Ginseng Oligopeptides Improve the Intestinal Physiology and Promote the Antioxidant Capacity of the Gut-on-a-Chip Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Culture and Treatments of the Gut-on-a-Chip
2.3. Observation of Morphological Structure
2.4. Detection of the Apparent Permeability Coefficient (Papp)
2.5. Detection of the TEER
2.6. Immunofluorescence Staining
2.7. QPCR Assay
2.8. Statistical Analysis
3. Results
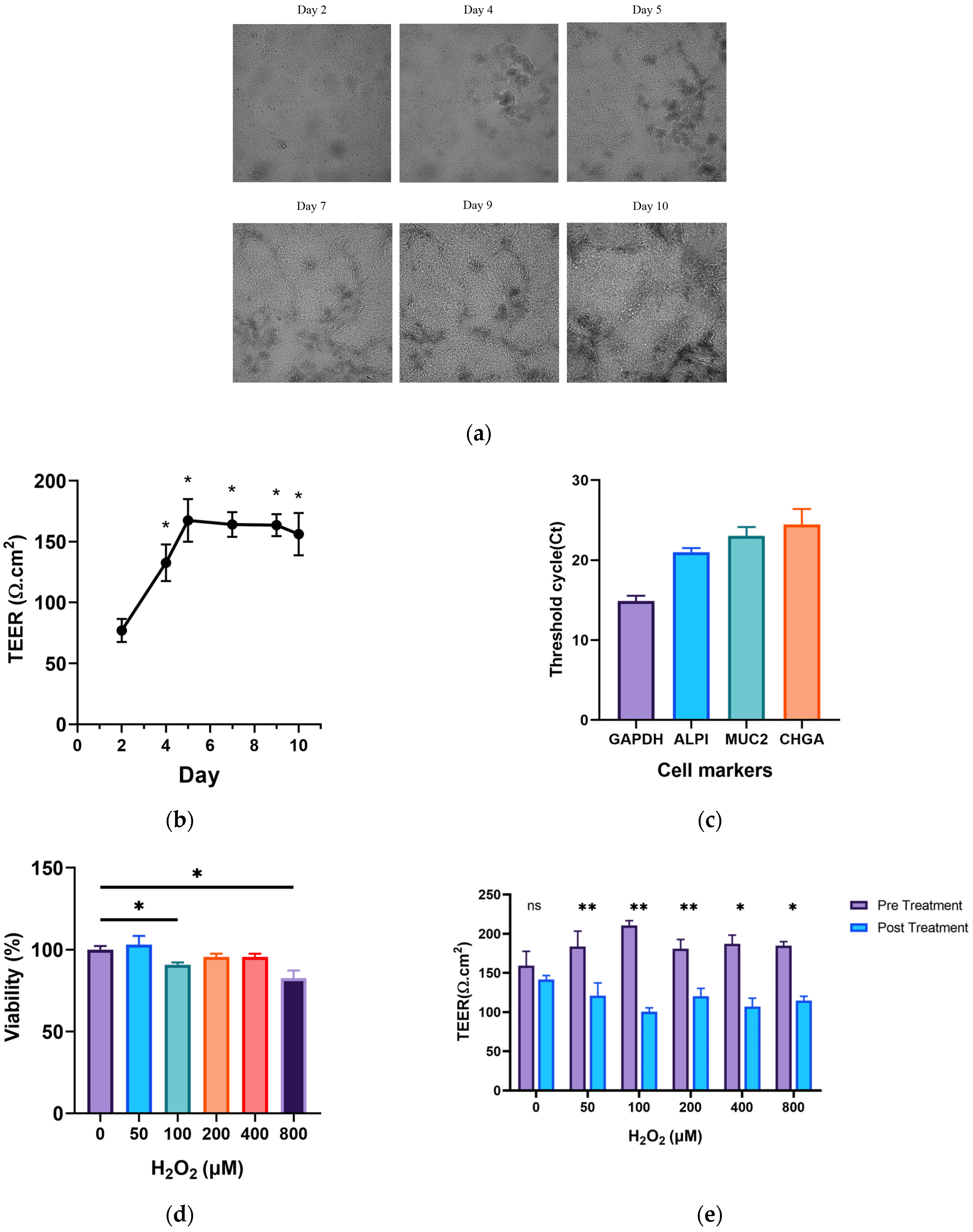
3.1. Culture and Construction of the Ageing Gut-on-a-Chip Model
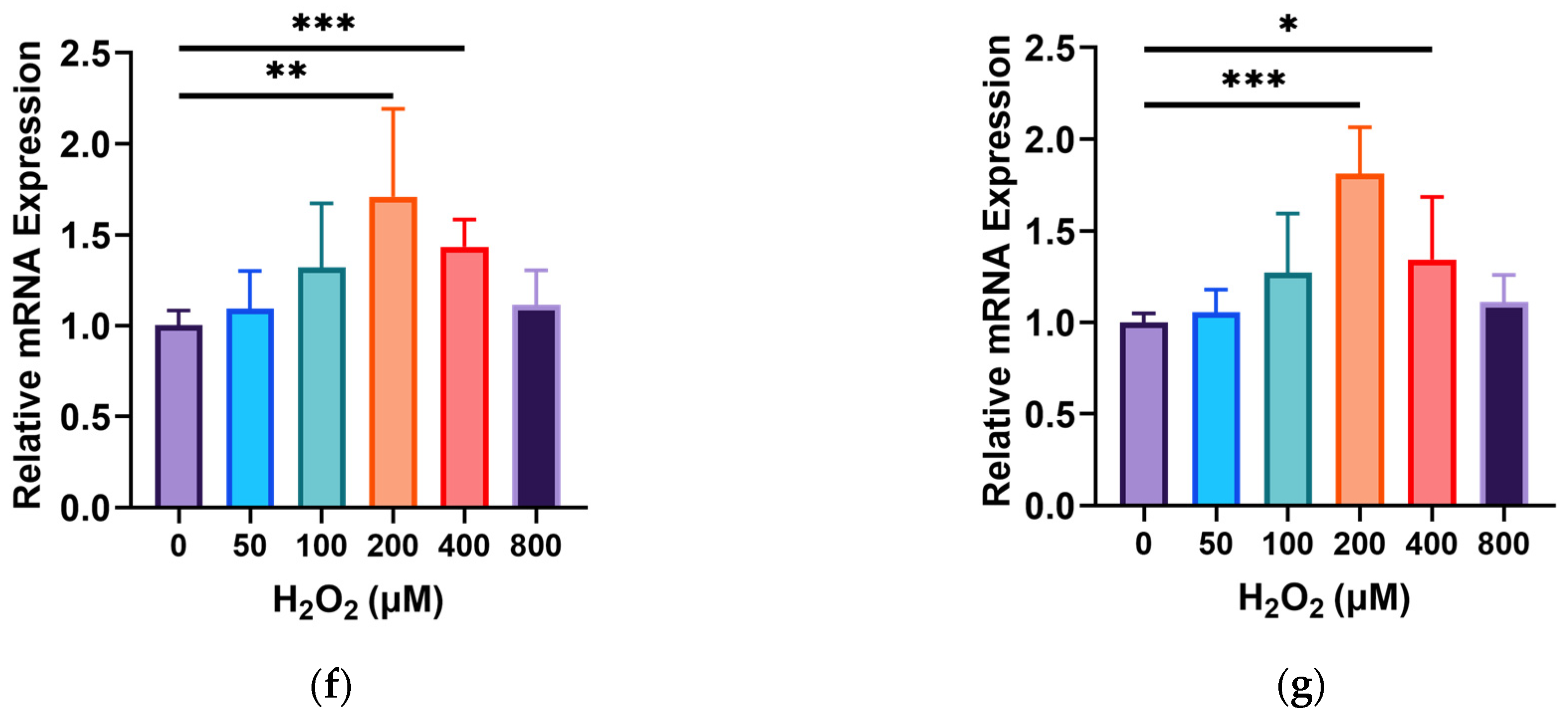
3.2. Effects of GOPs on the Barrier Integrity of the Gut-on-a-Chip
3.3. Effects of GOPs on the Cell Physiology of the Gut-on-a-Chip
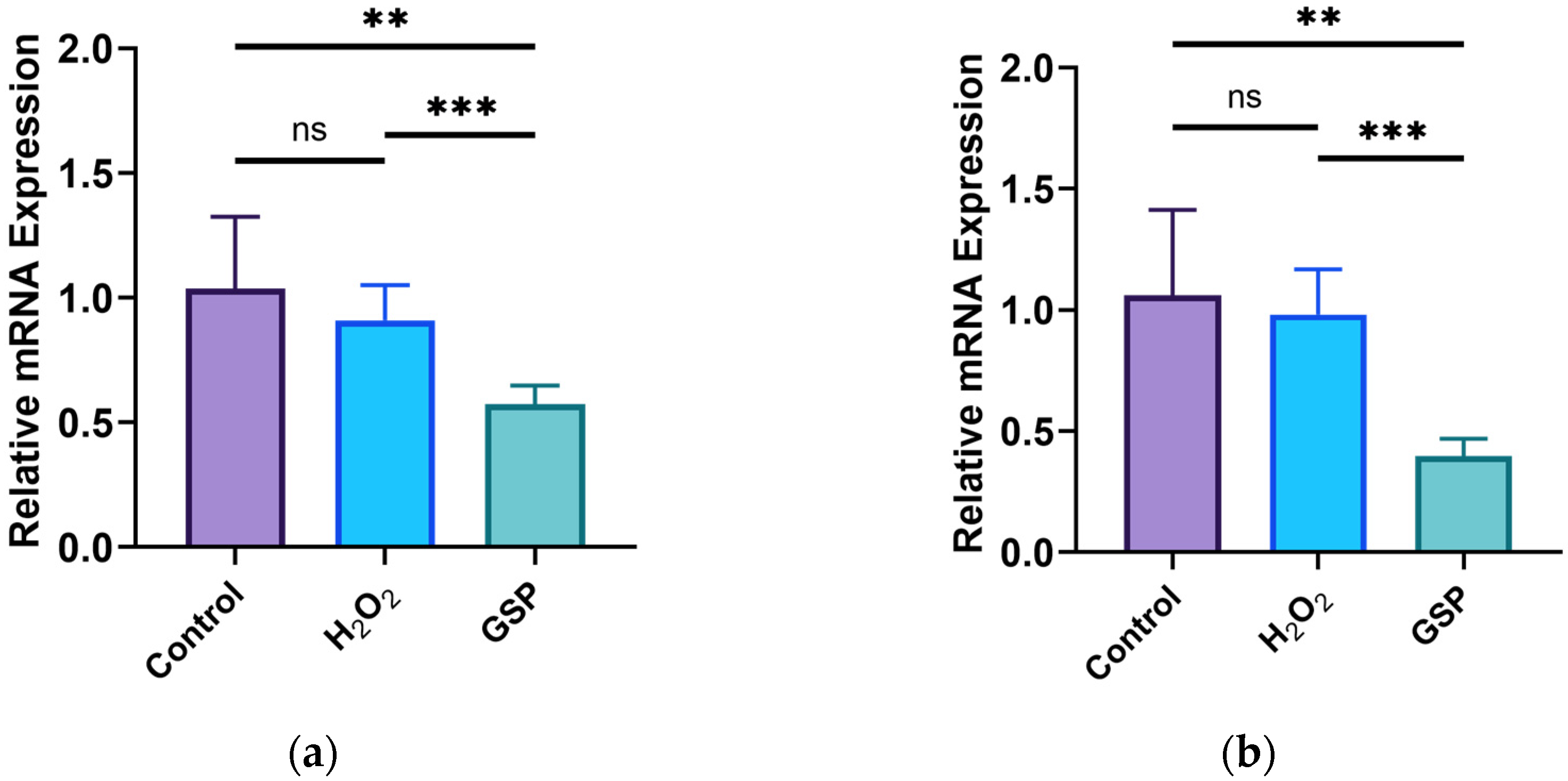
3.4. Effects of GOPs on Tight Junctions of the Gut-on-a-Chip
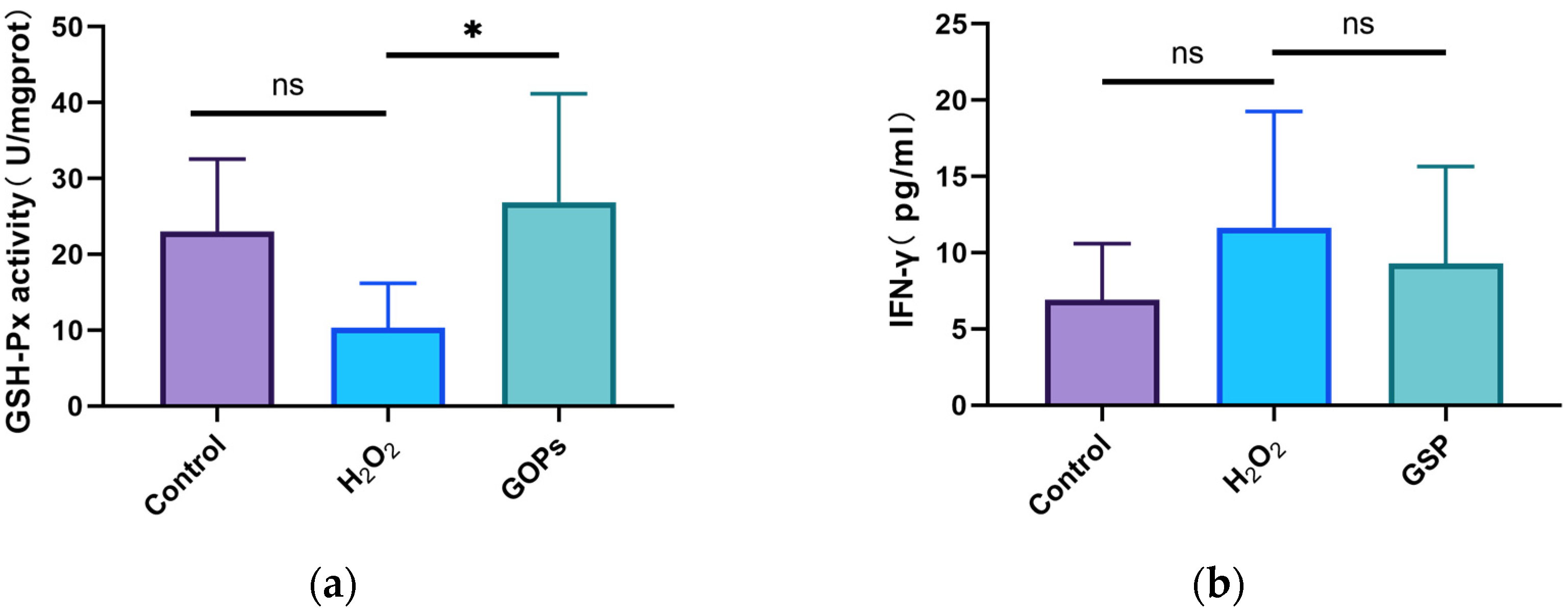
3.5. Effects of GOPs on Oxidative Stress and Inflammation of the Gut-on-a-Chip
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holt, P.R.; Tierney, A.R.; Kotler, D.P. Delayed Enzyme Expression: A Defect of Aging Rat Gut. Gastroenterology 1985, 89, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Salazar, A.M.; Aparicio, R.; Clark, R.I.; Rera, M.; Walker, D.W. Intestinal Barrier Dysfunction: An Evolutionarily Conserved Hallmark of Aging. Dis. Model. Mech. 2023, 16, 4. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Lv, H.; Wang, H.; Yang, H.; Li, Y.; Qian, J. Aging Increases the Severity of Colitis and the Related Changes to the Gut Barrier and Gut Microbiota in Humans and Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Thevaranjan, N.; Puchta, A.; Schulz, C.; Naidoo, A.; Szamosi, J.C.; Verschoor, C.P.; Loukov, D.; Schenck, L.P.; Jury, J.; Foley, K.P.; et al. Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell Host Microbe 2017, 21, 455–466.e4. [Google Scholar] [CrossRef] [PubMed]
- Broderick, N.A.; Buchon, N.; Lemaitre, B. Microbiota-Induced Changes in Drosophila Melanogaster Host Gene Expression and Gut Morphology. mBio 2014, 5, e01117-14. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Karpac, J.; Tran, S.L.; Jasper, H. Pgrp-Sc2 Promotes Gut Immune Homeostasis to Limit Commensal Dysbiosis and Extend Lifespan. Cell 2014, 156, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Rera, M.; Clark, R.I.; Walker, D.W. Intestinal Barrier Dysfunction Links Metabolic and Inflammatory Markers of Aging to Death in Drosophila. Proc. Natl. Acad. Sci. USA 2012, 109, 21528–21533. [Google Scholar] [CrossRef]
- Merchant, H.A.; Liu, F.; Gul, M.O.; Basit, A.W. Age-Mediated Changes in the Gastrointestinal Tract. Int. J. Pharm. 2016, 512, 382–395. [Google Scholar] [CrossRef]
- Benz, C.C.; Yau, C. Ageing, Oxidative Stress and Cancer: Paradigms in Parallax. Nat. Rev. Cancer 2008, 8, 875–879. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, Y.; Guo, H.; Hai, Y.; Luo, Y.; Yue, T. Mechanism and Intervention Measures of Iron Side Effects on the Intestine. Crit. Rev. Food Sci. Nutr. 2020, 60, 2113–2125. [Google Scholar] [CrossRef]
- Barzilai, A.; Yamamoto, K. DNA Damage Responses to Oxidative Stress. DNA Repair 2004, 3, 1109–1115. [Google Scholar] [CrossRef]
- Weiss, G.A.; Hennet, T. Mechanisms and Consequences of Intestinal Dysbiosis. Cell Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.N.; Kim, T.Y.; Park, E.K.; Kim, J.Y.; Jeong, G.S. Panax Ginseng Fruit Has Anti-Inflammatory Effect and Induces Osteogenic Differentiation by Regulating Nrf2/Ho-1 Signaling Pathway in In Vitro and In Vivo Models of Periodontitis. Antioxidants 2020, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Shao, S.; Zhang, Y.; Zhao, D.; Wang, M. Insight into Polysaccharides from Panax Ginseng C. A. Meyer in Improving Intestinal Inflammation: Modulating Intestinal Microbiota and Autophagy. Front. Immunol. 2021, 12, 683911. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.X.; Zhou, Q.L.; Yang, M.; Wang, Y.; Cui, Z.Y.; Liu, Y.Q.; Ikejima, T. Hypoglycemic Mechanism of Ginseng Glycopeptide. Acta Pharmacol. Sin. 2003, 24, 61–66. [Google Scholar] [PubMed]
- Li, H.; Song, J.; Zhang, J.; Wang, T.; Yan, Y.; Tao, Z.; Li, S.; Zhang, H.; Kang, T.; Yang, J. Ginseng Protein Reverses Amyloid Beta Peptide and H2O2 Cytotoxicity in Neurons, and Ameliorates Cognitive Impairment in Ad Rats Induced by a Combination of D-Galactose and Alcl3. Phytother. Res. 2017, 31, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Xu, M.H.; Li, Y. Bioactive Oligopeptides from Ginseng (Panax Ginseng Meyer) Suppress Oxidative Stress-Induced Senescence in Fibroblasts Via Nad+/Sirt1/Pgc-1α Signaling Pathway. Nutrients 2022, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Li, Y.; Xu, M. Beneficial Effects of Small-Molecule Oligopeptides Isolated from Panax Ginseng C. A. Meyer on Cellular Fates in Oxidative Stress-Induced Damaged Human Umbilical Vein Endothelial Cells and PC-12. Int. J. Mol. Sci. 2024, 25, 2906. [Google Scholar] [CrossRef]
- He, L.X.; Wang, J.B.; Sun, B.; Zhao, J.; Li, L.; Xu, T.; Li, H.; Sun, J.Q.; Ren, J.; Liu, R.; et al. Suppression of TNF-α and free radicals reduces systematic inflammatory and metabolic disorders: Radioprotective effects of ginseng oligopeptides on intestinal barrier function and antioxidant defense. J. Nutr. Biochem. 2017, 40, 53–61. [Google Scholar] [CrossRef]
- He, L.X.; Zhang, Z.F.; Zhao, J.; Li, L.; Xu, T.; Sun, B.; Ren, J.W.; Liu, R.; Chen, Q.H.; Wang, J.B.; et al. Ginseng oligopeptides protect against irradiation-induced immune dysfunction and intestinal injury. Sci. Rep. 2018, 8, 13916. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a Dish: Modeling Development and Disease Using Organoid Technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.K.; Nachun, D.; Martin, M.G.; Horvath, S.; Coppola, G.; Jones, D.L. DNA Methylation Analysis Validates Organoids as a Viable Model for Studying Human Intestinal Aging. Cell Mol. Gastroenterol. Hepatol. 2020, 9, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Huang, J.; Zhao, L.; Pan, X.; Liao, C.; Jiang, Q.; Lei, J.; Guo, F.; Cui, J.; Guo, Y.; et al. Dietary Genistein Increases Microbiota-Derived Short Chain Fatty Acid Levels, Modulates Homeostasis of the Aging Gut, and Extends Healthspan and Lifespan. Pharmacol. Res. 2023, 188, 106676. [Google Scholar] [CrossRef]
- Arnold, J.W.; Roach, J.; Fabela, S.; Moorfield, E.; Ding, S.; Blue, E.; Dagher, S.; Magness, S.; Tamayo, R.; Bruno-Barcena, J.M.; et al. The Pleiotropic Effects of Prebiotic Galacto-Oligosaccharides on the Aging Gut. Microbiome 2021, 9, 31. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Q.-H.; Ren, J.-W.; Sun, B.; Cai, X.-X.; Li, D.; Mao, R.-X.; Wu, X.; Li, Y. Ginseng (Panax Ginseng Meyer) Oligopeptides Protect against Binge Drinking-Induced Liver Damage through Inhibiting Oxidative Stress and Inflammation in Rats. Nutrients 2018, 10, 1665. [Google Scholar] [CrossRef] [PubMed]
- Arnauts, K.; Verstockt, B.; Ramalho, A.S.; Vermeire, S.; Verfaillie, C.; Ferrante, M. Ex Vivo Mimicking of Inflammation in Organoids Derived from Patients with Ulcerative Colitis. Gastroenterology 2020, 159, 1564–1567. [Google Scholar] [CrossRef]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.; Van Es, J.H.; Van den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-Term Expansion of Epithelial Organoids from Human Colon, Adenoma, Adenocarcinoma, and Barrett’s Epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef]
- Beaurivage, C.; Kanapeckaite, A.; Loomans, C.; Erdmann, K.S.; Stallen, J.; Janssen, R.A.J. Development of a Human Primary Gut-on-a-Chip to Model Inflammatory Processes. Sci. Rep. 2020, 10, 21475. [Google Scholar] [CrossRef]
- Maurer, M.; Gresnigt, M.S.; Last, A.; Wollny, T.; Berlinghof, F.; Pospich, R.; Cseresnyes, Z.; Medyukhina, A.; Graf, K.; Gröger, M.; et al. A Three-Dimensional Immunocompetent Intestine-on-Chip Model as in Vitro Platform for Functional and Microbial Interaction Studies. Biomaterials 2019, 220, 119396. [Google Scholar] [CrossRef]
- Qu, M.; Xiong, L.; Lyu, Y.; Zhang, X.; Shen, J.; Guan, J.; Chai, P.; Lin, Z.; Nie, B.; Li, C.; et al. Establishment of Intestinal Organoid Cultures Modeling Injury-Associated Epithelial Regeneration. Cell Res. 2021, 31, 259–271. [Google Scholar] [CrossRef]
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef] [PubMed]
- Pentinmikko, N.; Iqbal, S.; Mana, M.; Andersson, S.; Cognetta, A.B., 3rd; Suciu, R.; Roper, M.J.; Luopajarvi, K.; Markelin, E.; Tammela, S. Notum Produced by Paneth Cells Attenuates Regeneration of Aged Intestinal Epithelium. Nature 2019, 571, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Funk, M.C.; Zhou, J.; Boutros, M. Ageing, Metabolism and the Intestine. EMBO Rep. 2020, 21, e50047. [Google Scholar] [CrossRef]
- Ahmadi, S.; Wang, S.H.; Nagpal, R.; Wang, B.; Jain, S.; Razazan, A.; Mishra, S.P.; Zhu, X.; Wang, Z.; Kavanagh, K.; et al. A Human-Origin Probiotic Cocktail Ameliorates Aging-Related Leaky Gut and Inflammation Via Modulating the Microbiota/Taurine/Tight Junction Axis. JCI Insight 2020, 5, 132055. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Razazan, A.; Nagpal, R.; Jain, S.; Wang, B.; Mishra, S.P.; Wang, S.; Justice, J.; Ding, J.; A McClain, D.; et al. Metformin Reduces Aging-Related Leaky Gut and Improves Cognitive Function by Beneficially Modulating Gut Microbiome/Goblet Cell/Mucin Axis. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, e9–e21. [Google Scholar] [CrossRef]
- Bloom, S.I.; Islam, T.; Lesniewski, L.A.; Donato, A.J. Mechanisms and Consequences of Endothelial Cell Senescence. Nat. Rev. Cardiol. 2023, 20, 38–51. [Google Scholar] [CrossRef]
- Liang, D.; Su, W.; Tan, M. Advances of Microfluidic Intestine-on-a-Chip for Analyzing Anti-Inflammation of Food. Crit. Rev. Food Sci. Nutr. 2022, 62, 4418–4434. [Google Scholar] [CrossRef]
- Yun, J.; Hansen, S.; Morris, O.; Madden, D.T.; Libeu, C.P.; Kumar, A.J.; Wehrfritz, C.; Nile, A.H.; Zhang, Y.; Zhou, L.; et al. Senescent Cells Perturb Intestinal Stem Cell Differentiation through Ptk7 Induced Noncanonical Wnt and Yap Signaling. Nat. Commun. 2023, 14, 156. [Google Scholar] [CrossRef]
- Biragyn, A.; Ferrucci, L. Gut Dysbiosis: A Potential Link between Increased Cancer Risk in Ageing and Inflammaging. Lancet Oncol. 2018, 19, e295–e304. [Google Scholar] [CrossRef]
- Citi, S. Intestinal Barriers Protect against Disease. Science 2018, 359, 1097–1098. [Google Scholar] [CrossRef]
- Zhang, P.; Edgar, B.A. Lipoic Acid and Autophagy: New Insights into Stem Cell Aging. EMBO Rep. 2020, 21, e51175. [Google Scholar] [CrossRef]
- Holt, P.R.; Yeh, K.Y. Colonic Proliferation Is Increased in Senescent Rats. Gastroenterology 1988, 95, 1556–1563. [Google Scholar] [CrossRef]
- Sasaki, A.; Nishimura, T.; Takano, T.; Naito, S.; Yoo, S.K. White Regulates Proliferative Homeostasis of Intestinal Stem Cells During Ageing in Drosophila. Nat. Metab. 2021, 3, 546–557. [Google Scholar] [CrossRef]
- Resnik-Docampo, M.; Koehler, C.L.; Clark, R.I.; Schinaman, J.M.; Sauer, V.; Wong, D.M.; Lewis, S.; D’alterio, C.; Walker, D.W.; Jones, D.L. Tricellular Junctions Regulate Intestinal Stem Cell Behaviour to Maintain Homeostasis. Nat. Cell Biol. 2017, 19, 52–59. [Google Scholar] [CrossRef]
- Sang, X.; Wang, Q.; Ning, Y.; Wang, H.; Zhang, R.; Li, Y.; Fang, B.; Lv, C.; Zhang, Y.; Wang, X.; et al. Age-Related Mucus Barrier Dysfunction in Mice Is Related to the Changes in Muc2 Mucin in the Colon. Nutrients 2023, 15, 1830. [Google Scholar] [CrossRef]
- Correia-Melo, C.; Marques, F.D.M.; Anderson, R.; Hewitt, G.; Hewitt, R.; Cole, J.; Carroll, B.M.; Miwa, S.; Birch, J.; Merz, A.; et al. Mitochondria are Required for Pro-Ageing Features of the Senescent Phenotype. EMBO J. 2016, 35, 724–742. [Google Scholar] [CrossRef] [PubMed]
- Codoñer-Franch, P.; Valls-Bellés, V.; Arilla-Codoñer, A.; Alonso-Iglesias, E. Oxidant Mechanisms in Childhood Obesity: The Link between Inflammation and Oxidative Stress. Transl. Res. 2011, 158, 369–384. [Google Scholar] [CrossRef]
- Nunes, S.O.; Vargas, H.O.; Prado, E.; Barbosa, D.S.; de Melo, L.P.; Moylan, S.; Dodd, S.; Berk, M. The Shared Role of Oxidative Stress and Inflammation in Major Depressive Disorder and Nicotine Dependence. Neurosci. Biobehav. Rev. 2013, 37, 1336–1345. [Google Scholar] [CrossRef]
- Circu, M.L.; Aw, T.Y. Intestinal Redox Biology and Oxidative Stress. Semin. Cell Dev. Biol. 2012, 23, 729–737. [Google Scholar] [CrossRef]
- Huang, C.; Cao, X.; Chen, X.; Fu, Y.; Zhu, Y.; Chen, Z.; Luo, Q.; Li, L.; Song, X.; Jia, R.; et al. A Pectic Polysaccharide from Ligusticum Chuanxiong Promotes Intestine Antioxidant Defense in Aged Mice. Carbohydr. Polym. 2017, 174, 915–922. [Google Scholar] [CrossRef] [PubMed]



| Gene | Forward | Reverse |
|---|---|---|
| p16 | ATGGAGCCTTCGGCTGACT | GTAACTATTCGGTGCGTTGGG |
| p21 | AGGTGGACCTGGAGACTCTCAG | TCCTCTTGGAGAAGATCAGCCG |
| Ki67 | ACGCCTGGTTACTATCAAAAGG | CAGACCCATTTACTTGTGTTGGA |
| Lgr5 | GAGTTACGTCTTGCGGGAAAC | TGGGTACGTGTCTTAGCTGATTA |
| MUC2 | ACTCTCCACACCCAGCATCATC | GTGTCTCCGTATGTGCCGTTGT |
| P-gp | CCCATCATTGCAATAGCAGG | TGTTCAAACTTCTGCTCCTGA |
| ZO-1 | ACCAGTAAGTCGTCCTGATCC | TCGGCCAAATCTTCTCACTCC |
| Occludin | TGGGTACGTGTCTTAGCTGATTA | GTCATCCACAGGCGAAGTTAAT |
| Claudin-1 | GTCTTTGACTCCTTGCTGAATCTG | CACCTCATCGTCTTCCAAGCAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, M.; Xu, M. Ginseng Oligopeptides Improve the Intestinal Physiology and Promote the Antioxidant Capacity of the Gut-on-a-Chip Model. Nutrients 2024, 16, 845. https://doi.org/10.3390/nu16060845
You M, Xu M. Ginseng Oligopeptides Improve the Intestinal Physiology and Promote the Antioxidant Capacity of the Gut-on-a-Chip Model. Nutrients. 2024; 16(6):845. https://doi.org/10.3390/nu16060845
Chicago/Turabian StyleYou, Mei, and Meihong Xu. 2024. "Ginseng Oligopeptides Improve the Intestinal Physiology and Promote the Antioxidant Capacity of the Gut-on-a-Chip Model" Nutrients 16, no. 6: 845. https://doi.org/10.3390/nu16060845
APA StyleYou, M., & Xu, M. (2024). Ginseng Oligopeptides Improve the Intestinal Physiology and Promote the Antioxidant Capacity of the Gut-on-a-Chip Model. Nutrients, 16(6), 845. https://doi.org/10.3390/nu16060845






