Classification of Vitamin D Status Based on Vitamin D Metabolism: A Randomized Controlled Trial in Hypertensive Patients
Abstract
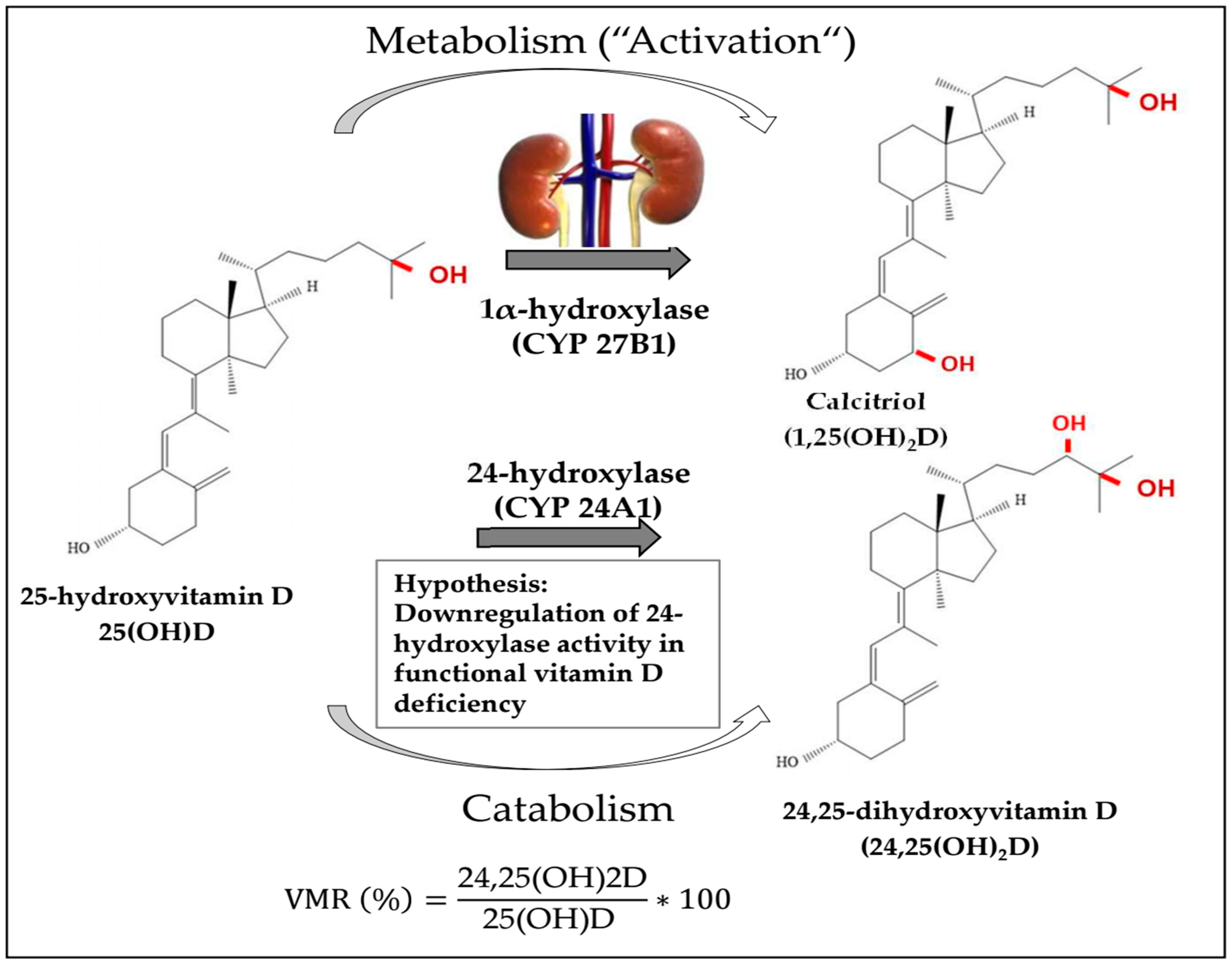
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Laboratory Measurements
2.3. Outcome Measures
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouillon, R. Comparative analysis of nutritional guidelines for vitamin D. Nat. Rev. Endocrinol. 2017, 13, 466–479. [Google Scholar] [CrossRef]
- Buttriss, J.L.; Lanham-New, S.A.; Steenson, S.; Levy, L.; Swan, G.E.; Darling, A.L.; Cashman, K.D.; Allen, R.E.; Durrant, L.R.; Smith, C.P.; et al. Implementation strategies for improving vitamin D status and increasing vitamin D intake in the UK: Current controversies and future perspectives: Proceedings of the 2nd Rank Prize Funds Forum on vitamin D. Br. J. Nutr. 2022, 127, 1567–1587. [Google Scholar] [CrossRef]
- Pludowski, P.; Takacs, I.; Boyanov, M.; Belaya, Z.; Diaconu, C.C.; Mokhort, T.; Zherdova, N.; Rasa, I.; Payer, J.; Pilz, S. Clinical Practice in the Prevention, Diagnosis and Treatment of Vitamin D Deficiency: A Central and Eastern European Expert Consensus Statement. Nutrients 2022, 14, 1483. [Google Scholar] [CrossRef]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Priemel, M.; von Domarus, C.; Klatte, T.O.; Kessler, S.; Schlie, J.; Meier, S.; Proksch, N.; Pastor, F.; Netter, C.; Streichert, T.; et al. Bone mineralization defects and vitamin D deficiency: Histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J. Bone Min. Res. 2010, 25, 305–312. [Google Scholar] [CrossRef]
- Thiering, E.; Bruske, I.; Kratzsch, J.; Hofbauer, L.C.; Berdel, D.; von Berg, A.; Lehmann, I.; Hoffmann, B.; Bauer, C.P.; Koletzko, S.; et al. Associations between serum 25-hydroxyvitamin D and bone turnover markers in a population based sample of German children. Sci. Rep. 2015, 5, 18138. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Cai, Q.; Jiang, D.; Wang, L.; Chen, S.; Jia, W. The Associations of Serum Vitamin D and Bone Turnover Markers with the Type and Severity of Hip Fractures in Older Women. Clin. Interv. Aging 2020, 15, 1971–1978. [Google Scholar] [CrossRef]
- Herrmann, M.; Zelzer, S.; Cavalier, E.; Kleber, M.; Drexler-Helmberg, C.; Schlenke, P.; Curcic, P.; Keppel, M.H.; Enko, D.; Scharnagl, H.; et al. Functional Assessment of Vitamin D Status by a Novel Metabolic Approach: The Low Vitamin D Profile Concept. Clin. Chem. 2023, 69, 1307–1316. [Google Scholar] [CrossRef]
- Francic, V.; Ursem, S.R.; Dirks, N.F.; Keppel, M.H.; Theiler-Schwetz, V.; Trummer, C.; Pandis, M.; Borzan, V.; Grubler, M.R.; Verheyen, N.D.; et al. The Effect of Vitamin D Supplementation on its Metabolism and the Vitamin D Metabolite Ratio. Nutrients 2019, 11, 2539. [Google Scholar] [CrossRef] [PubMed]
- Cavalier, E.; Huyghebaert, L.; Rousselle, O.; Bekaert, A.C.; Kovacs, S.; Vranken, L.; Peeters, S.; Le Goff, C.; Ladang, A. Simultaneous measurement of 25(OH)-vitamin D and 24,25(OH)2-vitamin D to define cut-offs for CYP24A1 mutation and vitamin D deficiency in a population of 1200 young subjects. Clin. Chem. Lab. Med. 2020, 58, 197–201. [Google Scholar] [CrossRef]
- Wagner, D.; Hanwell, H.E.; Schnabl, K.; Yazdanpanah, M.; Kimball, S.; Fu, L.; Sidhom, G.; Rousseau, D.; Cole, D.E.; Vieth, R. The ratio of serum 24,25-dihydroxyvitamin D(3) to 25-hydroxyvitamin D(3) is predictive of 25-hydroxyvitamin D(3) response to vitamin D(3) supplementation. J. Steroid Biochem. Mol. Biol. 2011, 126, 72–77. [Google Scholar] [CrossRef]
- Ginsberg, C.; Katz, R.; de Boer, I.H.; Kestenbaum, B.R.; Chonchol, M.; Shlipak, M.G.; Sarnak, M.J.; Hoofnagle, A.N.; Rifkin, D.E.; Garimella, P.S.; et al. The 24,25 to 25-hydroxyvitamin D ratio and fracture risk in older adults: The cardiovascular health study. Bone 2018, 107, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, C.; Hoofnagle, A.N.; Katz, R.; Becker, J.O.; Kritchevsky, S.B.; Shlipak, M.G.; Sarnak, M.J.; Ix, J.H. The Vitamin D Metabolite Ratio Is Independent of Vitamin D Binding Protein Concentration. Clin. Chem. 2021, 67, 385–393. [Google Scholar] [CrossRef]
- Ginsberg, C.; Hoofnagle, A.N.; Katz, R.; Hughes-Austin, J.; Miller, L.M.; Becker, J.O.; Kritchevsky, S.B.; Shlipak, M.G.; Sarnak, M.J.; Ix, J.H. The Vitamin D Metabolite Ratio Is Associated wsith Changes in Bone Density and Fracture Risk in Older Adults. J. Bone Min. Res. 2021, 36, 2343–2350. [Google Scholar] [CrossRef]
- Pilz, S.; Gaksch, M.; Kienreich, K.; Grubler, M.; Verheyen, N.; Fahrleitner-Pammer, A.; Treiber, G.; Drechsler, C.; Hartaigh, B.ó.; Obermayer-Pietsch, B.; et al. Effects of vitamin D on blood pressure and cardiovascular risk factors: A randomized controlled trial. Hypertension 2015, 65, 1195–1201. [Google Scholar] [CrossRef]
- Zelzer, S.; Meinitzer, A.; Enko, D.; Simstich, S.; Le Goff, C.; Cavalier, E.; Herrmann, M.; Goessler, W. Simultaneous determination of 24,25- and 25,26-dihydroxyvitamin D3 in serum samples with liquid-chromatography mass spectrometry—A useful tool for the assessment of vitamin D metabolism. J. Chromatogr. B 2020, 1158, 122394. [Google Scholar] [CrossRef] [PubMed]
- Pittas, A.G.; Jorde, R.; Kawahara, T.; Dawson-Hughes, B. Vitamin D Supplementation for Prevention of Type 2 Diabetes Mellitus: To D or Not to D? J. Clin. Endocrinol. Metab. 2020, 105, 3721–3733. [Google Scholar] [CrossRef] [PubMed]
- Scragg, R.; Sluyter, J.D. Is There Proof of Extraskeletal Benefits From Vitamin D Supplementation From Recent Mega Trials of Vitamin D? JBMR Plus 2021, 5, e10459. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Camargo, C.A., Jr.; Sluyter, J.D.; Aglipay, M.; Aloia, J.F.; Ganmaa, D.; Bergman, P.; Bischoff-Ferrari, H.A.; Borzutzky, A.; Damsgaard, C.T.; et al. Vitamin D supplementation to prevent acute respiratory infections: A systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021, 9, 276–292. [Google Scholar] [CrossRef]
- Pittas, A.G.; Dawson-Hughes, B.; Sheehan, P.; Ware, J.H.; Knowler, W.C.; Aroda, V.R.; Brodsky, I.; Ceglia, L.; Chadha, C.; Chatterjee, R.; et al. Vitamin D Supplementation and Prevention of Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Dawson-Hughes, B.; Staten, M.A.; Knowler, W.C.; Nelson, J.; Vickery, E.M.; LeBlanc, E.S.; Neff, L.M.; Park, J.; Pittas, A.G.; Group, D.d.R. Intratrial Exposure to Vitamin D and New-Onset Diabetes Among Adults With Prediabetes: A Secondary Analysis from the Vitamin D and Type 2 Diabetes (D2d) Study. Diabetes Care 2020, 43, 2916–2922. [Google Scholar] [CrossRef]
- Favresse, J.; Fangazio, M.; Cotton, F.; Wolff, F. Evaluation of four automated clinical analyzers for the determination of total 25(OH)D in comparison to a certified LC-MS/MS. Clin. Chem. Lab. Med. 2023, 61, 1420–1427. [Google Scholar] [CrossRef]
- Wise, S.A.; Camara, J.E.; Sempos, C.T.; Lukas, P.; Le Goff, C.; Peeters, S.; Burdette, C.Q.; Nalin, F.; Hahm, G.; Durazo-Arvizu, R.A.; et al. Vitamin D Standardization Program (VDSP) intralaboratory study for the assessment of 25-hydroxyvitamin D assay variability and bias. J. Steroid Biochem. Mol. Biol. 2021, 212, 105917. [Google Scholar] [CrossRef]
- Cappellani, D.; Brancatella, A.; Morganti, R.; Borsari, S.; Baldinotti, F.; Caligo, M.A.; Kaufmann, M.; Jones, G.; Marcocci, C.; Cetani, F. Hypercalcemia due to CYP24A1 mutations: A systematic descriptive review. Eur. J. Endocrinol. 2021, 186, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Tuckey, R.C.; Cheng, C.Y.S.; Slominski, A.T. The serum vitamin D metabolome: What we know and what is still to discover. J. Steroid Biochem. Mol. Biol. 2019, 186, 4–21. [Google Scholar] [CrossRef]
- Saponaro, F.; Saba, A.; Zucchi, R. An Update on Vitamin D Metabolism. Int. J. Mol. Sci. 2020, 21, 6573. [Google Scholar] [CrossRef]
- Vieth, R. Vitamin D supplementation: Cholecalciferol, calcifediol, and calcitriol. Eur. J. Clin. Nutr. 2020, 74, 1493–1497. [Google Scholar] [CrossRef]
| Characteristics | All (n = 505) | 25(OH)D < 50 nmol/L (n = 195) | 25(OH)D ≥ 50 nmol/L (n = 310) | p Value |
|---|---|---|---|---|
| Females (%) | 52.3 | 50.7 | 53.2 | 0.591 |
| Age (years) | 61.2 ± 10.6 | 59.9 ± 11.3 | 62.0 ± 10.0 | 0.026 |
| Body mass index (kg/m2) | 29.6 ± 5.1 | 30.8 ± 5.7 | 28.9 ± 4.6 | <0.001 |
| Office systolic BP (mm Hg) | 141 ± 16.6 | 141 ± 17.1 | 141 ± 16.3 | 0.734 |
| Office diastolic BP (mm Hg) | 86.3 ± 11.0 | 86.7 ± 11.7 | 86.1 ± 10.5 | 0.573 |
| 24 h systolic BP (mm Hg) | 128 ± 13.9 | 130 ± 14.3 | 126 ± 13.4 | 0.002 |
| 24 h diastolic BP (mm Hg) | 76.4 ± 9.5 | 77.5 ± 10.3 | 75.8 ± 9.0 | 0.049 |
| NT-proBNP (pg/mL) | 82 (42–153) | 83 (40.8–150) | 84 (45–158) | 0.428 |
| Corrected QT interval (ms) | 413 (391–441) | 414 (391–445) | 413 (391–439) | 0.865 |
| PRC (µU/mL) | 17.4 (10.1–48.3) | 18.6 (10.2–57.5) | 16.9 (9.6–44.3) | 0.596 |
| PAC (ng/dL) | 17.0 ± 10.3 | 16.5 ± 10.2 | 17.3 ± 10.4 | 0.39 |
| eGFR (mL/min/1.73 m2) | 79.2 ± 17.7 | 81.8 ± 18.7 | 78.1 ± 16.9 | 0.021 |
| 24 h UAE (mg/24 h) | 8.5 (6.0–19.8) | 9.0 (6.0–21.0) | 8.0 (6.0–17.0) | 0.064 |
| Diabetes mellitus (%) | 26.5 | 34.9 | 21.3 | <0.001 |
| Fasting glucose (mg/dL) | 112 ± 40.8 | 121 ± 49.8 | 107 ± 33.0 | <0.001 |
| HbA1c (mmol/mol) | 43.6 ± 11.7 | 46.2 ± 14.2 | 41.9 ± 9.4 | <0.001 |
| HOMA-IR | 1.73 (1.03–3.06) | 2.01 (1.24–3.93) | 1.56 (0.97–2.76) | 0.005 |
| Triglycerides (mg/dL) | 110 (75.0–154) | 117 (73.8–164) | 105 (75.0–151) | 0.311 |
| HDL cholesterol (mg/dL) | 58.6 ± 17.3 | 57.1 ± 17.4 | 59.5 ± 17.2 | 0.142 |
| LDL cholesterol (mg/dL) | 114 ± 38.4 | 113 ± 37.9 | 115 ± 38.7 | 0.470 |
| PWV (m/s) | 8.6 ± 2.4 | 8.5 ± 2.0 | 8.6 ± 2.6 | 0.861 |
| CRP (mg/L) | 1.80 (0.80–3.40) | 1.70 (0.85–3.40) | 1.9 (0.80–3.50) | 0.933 |
| 25(OH)D (nmol/L) | 60.4 ± 26.7 | 36.1 ± 9.6 | 75.7 ± 22.4 | <0.001 |
| 24,25(OH)2D (nmol/L) | 3.7 ± 2.6 | 1.9 ± 1.1 | 5.1 ± 2.4 | <0.001 |
| VMR (%) | 6.2 ± 2.3 | 5.2 ± 2.4 | 6.8 ± 2.0 | <0.001 |
| 1,25(OH)2D (pg/mL) | 52.5 ± 19.8 | 44.9 ± 17.4 | 57.2 ± 19.7 | <0.001 |
| Fibroblast growth factor-23 (pmol/L) | 0.85 (0.60–1.25) | 0.88 (0.61–1.37) | 0.83 (0.59–1.21) | 0.072 |
| bALP (µg/L) | 16.9 ± 6.2 | 17.3 ± 6.9 | 16.6 ± 5.7 | 0.380 |
| CTX (ng/mL) | 0.22 ± 0.18 | 0.20 ± 0.15 | 0.24 ± 0.20 | 0.058 |
| Osteocalcin (ng/mL) | 14.9 ± 8.4 | 14.4 ± 8.2 | 15.3 ± 8.5 | 0.216 |
| P1NP (ng/mL) | 42.0 ± 21.0 | 40.7 ± 18.3 | 42.8 ± 22.5 | 0.283 |
| PTH (pg/mL) | 51.1 ± 19.5 | 56.8 ± 22.0 | 47.4 ± 16.7 | <0.001 |
| Plasma calcium (mmol/L) | 2.38 ± 0.11 | 2.37 ± 0.11 | 2.39 ± 0.10 | 0.017 |
| 24 h UCaE (mmol/24 h) | 3.72 ± 2.48 | 3.59 ± 2.62 | 3.80 ± 2.39 | 0.397 |
| Calcium supplement (%) | 9.9 | 5.1 | 12.9 | 0.005 |
| Vitamin D supplement (%) | 10.9 | 5.1 | 14.5 | 0.001 |
| Characteristics | 25(OH)D < 50 nmol/L (n = 66) with Functional Deficiency | 25(OH)D < 50 nmol/L (n = 126) without Functional Deficiency | p Value |
|---|---|---|---|
| Females (%) | 50.0 | 51.2 | 0.741 |
| Age (years) | 62.3 ± 10.2 | 58.7 ± 11.7 | 0.035 |
| Body mass index (kg/m2) | 31.0 ± 5.8 | 30.7 ± 5.6 | 0.722 |
| Office systolic BP (mm Hg) | 144 ± 18.1 | 140 ± 16.6 | 0.187 |
| Office diastolic BP (mm Hg) | 86.4 ± 11.6 | 86.9 ± 11.8 | 0.780 |
| 24 h systolic BP (mm Hg) | 131 ± 15.8 | 130 ± 13.5 | 0.550 |
| 24 h diastolic BP (mm Hg) | 76.7 ± 9.6 | 77.9 ± 10.6 | 0.470 |
| NT-proBNP (pg/mL) | 82.5 (39.0–171) | 87.5 (37.3–165) | 0.717 |
| Corrected QT interval (ms) | 407 (388–437) | 416 (392–442) | 0.150 |
| PRC (µU/mL) | 19.0 (12.4–79.3) | 17.4 (8.5–51.8) | 0.127 |
| PAC (ng/dL) | 18.5 ± 9.4 | 15.5 ± 10.6 | 0.055 |
| eGFR (mL/min/1.73 m2) | 76.5 ± 21.1 | 84.6 ± 16.8 | 0.004 |
| 24 h UAE (mg/24 h) | 9.0 (6.0–24.5) | 9.0 (6.0–21.0) | 0.604 |
| Diabetes mellitus (%) | 54.4 | 45.6 | <0.001 |
| Fasting glucose (mg/dL) | 141 ± 61.2 | 110 ± 39.0 | <0.001 |
| HbA1c (mmol/mol) | 52.6 ± 17.5 | 42.8 ± 10.8 | <0.001 |
| HOMA-IR | 2.6 (1.5–5.7) | 2.1 (1.3–3.5) | 0.061 |
| Triglycerides (mg/dL) | 117 (70.5–178) | 118 (81.8–150) | 0.504 |
| HDL cholesterol (mg/dL) | 55.8 ± 20.3 | 57.8 ± 15.7 | 0.437 |
| LDL cholesterol (mg/dL) | 107 ± 38.7 | 116 ± 37.2 | 0.115 |
| PWV (m/s) | 8.8 ± 2.0 | 8.4 ± 2.1 | 0.240 |
| CRP (mg/L) | 1.95 (1.10–4.75) | 1.90 (0.90–3.40) | 0.017 |
| 25(OH)D (nmol/L) | 31.5 ± 9.7 | 38.4 ± 8.8 | <0.001 |
| 24,25(OH)2D (nmol/L) | 0.85 ± 0.46 | 2.38 ± 0.96 | <0.001 |
| VMR (%) | 2.76 ± 0.93 | 6.43 ± 1.85 | <0.001 |
| 1,25(OH)2D (pg/mL) | 39.4 ± 18.0 | 47.7 ± 16.4 | 0.001 |
| Fibroblast growth factor-23 (pmol/L) | 1.00 (0.69–1.44) | 0.89 (0.57–1.37) | 0.383 |
| bALP (µg/L) | 18.1 ± 7.2 | 17.0 ± 6.7 | 0.556 |
| CTX (ng/mL) | 0.19 ± 0.14 | 0.21 ± 0.15 | 0.454 |
| Osteocalcin (ng/mL) | 14.0 ± 9.4 | 14.5 ± 7.5 | 0.681 |
| P1NP (ng/mL) | 37.4 ± 14.4 | 42.3 ± 19.8 | 0.083 |
| PTH (pg/mL) | 57.1 ± 26.7 | 56.7 ± 19.3 | 0.917 |
| Plasma calcium (mmol/L) | 2.37 ± 0.11 | 2.37 ± 0.11 | 0.958 |
| 24 h UCaE (mmol/24 h) | 3.44 ± 2.79 | 3.67 ± 2.53 | 0.592 |
| Calcium supplement (%) | 2.0 | 6.3 | 0.499 |
| Vitamin D supplement (%) | 4.5 | 5.6 | 0.900 |
| Characteristics | Baseline | Follow-Up | Treatment Effect | p Value |
|---|---|---|---|---|
| 25-hydroxyvitamin D (nmol/L) | ||||
| Vitamin D (n = 27) | 38.9 ± 14.3 | 68.8 ± 11.2 | 30.9 (24.4 to 37.5) | <0.001 |
| Placebo (n = 22) | 38.9 ± 12.9 | 37.1 ± 14.0 | ||
| 24,25(OH)2D (nmol/L) | ||||
| Vitamin D (n = 25) | 1.16 ± 0.62 | 4.54 ± 1.14 | 2.6 (2.0 to 3.3) | <0.001 |
| Placebo (n = 22) | 1.15 ± 0.57 | 1.88 ± 1.11 | ||
| Vitamin D metabolite ratio (%) | ||||
| Vitamin D (n = 25) | 3.00 ± 0.95 | 6.79 ± 1.67 | 1.9 (1.1 to 2.6) | <0.001 |
| Placebo (n = 22) | 3.00 ± 0.67 | 5.04 ± 1.59 | ||
| 1,25-dihydroxyvitamin D (pg/mL) | ||||
| Vitamin D (n = 19) | 50.5 ± 20.9 | 61.8 ± 27.6 | 6.2 (−7.8 to 20.1) | 0.377 |
| Placebo (n = 18) | 38.3 ± 17.7 | 43.3 ± 17.2 | ||
| Fibroblast growth factor-23 (pmol/L) | ||||
| Vitamin D (n = 18) | 0.83 ± 0.71 | 2.01 ± 4.35 | 1.5 (−0.56 to 3.61) | 0.147 |
| Placebo (n = 17) | 1.34 ± 1.30 | 1.14 ± 0.49 | ||
| Bone-specific alkaline phosphatase (µg/L) | ||||
| Vitamin D (n = 17) | 16.5 ± 7.6 | 16.6 ± 5.7 | 0.67 (−2.95 to 1.61) | 0.553 |
| Placebo (n = 16) | 19.8 ± 6.4 | 19.2 ± 6.5 | ||
| β-CrossLaps (ng/mL) | ||||
| Vitamin D (n = 14) | 0.19 ± 0.12 | 0.18 ± 0.11 | −0.011 (−0.078 to 0.057) | 0.747 |
| Placebo (n = 16) | 0.19 ± 0.14 | 0.23 ± 0.16 | ||
| Osteocalcin (ng/mL) | ||||
| Vitamin D (n = 18) | 12.6 ± 6.9 | 13.2 ± 5.9 | 0.46 (−2.060 to 2.986) | 0.711 |
| Placebo (n = 17) | 13.4 ± 6.7 | 14.4 ± 5.3 | ||
| Procollagen typ 1 amino-terminal propetide (ng/mL) | ||||
| Vitamin D (n = 18) | 35.6 ± 17.5 | 38.5 ± 16.6 | −1.435 (−6.52 to 9.39) | 0.715 |
| Placebo (n = 17) | 40.2 ± 13.9 | 41.1 ± 11.7 | ||
| Parathyroid hormone (pg/mL)# | ||||
| Vitamin D (n = 19) | 52.2 ± 23.4 | 46.9 ± 15.7 | −12.2 (−22.1 to −2.3) | 0.017 |
| Placebo (n = 20) | 50.9 ± 21.6 | 55.4 ± 29.6 | ||
| Plasma calcium (mmol/L) | ||||
| Vitamin D (n = 19) | 2.38 ± 0.08 | 2.36 ± 0.07 | −0.008 (−0.055 to 0.040) | 0.747 |
| Placebo (n = 20) | 2.38 ± 0.14 | 2.37 ± 0.13 | ||
| 24 h urinary calcium excretion (mmol/24 h) # | ||||
| Vitamin D (n = 12) | 3.30 (2.10–7.20) | 3.74 (2.05–6.86) | −0.19 (−1.34 to 1.72) | 0.802 |
| Placebo (n = 15) | 2.05 (1.28–5.40) | 2.53 (0.65–6.24) | ||
| Characteristics | Baseline | Follow-Up | Treatment Effect | p Value |
|---|---|---|---|---|
| 24 h systolic blood pressure (mm Hg) | ||||
| Vitamin D (n = 24) | 131 ± 9.5 | 129 ± 9.3 | −1.14 (−6.10 to 3.83) | 0.647 |
| Placebo (n = 24) | 134 ± 7.4 | 132 ± 10.6 | ||
| 24 h diastolic blood pressure (mm Hg) | ||||
| Vitamin D (n = 24) | 78.7 ± 7.6 | 76.7 ± 8.7 | −0.64 (−3.64 to 2.36) | 0.668 |
| Placebo (n = 24) | 77.8 ± 7.3 | 76.4 ± 8.7 | ||
| N-terminal pro-B-type natriuretic peptide (ng/L) # | ||||
| Vitamin D (n = 26) | 45.0 (29.5–76.3) | 58.5 (35.8–134) | 2.55 (−69.5 to 74.6) | 0.944 |
| Placebo (n = 25) | 99.0 (45.5–228) | 98.0 (50.5–188) | ||
| Corrected QT interval (ms) | ||||
| Vitamin D (n = 23) | 409 ± 30.8 | 422 ± 30.8 | 19.4 (−32.4 to 71.2) | 0.455 |
| Placebo (n = 24) | 417 ± 33.9 | 423 ± 29.9 | ||
| Plasma renin concentration (µU/mL) # | ||||
| Vitamin D (n = 24) | 18.3 (11.4–70.7) | 15.0 (10.6–47.0) | −7.78 (−23.5 to 7.99) | 0.326 |
| Placebo (n = 23) | 14.9 (9.0–37.2) | 17.1 (8.8–46.7) | ||
| Plasma aldosterone concentration (ng/dL) | ||||
| Vitamin D (n = 26) | 16.8 ± 7.1 | 17.4 ± 6.6 | 2.53 (−1.55 to 6.62) | 0.218 |
| Placebo (n = 25) | 19.9 ± 10.9 | 22.8 ± 14.3 | ||
| 24 h urinary albumin concentration (mg/24 h) # | ||||
| Vitamin D (n = 12) | 7.0 (6.0–13.0) | 8.0 (7.9–11.9) | 6.99 (−14.7 to 28.7) | 0.509 |
| Placebo (n = 14) | 8.0 (4.0–120) | 8.0 (4.8–23.4) | ||
| Homeostasis Model Assessment—Insulin Resistance # | ||||
| Vitamin D (n = 26) | 2.38 (1.11–5.48) | 2.53 (1.38–4.75) | 0.41 (−3.1 to 3.9) | 0.812 |
| Placebo (n = 23) | 3.08 (1.40–6.80) | 3.79 (1.60–7.28) | ||
| Triglycerides (mg/dL) | ||||
| Vitamin D (n = 26) | 132 ± 78.0 | 135 ± 80.8 | 2.63 (−25.2 to 30.5) | 0.850 |
| Placebo (n = 25) | 136 ± 72.4 | 140 ± 64.7 | ||
| HDL cholesterol (mg/dL) | ||||
| Vitamin D (n = 26) | 54.7 ± 15.7 | 54.9 ± 17.4 | 0.42 (−3.90 to 4.75) | 0.845 |
| Placebo (n = 25) | 53.2 ± 15.8 | 53.8 ± 17.2 | ||
| Pulse wave velocity (m/s) | ||||
| Vitamin D (n = 23) | 8.68 ± 1.76 | 8.93 ± 2.32 | 0.59 (−0.57 to 1.76) | 0.308 |
| Placebo (n = 15) | 9.08 ± 2.58 | 8.41 ± 2.46 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zelzer, S.; Meinitzer, A.; Enko, D.; Keppel, M.H.; Herrmann, M.; Theiler-Schwetz, V.; Trummer, C.; Schmitt, L.; Tomaschitz, A.; Sadoghi, P.; et al. Classification of Vitamin D Status Based on Vitamin D Metabolism: A Randomized Controlled Trial in Hypertensive Patients. Nutrients 2024, 16, 839. https://doi.org/10.3390/nu16060839
Zelzer S, Meinitzer A, Enko D, Keppel MH, Herrmann M, Theiler-Schwetz V, Trummer C, Schmitt L, Tomaschitz A, Sadoghi P, et al. Classification of Vitamin D Status Based on Vitamin D Metabolism: A Randomized Controlled Trial in Hypertensive Patients. Nutrients. 2024; 16(6):839. https://doi.org/10.3390/nu16060839
Chicago/Turabian StyleZelzer, Sieglinde, Andreas Meinitzer, Dietmar Enko, Martin H. Keppel, Markus Herrmann, Verena Theiler-Schwetz, Christian Trummer, Lisa Schmitt, Andreas Tomaschitz, Patrick Sadoghi, and et al. 2024. "Classification of Vitamin D Status Based on Vitamin D Metabolism: A Randomized Controlled Trial in Hypertensive Patients" Nutrients 16, no. 6: 839. https://doi.org/10.3390/nu16060839
APA StyleZelzer, S., Meinitzer, A., Enko, D., Keppel, M. H., Herrmann, M., Theiler-Schwetz, V., Trummer, C., Schmitt, L., Tomaschitz, A., Sadoghi, P., Dierkes, J., Pludowski, P., Zittermann, A., März, W., & Pilz, S. (2024). Classification of Vitamin D Status Based on Vitamin D Metabolism: A Randomized Controlled Trial in Hypertensive Patients. Nutrients, 16(6), 839. https://doi.org/10.3390/nu16060839











