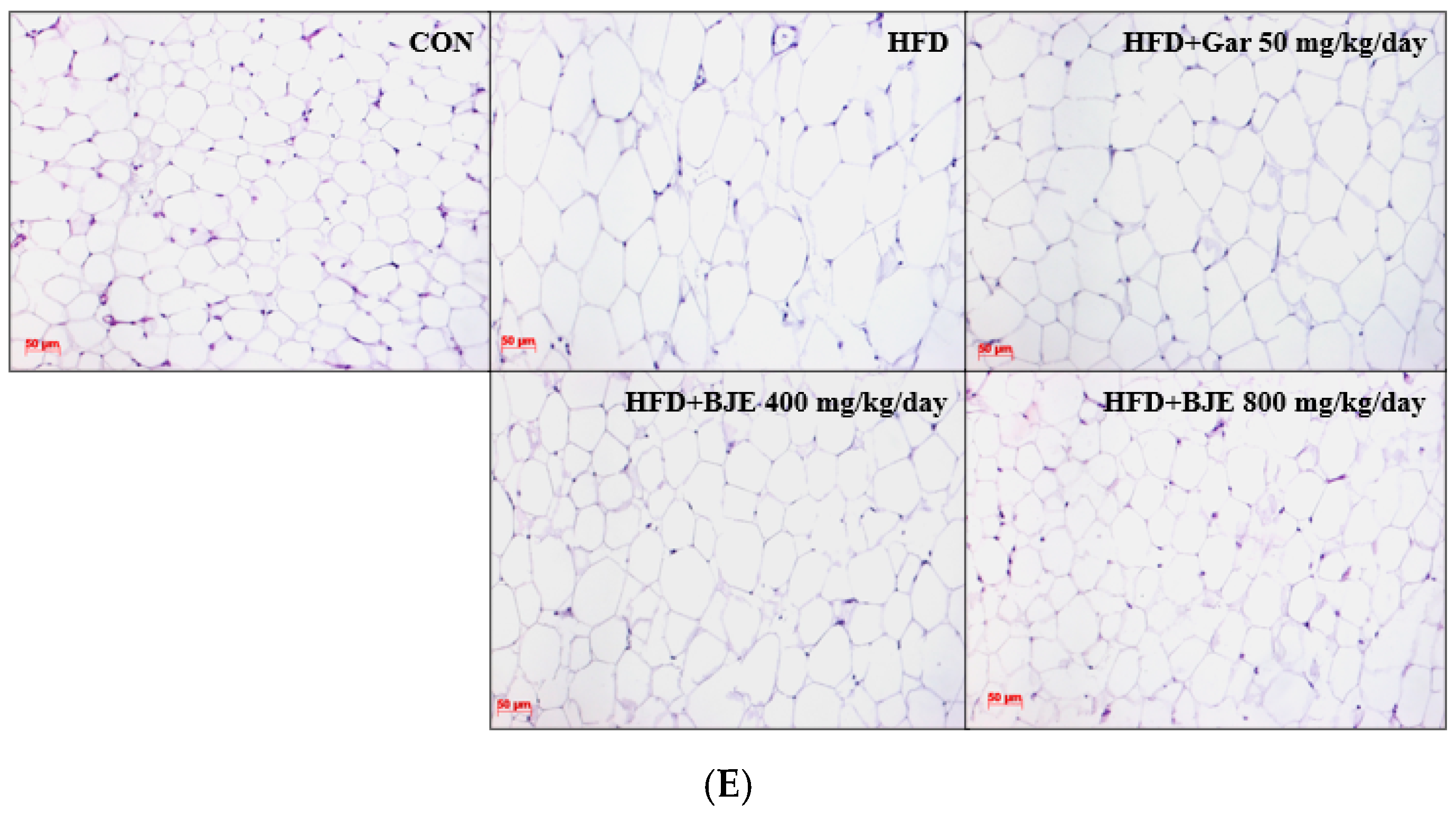
3.4. Effect of BJE on Body Weight, Food Efficiency, Adipose Tissue Mass, Organ Mass, and Adipose Tissue Size in HFD-Induced Obese C57BL/6J Mice
3.5. Effect of BJE on Serum Biochemical Indexes in HFD-Induced Obese C57BL/6J Mice
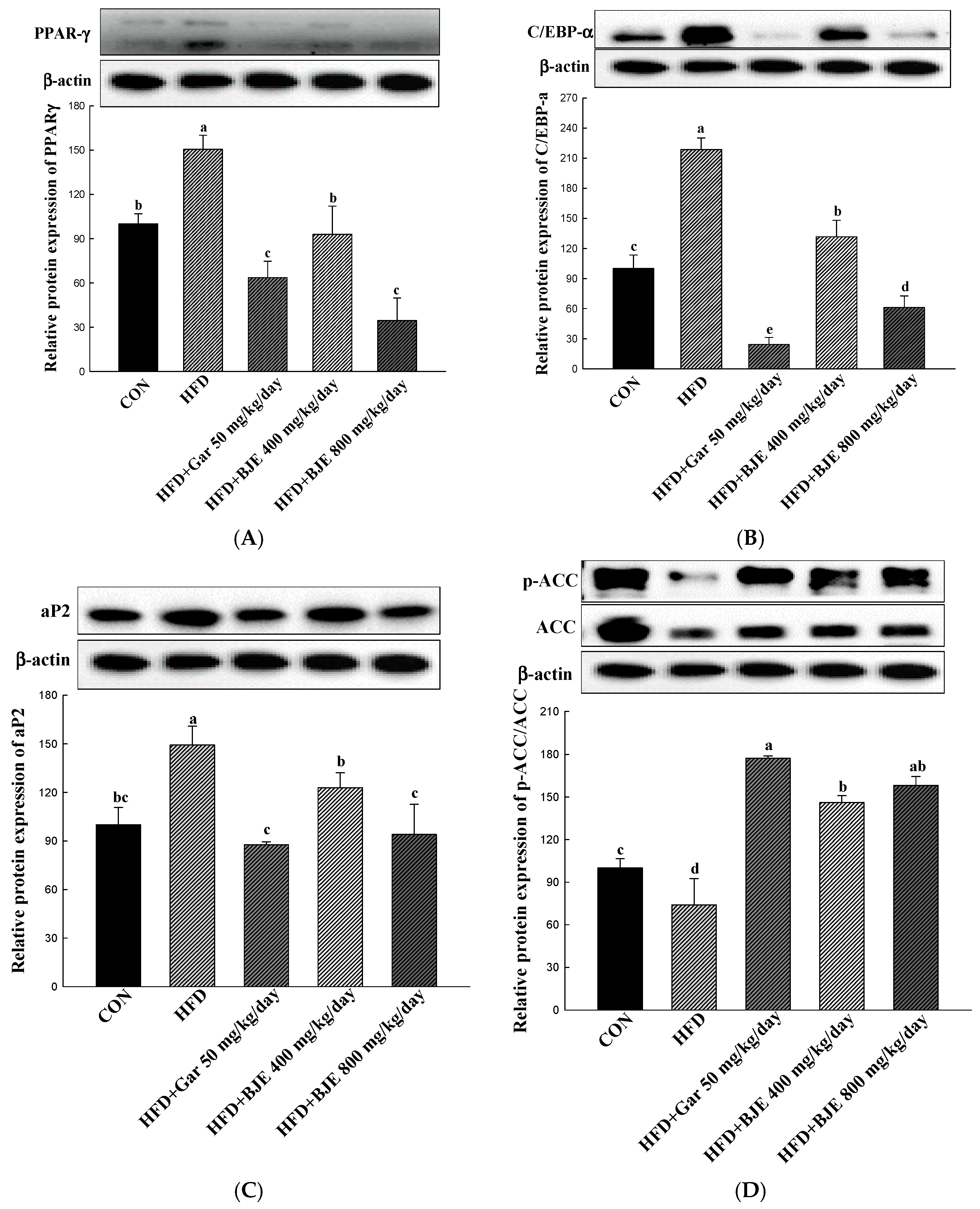
3.6. The Effect of BJE on the Regulation of Protein Expression Associated with Adipogenesis, Lipid Synthesis, Heat Generation, and Fatty Acid Oxidation in HFD-Induced Obese Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, Y.; Zhang, L.; Bao, X.; Qian, X.; Dong, W.; Jiang, M. Palmitoleic acid-rich oleaginous yeast Scheffersomyces segobiensis DSM 27193 exerts anti-obesity effects by ameliorating hepatic steatosis and adipose tissue hypertrophy. J. Sci. Food Agric. 2024, 104, 2156–2164. [Google Scholar] [CrossRef]
- Kim, H.R.; Jung, B.K.; Yeo, M.H.; Yoon, W.J.; Chang, K.S. Inhibition of lipid accumulation by the ethyl acetate fraction of Distylium racemosum in vitro and in vivo. Toxicol. Rep. 2019, 6, 215–221. [Google Scholar] [CrossRef]
- Wu, C.H.; Yang, M.Y.; Chan, K.C.; Chung, P.J.; Ou, T.T.; Wang, C.J. Improvement in high-fat diet-induced obesity and body fat accumulation by a Nelumbo nucifera leaf flavonoid-rich extract in mice. J. Agric. Food Chem. 2010, 58, 7075–7081. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Choi, J.H.; Kim, Y.G.; Lee, C.H. Effect of dietary intake of Salicornia herbacea L. hot water extract on anti-obesity in diet-induced obese rats. J. Korean Soc. Food Sci. Nutr. 2012, 41, 950–956. [Google Scholar] [CrossRef]
- Jiao, X.; Wang, Y.; Lin, Y.; Lang, Y.; Li, E.; Zhang, X.; Zhang, Q.; Feng, Y.; Meng, X.; Li, B. Blueberry polyphenols extract as a potential prebiotic with anti-obesity effects on C57BL/6J mice by modulating the gut microbiota. J. Nutr. Biochem. 2019, 64, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Guo, L.; Kang, H.M.; Son, B.G.; Kang, J.S.; Lee, Y.J.; Park, Y.H.; Je, B.I.; Choi, Y.W. Leaves of Cudrania tricuspidata on the shoot positional sequence show different inhibition of adipogenesis activity in 3T3-L1 cells. J. Life Sci. 2021, 31, 209–218. [Google Scholar]
- Lee, Y.S.; Seo, Y.H.; Kim, J.Y. Antiobesity effect of radish leaf extracts on high fat diet-induced obesity in mice. Korean J. Food Sci. Technol. 2022, 54, 297–305. [Google Scholar]
- Greenberg, A.S.; Obin, M.S. Obesity and the role of adipose tissue in inflammation and metabolism. Am. J. Clin. Nutr. 2006, 83, 461S–465S. [Google Scholar] [CrossRef]
- Pagliassotti, M.J.; Kim, P.Y.; Estrada, A.L.; Stewart, C.M.; Gentile, C.L. Endoplasmic reticulum stress in obesity and obesity-related disorders: An expanded view. Metabolism 2016, 65, 1238–1246. [Google Scholar] [CrossRef]
- Kim, H.S.; Han, E.J.; Fernando, I.P.S.; Sanjeewa, K.K.A.; Jayawardena, T.U.; Kim, H.J.; Jee, Y.; Kang, S.; Jang, J.; Jang, J.; et al. Anti-allergy effect of mojabanchromanol isolated from Sargassum horneri in bone marrow-derived cultured mast cells. Algal Res. 2020, 48, 101898. [Google Scholar] [CrossRef]
- Kim, G.C.; Kim, J.S.; Kim, G.M.; Choi, S.Y. Anti-adipogenic effects of Tropaeolum majus (nasturtium) ethanol extract on 3T3-L1 cells. Food Nutr. Res. 2017, 61, 1339555. [Google Scholar] [CrossRef]
- Lee, S.G.; Kang, H. Anti-obesity, and lipid metabolism effects of Ulmus davidiana var. japonica in mice fed a high-fat diet. J. Microbiol. Biotechnol. 2021, 31, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Nam, W.; Nam, S.H.; Kim, S.P.; Levin, C.; Friedman, M. Anti-adipogenic and anti-obesity activities of purpurin in 3T3-L1 preadipocyte cells and in mice fed a high-fat diet. BMC Complement. Altern. Med. 2019, 19, 364. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.J.; Kim, K.J.; Choi, J.; Koh, E.J.; Lee, B.Y. Spirulina maxima extract reduces obesity through suppression of adipogenesis and activation of browning in 3T3-L1 cells and high-fat diet-induced obese mice. Nutrients 2018, 10, 712. [Google Scholar] [CrossRef]
- Choi, D.H.; Han, J.H.; Yu, K.H.; Hong, M.; Lee, S.Y.; Park, K.H.; Lee, S.U.; Kwon, T.H. Antioxidant and anti-obesity activities of Polygonum cuspidatum extract through alleviation of lipid accumulation on 3T3-L1 adipocytes. J. Microbiol. Biotechnol. 2020, 30, 21–30. [Google Scholar] [CrossRef]
- Cho, H.H.; Lee, S.J.; Kim, S.H.; Jang, S.H.; Won, C.W.; Kim, H.D.; Cho, J.H. Antiobesity activity of Acer tegmentosum Maxim on 3T3-L1 preadipocyte and high-fat diet-induced obese rats. Biol. Pharm. Bull. 2008, 31, 1415–1421. [Google Scholar]
- Makowski, L.; Brittingham, K.C.; Reynolds, J.M.; Suttles, J.; Hotamisligil, G.S. The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity: Macrophage expression of aP2 impacts peroxisome proliferator-activated receptor γ and IκB kinase activities. J. Biol. Chem. 2005, 280, 12888–12895. [Google Scholar] [CrossRef]
- Moseti, D.; Regassa, A.; Kim, W.K. Molecular Regulation of Adipogenesis and Potential Anti-Adipogenic Bioactive Molecules. Int. J. Mol. Sci. 2016, 17, 124. [Google Scholar] [CrossRef]
- Ho, J.N.; Choi, J.W.; Lim, W.C.; Kim, M.K.; Lee, I.Y.; Cho, H.Y. Kefir inhibits 3T3-L1 adipocyte differentiation through down-regulation of adipogenic transcription factor expression. J. Sci. Food Agric. 2013, 93, 485–490. [Google Scholar] [CrossRef]
- Choi, R.Y.; Lee, H.I.; Yun, K.W.; Ham, J.R.; Lee, M.K. Inhibitory Effects of Aralia elata Sprout Hot-Water Extract on Adipocyte Differentiation and Triglyceride Synthesis in 3T3-L1 cells. J. Korean Soc. Food Sci. Nutr. 2020, 49, 631–637. [Google Scholar] [CrossRef]
- Cha, J.Y.; Nepali, S.; Lee, H.Y.; Hwang, S.W.; Choi, S.Y.; Yeon, J.M.; Song, B.J.; Kim, D.K.; Lee, Y.M. Chrysanthemum indicum L. ethanol extract reduces high-fat diet-induced obesity in mice. Exp. Ther. Med. 2018, 15, 5070–5076. [Google Scholar]
- Goktas, Z.; Zu, Y.; Abbasi, M.; Galyean, S.; Wu, D.; Fan, Z.; Wang, S. Recent advances in nanoencapsulation of phytochemicals to combat obesity and its comorbidities. J. Agric. Food Chem. 2020, 68, 8119–8131. [Google Scholar] [CrossRef]
- Park, H.J.; Han, Y.S. Effect of mustard leaf on quality and sensory characteristics of Kimchi. J. Korean Soc. Food Sci. Nutr. 1994, 23, 618–624. [Google Scholar]
- Kwon, H.Y.; Choi, S.I.; Cho, B.Y.; Choi, S.H.; Sim, W.S.; Han, X.; Jang, G.W.; Choi, Y.E.; Yeo, J.H.; Cho, J.H.; et al. Analysis of nutritional components and cell-based antioxidant activity on Brassica juncea cultivated in Jeongseon, South Korea. Korean J. Food Nutr. 2019, 32, 462–472. [Google Scholar]
- Popova, I.E.; Morra, M.J. Simultaneous quantification of sinigrin, sinalbin, and anionic glucosinolate hydrolysis products in Brassica juncea and sinapis alba seed extracts using ion chromatography. J. Agric. Food Chem. 2014, 62, 10687–10693. [Google Scholar] [CrossRef]
- Talalay, P.; Fahey, J.W. Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. J. Nutr. 2001, 131, 3027S–3033S. [Google Scholar] [CrossRef]
- Blažević, I.; Radonić, A.; Mastelić, J.; Marina, Z.; Skočibušić, M.; Maravić, A. Glucosinolates, glycosidically bound volatiles and antimicrobial activity of Aurinia sinuata (Brassicaceae). Food Chem. 2010, 121, 1020–1028. [Google Scholar] [CrossRef]
- Oh, S.K.; Kim, K.W.; Bea, S.O.; Choi, M.R. Sinigrin content of different parts of Dolsan leaf mustard. Korean J. Food Preserv. 2015, 22, 553–558. [Google Scholar] [CrossRef]
- Wang, T.; Liang, H.; Yuan, Q. Optimization of ultrasonic-stimulated solvent extraction of sinigrin from Indian mustard seed (Brassica Juncea L.) using response surface methodology. Phytochem. Anal. 2011, 22, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.Y.; Choi, S.I.; Han, X.; Men, X.; Jang, G.W.; Choi, Y.E.; Lee, O.H. Antiobesity effect of Brassica juncea cultivated in Jeonseon with optimized sinigrin content using 3T3-L1 adipocytes. J. Food Biochem. 2021, 45, e13650. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Kim, H.A.; Lee, J.M. The effects of Brassica juncea L. leaf extract on obesity and lipid profiles of rats fed a high-fat/high-cholesterol diet. Nutr. Res. Pract. 2018, 12, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Trigueros, L.; Peña, S.; Ugidos, A.V.; Sayas-Barberá, E.; Pérez-Álvarez, J.A.; Sendra, E. Food Ingredients as Anti-Obesity Agents: A Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Dulloo, A.G. The search for compounds that stimulate thermogenesis in obesity management: From pharmaceuticals to functional food ingredients. Obes. Rev. 2011, 12, 866–883. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Lee, J.J.; Son, H.K.; Kim, B.H.; Byun, J.; Ha, J.H. Antiobesity Effects of Extract from Spergularia marina Griseb in Adipocytes and High-Fat Diet-Induced Obese Rats. Nutrients 2020, 12, 336. [Google Scholar] [CrossRef]
- Choi, B.R.; Kim, H.; Lee, Y.J.; Ku, S.K. Anti-diabetic Obesity Effects of Wasabia japonica Matsum Leaf Extract on 45% Kcal High-Fat Diet-Fed Mice. Nutrients 2020, 12, 2837. [Google Scholar] [CrossRef]
- Ko, J.W.; Chung, Y.S.; Kwak, C.S.; Kwon, Y.H. Doenjang, A Korean Traditional Fermented Soybean Paste, Ameliorates Neuroinflammation and Neurodegeneration in Mice Fed a High-Fat Diet. Nutrients 2019, 11, 1702. [Google Scholar] [CrossRef]
- Choi, S.K.; Park, S.; Jang, S.; Cho, H.H.; Lee, S.; You, S.; Kim, S.H.; Moon, H.S. Cascade regulation of PPARγ2 and C/EBPα signaling pathways by celastrol impairs adipocyte differentiation and stimulates lipolysis in 3T3-L1 adipocytes. Metabolism 2016, 65, 646–654. [Google Scholar] [CrossRef]
- Brownsey, R.W.; Boone, A.N.; Elliott, J.E.; Kulpa, J.E.; Lee, W.M. Regulation of acetyl-CoA carboxylase. Biochem. Soc. Trans. 2006, 24, 223–227. [Google Scholar] [CrossRef]
- Park, Y.H.; An, M.; Kim, J.K.; Lim, Y.H. Antiobesity effect of ethanolic extract of Ramulus mori in differentiated 3T3-L1 adipocytes and high-fat diet-induced obese mice. J. Ethnopharmacol. 2020, 251, 112542. [Google Scholar] [CrossRef]
- Schreurs, M.; Kuipers, F.; Van Der Leij, F.R. Regulatory enzymes of mitochondrial β-oxidation as targets for treatment of the metabolic syndrome. Obes. Rev. 2010, 11, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Gleyzer, N.; Vercauteren, K.; Scarpulla, R.C. Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol. Cell. Biol. 2005, 25, 1354–1366. [Google Scholar] [CrossRef]
- Chun, M.R.; Lee, Y.J.; Kim, K.H.; Kim, Y.W.; Park, S.Y.; Lee, K.M.; Kim, J.Y.; Park, Y.K. Differential effects of high-carbohydrate and high-fat diet composition on muscle insulin resistance in rats. J. Korean Med. Sci. 2010, 25, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Hariri, N.; Thibault, L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010, 23, 270–299. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, J.H.; Noh, S.; Hur, H.J.; Sung, M.J.; Hwang, J.T.; Park, J.H.; Yang, H.J.; Kim, M.S.; Kwon, D.Y.; et al. Metabolomic analysis of livers and serum from high-fat diet induced obese mice. J. Proteome Res. 2011, 10, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yamashita, Y.; Yasuda, M.; Yamamoto, N.; Ashida, H. Ashitaba (Angelica keiskei) extract prevents adiposity in high-fat diet-fed C57BL/6 mice. Food Funt. 2015, 6, 134–144. [Google Scholar] [CrossRef]
- Coulter, A.A.; Bearden, C.M.; Liu, X.; Koza, R.A.; Kozak, L.P. Dietary fat interacts with QTLs controlling induction of PGC-1 alpha and UCP1 during conversion of white to brown fat. Physiol. Genom. 2003, 14, 139–147. [Google Scholar] [CrossRef]
- Tung, Y.C.; Lin, Y.H.; Chen, H.J.; Chou, S.C.; Cheng, A.C.; Kalyanam, N.; Ho, C.T.; Pan, M.H. Piceatannol exerts anti-obesity effects in C57BL/6 mice through modulating adipogenic proteins and gut microbiota. Molecules 2016, 21, 1419. [Google Scholar] [CrossRef]
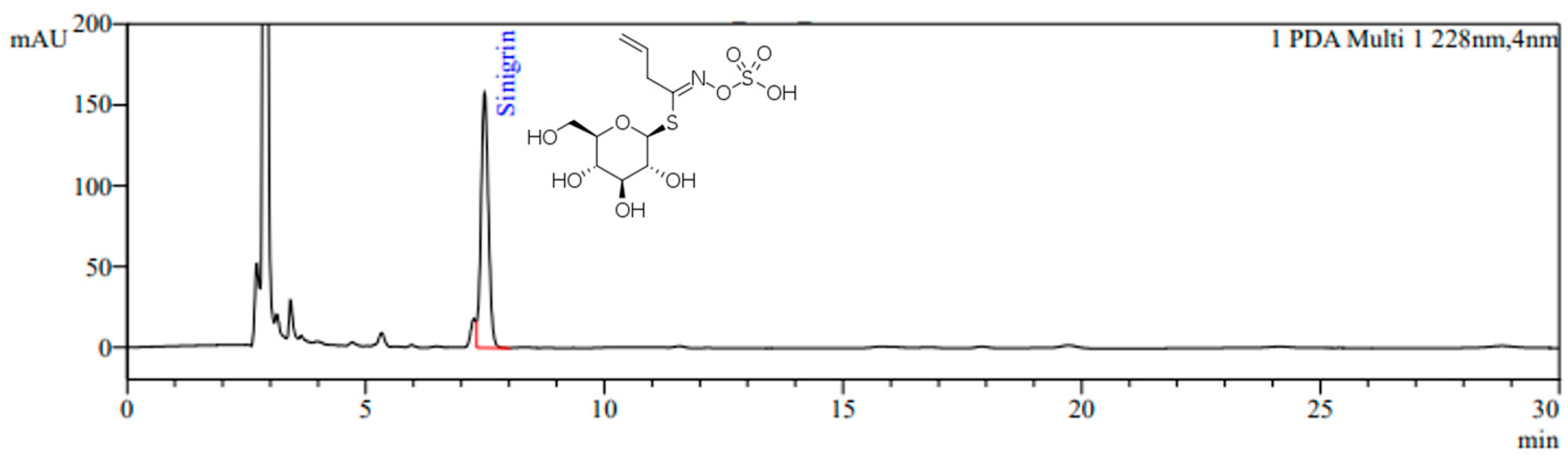


| Instrument | Conditions |
|---|---|
| Column | CAPCELL PAK C18, UG120 (5.0 μm, 4.6 mm × 250 mm) |
| Column temp. | 30 °C |
| Mobile phase (isocratic) | Isocratic HPLC water containing 0.1 M ammonium sulfate |
| Detector | PDA detector (228 nm) |
| Flow rate | 1.0 mL/min |
| Injection volume | 40 μL |
| Run time | 30 min |
| Parameters | Groups | ||||
|---|---|---|---|---|---|
| CON | HFD | HFD + Gar 50 mg/kg/day | HFD + BJE 400 mg/kg/day | HFD + BJE 800 mg/kg/day | |
| ALT (U/L) | 32.00 ± 5.24 | 46.00 ± 12.36 | 38.00 ± 8.02 | 30.00 ± 5.82 * | 29.00 ± 1.23 * |
| AST (U/L) | 117.00 ± 20.37 | 243.00 ± 74.80 # | 134.00 ± 64.60 | 126.00 ± 58.65 * | 110.00 ± 27.00 * |
| TC (mg/dL) | 106.00 ± 4.53 | 161.00 ± 9.66 ### | 173.00 ± 11.06 | 163.00 ± 12.16 | 142.00 ± 5.64 * |
| TG (mg/dL) | 40.00 ± 7.65 | 86.00 ± 5.81 ### | 64.00 ± 8.71 ** | 71.00 ± 9.93 | 57.00 ± 7.86 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.-S.; Im, J.-H.; Han, X.; Men, X.; Oh, G.; Fu, X.; Hwang, W.; Choi, S.-I.; Lee, O.-H. The Mechanism of the Anti-Obesity Effects of a Standardized Brassica juncea Extract in 3T3-L1 Preadipocytes and High-Fat Diet-Induced Obese C57BL/6J Mice. Nutrients 2024, 16, 846. https://doi.org/10.3390/nu16060846
Lim J-S, Im J-H, Han X, Men X, Oh G, Fu X, Hwang W, Choi S-I, Lee O-H. The Mechanism of the Anti-Obesity Effects of a Standardized Brassica juncea Extract in 3T3-L1 Preadipocytes and High-Fat Diet-Induced Obese C57BL/6J Mice. Nutrients. 2024; 16(6):846. https://doi.org/10.3390/nu16060846
Chicago/Turabian StyleLim, June-Seok, Ji-Hyun Im, Xionggao Han, Xiao Men, Geon Oh, Xiaolu Fu, Woonsang Hwang, Sun-Il Choi, and Ok-Hwan Lee. 2024. "The Mechanism of the Anti-Obesity Effects of a Standardized Brassica juncea Extract in 3T3-L1 Preadipocytes and High-Fat Diet-Induced Obese C57BL/6J Mice" Nutrients 16, no. 6: 846. https://doi.org/10.3390/nu16060846
APA StyleLim, J.-S., Im, J.-H., Han, X., Men, X., Oh, G., Fu, X., Hwang, W., Choi, S.-I., & Lee, O.-H. (2024). The Mechanism of the Anti-Obesity Effects of a Standardized Brassica juncea Extract in 3T3-L1 Preadipocytes and High-Fat Diet-Induced Obese C57BL/6J Mice. Nutrients, 16(6), 846. https://doi.org/10.3390/nu16060846







