Live and Heat-Inactivated Streptococcus thermophilus MN-ZLW-002 Mediate the Gut–Brain Axis, Alleviating Cognitive Dysfunction in APP/PS1 Mice
Abstract
1. Introduction
2. Materials and Methods
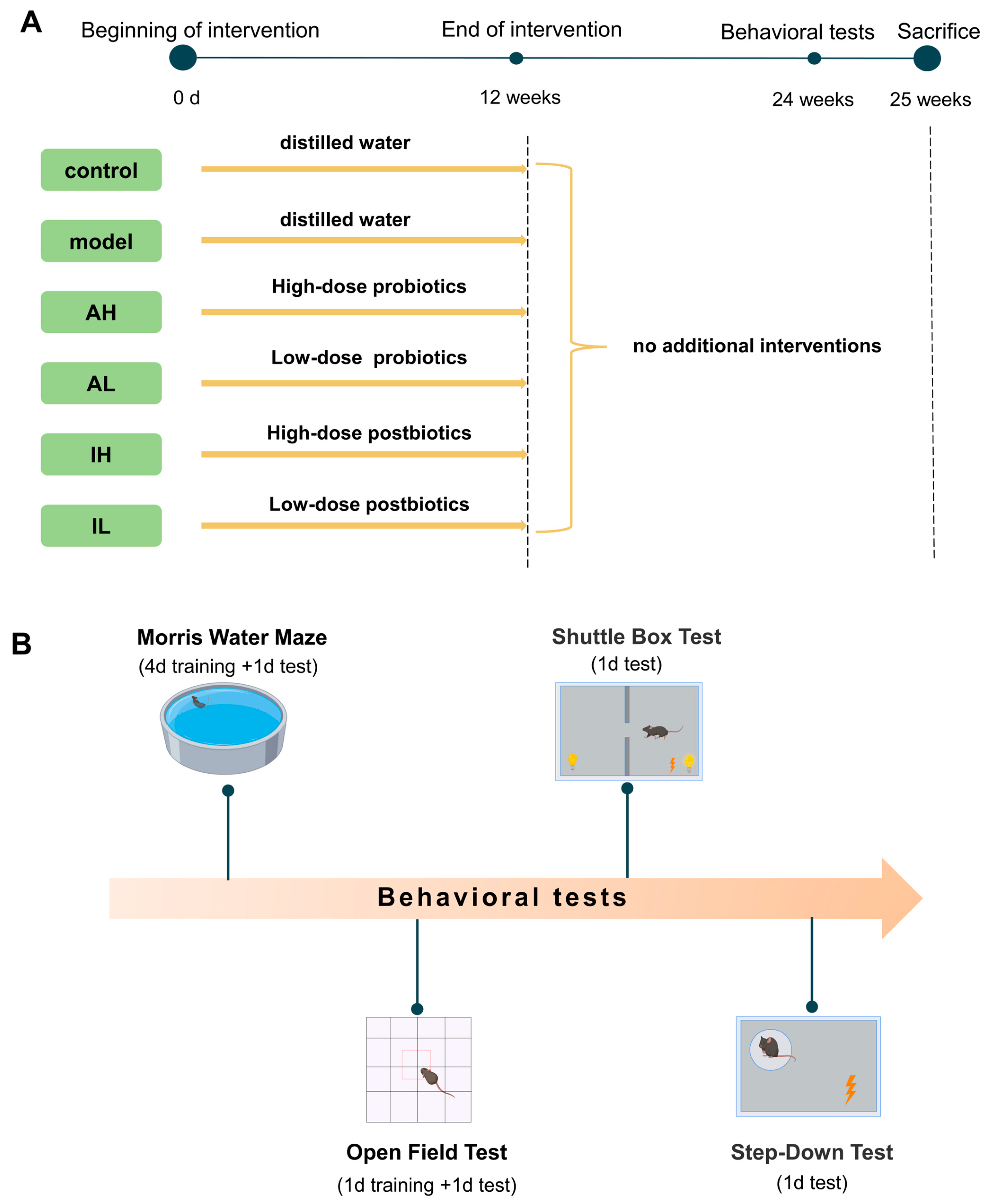
2.1. Animals
2.2. Probiotic Treatment
2.3. Morris Water Maze Test (MWM)
2.4. Open Field Test (OFT)
2.5. Shuttle Box Test
2.6. Step-Down Test
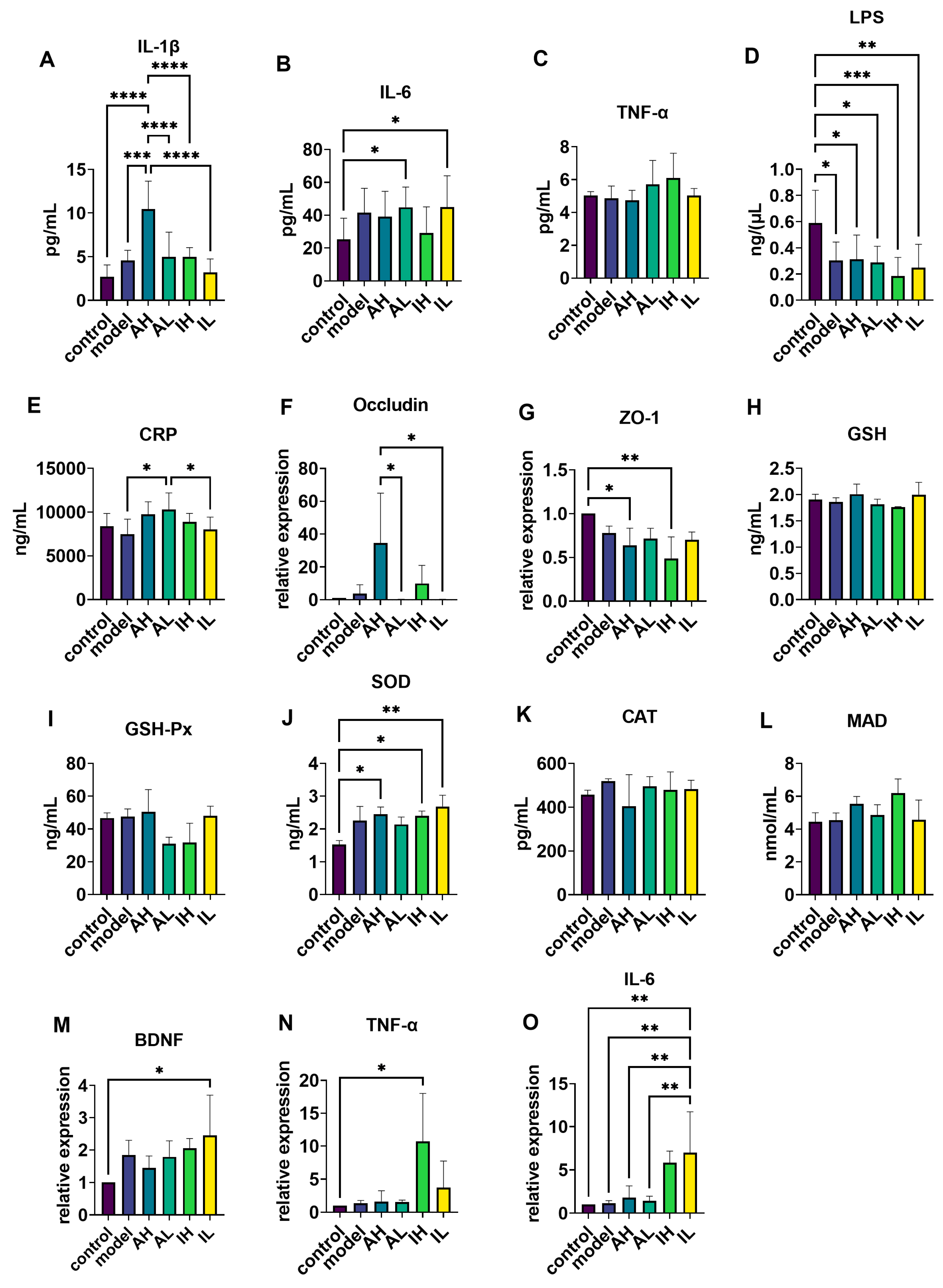
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
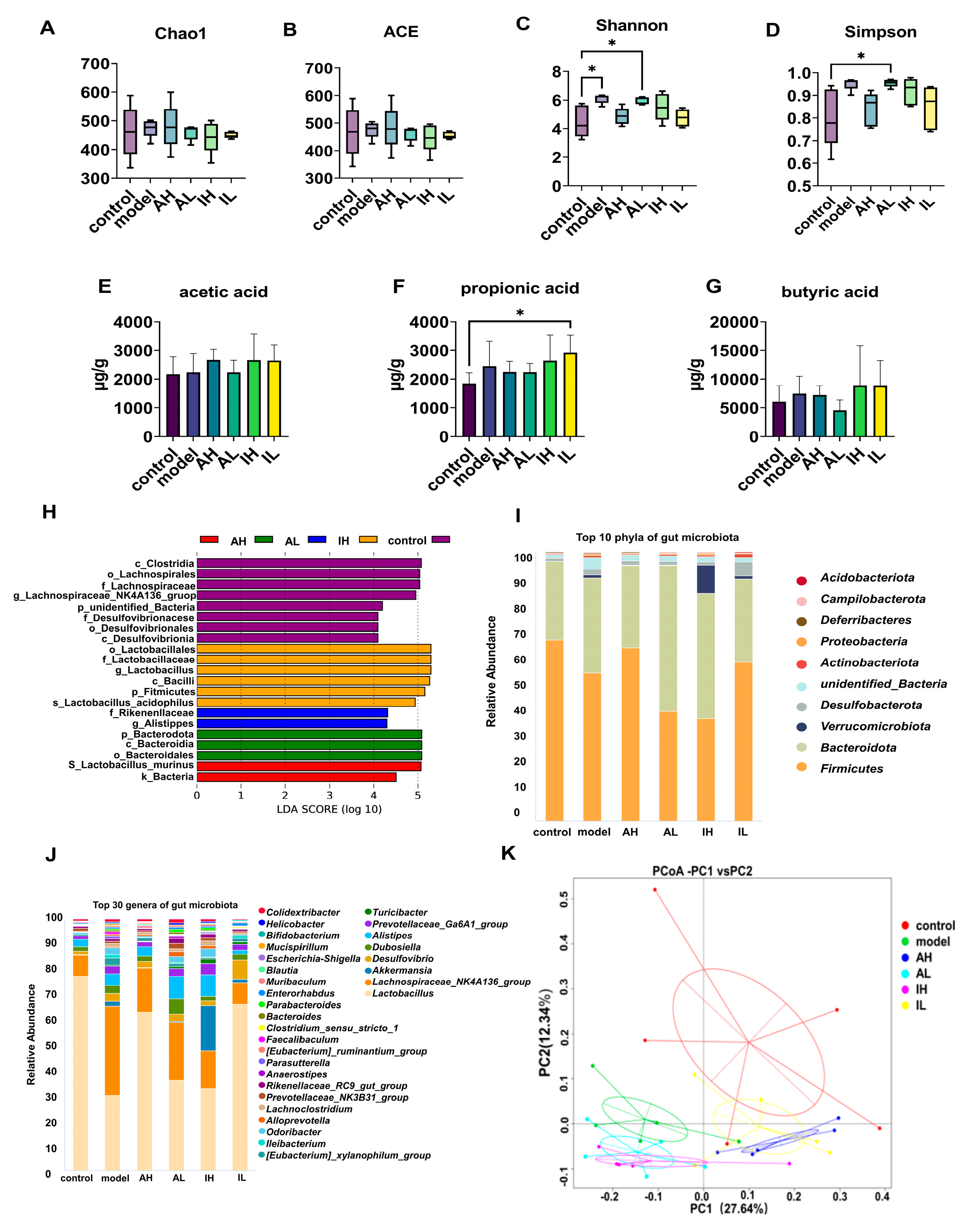
2.9. 16S rRNA-Encoding Gene Sequencing and Bioinformatics Analysis
2.10. Immunofluorescence and Immunohistochemistry
2.11. Detection of Short-Chain Fatty Acids in Colonic Contents
2.12. Statistical Analysis
3. Results
3.1. Changes in Spatial Memory, Anxiety, and Short-Term Memory
3.2. Changes in Hippocampal Deposits of Aβ40, Astrogliosis, and Microgliosis
3.3. Alterations in Gut Microbiota and Their Metabolites
3.4. Changes in Serum Inflammatory Markers
3.5. Changes in the mRNA Expression of Ileum Tight Junction Proteins
3.6. Changes in Antioxidation-Related Enzymes, mRNA Levels of BDNF, and Inflammatory Marker Expression in the Hippocampus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, S.; Barve, K.H.; Kumar, M.S. Recent Advancements in Pathogenesis, Diagnostics and Treatment of Alzheimer’s Disease. Curr. Neuropharmacol. 2020, 18, 1106–1125. [Google Scholar] [CrossRef]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Edwards, S.M.; Cunningham, S.A.; Dunlop, A.L.; Corwin, E.J. The Maternal Gut Microbiome During Pregnancy. MCN Am. J. Matern. Child. Nurs. 2017, 42, 310–317. [Google Scholar] [CrossRef]
- Valle Gottlieb, M.G.; Closs, V.E.; Junges, V.M.; Schwanke, C.H.A. Impact of human aging and modern lifestyle on gut microbiota. Crit. Rev. Food Sci. Nutr. 2018, 58, 1557–1564. [Google Scholar] [CrossRef]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef]
- De Pessemier, B.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut-Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms 2021, 9, 353. [Google Scholar] [CrossRef]
- Saint-Criq, V.; Lugo-Villarino, G.; Thomas, M. Dysbiosis, malnutrition and enhanced gut-lung axis contribute to age-related respiratory diseases. Ageing Res. Rev. 2021, 66, 101235. [Google Scholar] [CrossRef]
- Mancuso, C.; Santangelo, R. Alzheimer’s disease and gut microbiota modifications: The long way between preclinical studies and clinical evidence. Pharmacol. Res. 2018, 129, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Kesika, P.; Suganthy, N.; Sivamaruthi, B.S.; Chaiyasut, C. Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer’s disease. Life Sci. 2021, 264, 118627. [Google Scholar] [CrossRef] [PubMed]
- Ou, Z.; Deng, L.; Lu, Z.; Wu, F.; Liu, W.; Huang, D.; Peng, Y. Protective effects of Akkermansia muciniphila on cognitive deficits and amyloid pathology in a mouse model of Alzheimer’s disease. Nutr. Diabetes 2020, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Hu, A.; Shu, X.; Huang, W.; Liu, J.; Wang, B.; Zhang, R.; Yue, M.; Yang, C. Lactobacillus plantarum-derived postbiotics prevent Salmonella-induced neurological dysfunctions by modulating gut-brain axis in mice. Front. Nutr. 2022, 9, 946096. [Google Scholar] [CrossRef] [PubMed]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics-A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Vinderola, G.; Shamir, R.; Swann, J.R.; Szajewska, H.; Sanders, M.E.; Lebeer, S.; Quigley, E.M.M.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Nimgampalle, M.; Kuna, Y. Anti-Alzheimer Properties of Probiotic, Lactobacillus plantarum MTCC 1325 in Alzheimer’s Disease induced Albino Rats. J. Clin. Diagn. Res. JCDR 2017, 11, Kc01–Kc05. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Cha, L.; Sim, M.; Jung, S.; Chun, W.Y.; Baik, H.W.; Shin, D.M. Probiotic Supplementation Improves Cognitive Function and Mood with Changes in Gut Microbiota in Community-Dwelling Older Adults: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 32–40. [Google Scholar] [CrossRef]
- McFarland, L.V.; Evans, C.T.; Goldstein, E.J.C. Strain-Specificity and Disease-Specificity of Probiotic Efficacy: A Systematic Review and Meta-Analysis. Front. Med. 2018, 5, 124. [Google Scholar] [CrossRef]
- Kang, X.; Liang, H.; Luo, Y.; Li, Z.; He, F.; Han, X.; Zhang, L. Streptococcus thermophilus MN-ZLW-002 Can Inhibit Pre-adipocyte Differentiation through Macrophage Activation. Biol. Pharm. Bull. 2021, 44, 316–324. [Google Scholar] [CrossRef]
- Kang, X.; Liang, H.; Zhang, L.; Luo, Y.; Li, Z.; He, F.; Han, X. Anti-adipogenesis and metabolism-regulating effects of heat-inactivated Streptococcus thermophilus MN-ZLW-002. Lett. Appl. Microbiol. 2021, 72, 677–687. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, H.; Wang, Y.; Cheng, R.; Pu, F.; Yang, Y.; Li, J.; Wu, S.; Shen, X.; He, F. Heat-inactivated Lacticaseibacillus paracasei N1115 alleviates the damage due to brain function caused by long-term antibiotic cocktail exposure in mice. BMC Neurosci. 2022, 23, 38. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Sugahara, H.; Shimada, K.; Mitsuyama, E.; Kuhara, T.; Yasuoka, A.; Kondo, T.; Abe, K.; Xiao, J.Z. Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer’s disease. Sci. Rep. 2017, 7, 13510. [Google Scholar] [CrossRef]
- Stoeva, M.K.; Garcia-So, J.; Tyagi, S.; Nemchek, M.; McMurdie, P.J.; Myers, J.; Kolterman, O.; Justice, N.; Eid, J. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes 2021, 13, 1–28. [Google Scholar] [CrossRef]
- Chudzik, A.; Orzyłowska, A.; Stanisz, G.J.; Rola, R. Probiotics, Prebiotics and Postbiotics on Mitigation of Depression Symptoms: Modulation of the Brain-Gut-Microbiome Axis. Biomol. Ther. 2021, 11, 1000. [Google Scholar] [CrossRef]
- Varian, B.J.; Poutahidis, T.; DiBenedictis, B.T.; Levkovich, T.; Ibrahim, Y.; Didyk, E.; Shikhman, L.; Cheung, H.K.; Hardas, A.; Ricciardi, C.E.; et al. Microbial lysate upregulates host oxytocin. Brain. Behav. Immun. 2017, 61, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Bäuerl, C.; Collado, M.C.; Diaz Cuevas, A.; Viña, J.; Pérez Martínez, G. Shifts in gut microbiota composition in an APP/PSS1 transgenic mouse model of Alzheimer’s disease during lifespan. Lett. Appl. Microbiol. 2018, 66, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain. Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef]
- Ding, X.; Xu, Y.; Zhang, X.; Zhang, L.; Duan, G.; Song, C.; Li, Z.; Yang, Y.; Wang, Y.; Wang, X.; et al. Gut microbiota changes in patients with autism spectrum disorders. J. Psychiatr. Res. 2020, 129, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Shin, Y.C.; Kim, T.Y.; Kim, Y.; Lee, Y.S.; Lee, S.H.; Kim, M.N.; O, E.; Kim, K.S.; Kweon, M.N. Mucin degrader Akkermansia muciniphila accelerates intestinal stem cell-mediated epithelial development. Gut Microbes 2021, 13, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, N.; Tan, H.Y.; Li, S.; Zhang, C.; Feng, Y. Function of Akkermansia muciniphila in Obesity: Interactions With Lipid Metabolism, Immune Response and Gut Systems. Front. Microbiol. 2020, 11, 219. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Cho, C.H.; Yun, M.S.; Jang, S.J.; You, H.J.; Kim, J.H.; Han, D.; Nam, T.W.; Ko, G.; Moon, S.H.; et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat. Microbiol. 2021, 6, 563–573. [Google Scholar] [CrossRef]
- Hou, Y.F.; Shan, C.; Zhuang, S.Y.; Zhuang, Q.Q.; Ghosh, A.; Zhu, K.C.; Kong, X.K.; Wang, S.M.; Gong, Y.L.; Yang, Y.Y.; et al. Gut microbiota-derived propionate mediates the neuroprotective effect of osteocalcin in a mouse model of Parkinson’s disease. Microbiome 2021, 9, 34. [Google Scholar] [CrossRef]
- González Olmo, B.M.; Butler, M.J.; Barrientos, R.M. Evolution of the Human Diet and Its Impact on Gut Microbiota, Immune Responses, and Brain Health. Nutrients 2021, 13, 196. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The microbiome and innate immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef]
- Hao, X.; Ding, N.; Zhang, Y.; Yang, Y.; Zhao, Y.; Zhao, J.; Li, Y.; Li, Z. Benign regulation of the gut microbiota: The possible mechanism through which the beneficial effects of manual acupuncture on cognitive ability and intestinal mucosal barrier function occur in APP/PS1 mice. Front. Neurosci. 2022, 16, 960026. [Google Scholar] [CrossRef]
- Pepys, M.B.; Hirschfield, G.M. C-reactive protein: A critical update. J. Clin. Investig. 2003, 111, 1805–1812. [Google Scholar] [CrossRef]
- Chakrabarty, P.; Jansen-West, K.; Beccard, A.; Ceballos-Diaz, C.; Levites, Y.; Verbeeck, C.; Zubair, A.C.; Dickson, D.; Golde, T.E.; Das, P. Massive gliosis induced by interleukin-6 suppresses Abeta deposition in vivo: Evidence against inflammation as a driving force for amyloid deposition. FASEB J. 2010, 24, 548–559. [Google Scholar] [CrossRef]
- Bolmont, T.; Haiss, F.; Eicke, D.; Radde, R.; Mathis, C.A.; Klunk, W.E.; Kohsaka, S.; Jucker, M.; Calhoun, M.E. Dynamics of the microglial/amyloid interaction indicate a role in plaque maintenance. J. Neurosci. 2008, 28, 4283–4292. [Google Scholar] [CrossRef]
- Poo, M.M. Neurotrophins as synaptic modulators. Nat. Rev. Neurosci. 2001, 2, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Bramham, C.R.; Messaoudi, E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Prog. Neurobiol. 2005, 76, 99–125. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef] [PubMed]
- Multhaup, G.; Ruppert, T.; Schlicksupp, A.; Hesse, L.; Beher, D.; Masters, C.L.; Beyreuther, K. Reactive oxygen species and Alzheimer’s disease. Biochem. Pharmacol. 1997, 54, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Aborode, A.T.; Pustake, M.; Awuah, W.A.; Alwerdani, M.; Shah, P.; Yarlagadda, R.; Ahmad, S.; Silva Correia, I.F.; Chandra, A.; Nansubuga, E.P.; et al. Targeting Oxidative Stress Mechanisms to Treat Alzheimer’s and Parkinson’s Disease: A Critical Review. Oxid. Med. Cell. Longev. 2022, 2022, 7934442. [Google Scholar] [CrossRef] [PubMed]


| Target | Sequences |
|---|---|
| GAPDH | F: 5′-CCT TCC GTG TTC CTA CCC C-3′ R: 5′-GCC CAA GAT GCC CTT CAG T-3′ |
| BDNF | F: 5′-TGG AAC TCG CAA TGC CGA ACT AC-3′ R: 5′-TCC TTA TGA ATC GCC AGC CAA TTC TC-3′ |
| IL-6 | F: 5′-GTC ACA GAA GGA GTG GCT A-3′ R: 5′-AGA GAA CAA CAT AAG TCA GAT ACC-3′ |
| TNF-α | F: 5′-CTC TTC AAG GGA CAA GGC TG-3′ R: 5′-CGG ACT CCG CAA AGT CTA AG-3′ |
| Occludin | F: 5′-GCG AGG AGC TGG AGG AGG AC-3′ R: 5′-CGT CGT CTA GTT CTG CCT GTA AGC-3′ |
| ZO-1 | F: 5′-GCG AAC AGA AGG AGC GAG AAG AG-3′ R: 5′-GCT TTG CGG GCT GAC TGG AG-3′ |
| Control (%) | Model (%) | AH (%) | AL (%) | IH (%) | IL (%) | |
|---|---|---|---|---|---|---|
| Actinobacteriota | 0.36 | 0.70 | 0.38 | 0.55 | 0.59 | 1.42 * |
| Bacteroidota | 29.03 | 34.19 | 30.02 | 53.12 *# | 45.93 | 30.60 # |
| Campilobacterota | 0.06 | 0.26 | 0.08 | 0.30 * | 0.30 | 0.05 |
| Deferribacteres | 0.05 | 0.38 ** | 0.07 # | 0. 10 # | 0.10 # | 0.04 ## |
| Firmicutes | 66.40 | 53.07 | 63.25 | 40.04 * | 37.30 * | 58.58 |
| Verrumicrobiota | 0.04 | 1.35 * | 0.19 | 0.19 | 10.26 ** | 1.14 * |
| Control (%) | Model (%) | AH (%) | AL (%) | IH (%) | IL (%) | |
|---|---|---|---|---|---|---|
| Akkermansia | 0.04 | 1.10 * | 0.19 | 0.19 | 10.26 ** | 1.12 |
| Bifidobacterium | 0.06 | 0.26 * | 0.02 ## | 0.09 *# | 0.08 # | 0.24 |
| Blautia | 0.04 | 0.10 * | 0.12 ** | 0.02 | 0.25 ** | 0.07 |
| Enterorhabdus | 0.20 | 0.34 | 0.20 | 0.37 | 0.36 | 0.68 * |
| Helicobacter | 0.05 | 0.26 | 0.08 | 0.35 * | 0.30 | 0.05 |
| Ileibacterium | 0.03 | 0.80 ** | 0. 12 | 0.16 * | 0.15 | 0.69 |
| Intestinimonas | 0.07 | 0.27 ** | 0.02 | 0.26 ** | 0.15 | 0.05 |
| Lachnoclostridium | 0.24 | 0.87 * | 0.57 | 0.58 | 1.02 | 0.57 |
| Lactobacillus | 54.73 | 18.21 * | 41.84 | 16.57 ** | 18.63 * | 44.08 |
| Monoglobus | 0.02 | 0.04 ** | 0.03 | 0.03 | 0.03 | 0.04 |
| Mucispirillum | 0.05 | 0.38 ** | 0.07 # | 0.10 *# | 0.10# | 0.04 # |
| Negativibacillus | 0.01 | 0.03 * | 0.03 | 0.02 * | 0.02 | 0.01 |
| Odoribacter | 0.56 | 0.55 ** | 0.41 | 2.11 | 1.90 * | 0. 35 |
| Oscillibacter | 0.12 | 0.18 | 0.18 | 0.20 * | 0.20 | 0.06 ## |
| Parasutterella | 0.08 | 0.50 ** | 0.07 ## | 0.21 | 0.39 | 0.10 # |
| Parvibacter | <0.001 | 0.01 | <0.001 # | 0.01 * | 0.01 | 0.01 * |
| Streptococcus | 0.03 | 0.02 | 0.02 | 0.01 | 0.18 ** | 0.01 * |
| Turicibacter | 0.07 | 0.22 * | 0.16 | 0.42 * | 1.05 | 0.70 |
| Tuzzerella | 0.02 | 0.04 | 0.04 | 0.08 * | 0.01 * | 0.01 ## |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, Y.; Zhou, Z.; Yang, Y.; Zhao, J.; Kang, X.; Li, Z.; Shen, X.; He, F.; Cheng, R. Live and Heat-Inactivated Streptococcus thermophilus MN-ZLW-002 Mediate the Gut–Brain Axis, Alleviating Cognitive Dysfunction in APP/PS1 Mice. Nutrients 2024, 16, 844. https://doi.org/10.3390/nu16060844
Zhang Y, Wang Y, Zhou Z, Yang Y, Zhao J, Kang X, Li Z, Shen X, He F, Cheng R. Live and Heat-Inactivated Streptococcus thermophilus MN-ZLW-002 Mediate the Gut–Brain Axis, Alleviating Cognitive Dysfunction in APP/PS1 Mice. Nutrients. 2024; 16(6):844. https://doi.org/10.3390/nu16060844
Chicago/Turabian StyleZhang, Yujie, Yimei Wang, Zhimo Zhou, Yang Yang, Jincheng Zhao, Xiaohong Kang, Zhouyong Li, Xi Shen, Fang He, and Ruyue Cheng. 2024. "Live and Heat-Inactivated Streptococcus thermophilus MN-ZLW-002 Mediate the Gut–Brain Axis, Alleviating Cognitive Dysfunction in APP/PS1 Mice" Nutrients 16, no. 6: 844. https://doi.org/10.3390/nu16060844
APA StyleZhang, Y., Wang, Y., Zhou, Z., Yang, Y., Zhao, J., Kang, X., Li, Z., Shen, X., He, F., & Cheng, R. (2024). Live and Heat-Inactivated Streptococcus thermophilus MN-ZLW-002 Mediate the Gut–Brain Axis, Alleviating Cognitive Dysfunction in APP/PS1 Mice. Nutrients, 16(6), 844. https://doi.org/10.3390/nu16060844








