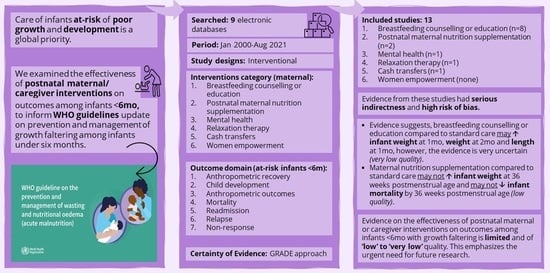
Effectiveness of Postnatal Maternal or Caregiver Interventions on Outcomes among Infants under Six Months with Growth Faltering: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Ethics
2.2. Eligibility Criteria
- (i)
- wasting (weight-for-length z scores < −2 (WHO 2006)) or
- (ii)
- underweight (weight-for-age z scores < −2 (WHO 2006)) or
- (iii)
- low mid-upper arm circumference (MUAC) or
- (iv)
- small size at birth (preterm (<37 weeks of gestation) or
- (v)
- small for gestational age at birth (<10th centile) or
- (vi)
- low birth weight (<2500 g) or
- (vii)
- losing weight (study authors’ definition).
2.3. Information Source and Search Strategy
2.4. Data Collection and Analysis
2.5. Assessment of Risk of Bias
2.6. Measures of Treatment Effect
2.7. Assessment of Heterogeneity
2.8. Assessment of Reporting Biases
2.9. Data Synthesis
2.10. Subgroup and Sensitivity Analysis
2.11. Certainty of Evidence
3. Results
3.1. Study Flow
3.2. Characteristics of Included Studies
- Child development: child development score
- Anthropometric outcomes: weight, length, and head circumference at different time points; and change in weight and head circumference z scores
- Mortality outcome: mortality (different time points)
- Readmission outcome: readmission (different time points)
3.3. Risk of Bias of Included Studies
3.4. Summary of Included Studies
3.4.1. Breastfeeding Counselling or Education
3.4.2. Maternal Nutrition Supplementation
3.4.3. Mental Health
3.4.4. Relaxation Therapy
3.4.5. Cash Transfer
3.5. Summary of Comparisons
3.5.1. Effect of Breastfeeding Counselling or Education Versus Standard Care
Anthropometric Outcomes
Readmission
3.5.2. Effect of Maternal Nutritional Supplementation Versus Standard Care
Anthropometric Outcomes
Mortality Outcome
3.5.3. Effect of Mental Health Intervention Versus Standard Care
Anthropometric Outcomes
3.5.4. Effect of Relaxation Therapy Versus Standard Care
Anthropometric Outcomes
3.5.5. Effect of Cash Transfer Versus Standard Care
Child Development Outcomes
Anthropometric Outcomes
Readmission Outcome
4. Discussion
4.1. Summary of Evidence
4.2. Overall Completeness and Applicability of Evidence
4.3. Review Findings in Context of Other Reviews
4.4. Potential Biases in the Review Process
4.5. Implications for Guidelines and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Victora, C.G.; De Onis, M.; Hallal, P.C.; Blössner, M.; Shrimpton, R. Worldwide timing of growth faltering: Revisiting implications for interventions. Pediatrics 2010, 125, e473–e480. [Google Scholar] [CrossRef] [PubMed]
- Kerac, M.; James, P.T.; McGrath, M.G.; Brennan, E.; Opondo, C.; Frison, S. Infant malnutrition in low- and middle-income countries: Assessment and prevalence of small and nutritionally at-risk infants aged under 6 months in 54 Demographic & Health Survey datasets. medRxiv 2021. [Google Scholar] [CrossRef]
- MAMI Project Summary (Management of Small & Nutritionally At-Risk Infants aged u6months and Their Mothers). Available online: http://www.ennonline.net/ourwork/research/mami (accessed on 23 February 2021).
- Aneja, S.; Kumar, P.; Choudhary, T.S.; Srivastava, A.; Chowdhury, R.; Taneja, S.; Bhandari, N.; Daniel, A.; Menon, P.; Chellani, H.; et al. Growth faltering in early infancy: Highlights from a two-day scientific consultation. BMC Proc. 2020, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.C.; Blencowe, H.; Lawn, J.E. Small babies, big numbers: Global estimates of preterm birth. Lancet Glob. Health 2019, 7, e2–e3. [Google Scholar] [CrossRef] [PubMed]
- Blencowe, H.; Krasevec, J.; De Onis, M.; Black, R.E.; An, X.; Stevens, G.A.; Borghi, E.; Hayashi, C.; Estevez, D.; Cegolon, L. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: A systematic analysis. Lancet Glob. Health 2019, 7, e849–e860. [Google Scholar] [CrossRef] [PubMed]
- Mwangome, M.; Ngari, M.; Bwahere, P.; Kabore, P.; McGrath, M.; Kerac, M.; Berkley, J.A. Anthropometry at birth and at age of routine vaccination to predict mortality in the first year of life: A birth cohort study in BukinaFaso. PLoS ONE 2019, 14, e0213523. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Guideline on the Prevention and Management of Wasting and Nutritional Oedema (Acute Malnutrition) in Infants and Children under 5 Year. Available online: https://www.who.int/publications-detail-redirect/9789240082830 (accessed on 15 January 2024).
- Kerac, M.; McGrath, M. Management of Acute Malnutrition in Infants under 6 Months of Age. In The Biology of the First 1,000 Days; Karakochuk, C.D., Whitfield, K.C., Green, T.J., Kraemer, K., Eds.; CRC Press: Boca Raton, FL, USA, 2017; Chapter 14; pp. 207–220. [Google Scholar] [CrossRef]
- Grey, K.; Rana, R.; McGrath, M.; Kerac, M. Key considerations for developing patient management tools for small and nutritionally at-risk infants: A scoping review. World Nutr. 2023, 14, 58–75. [Google Scholar] [CrossRef]
- Kirolos, A.; Goyheneix, M.; Kalmus Eliasz, M.; Chisala, M.; Lissauer, S.; Gladstone, M.; Kerac, M. Neurodevelopmental, cognitive, behavioural and mental health impairments following childhood malnutrition: A systematic review. BMJ Glob. Health 2022, 7, e009330. [Google Scholar] [CrossRef]
- van den Heuvel, M.; Voskuijl, W.; Chidzalo, K.; Kerac, M.; Reijneveld, S.A.; Bandsma, R.; Gladstone, M. Developmental and behavioural problems in children with severe acute malnutrition in Malawi: A cross-sectional study. J. Glob. Health 2017, 7, 020416. [Google Scholar] [CrossRef]
- WHO. Updates on the Management of Severe Acute Malnutrition in Infants and Children (Guideline). Available online: https://www.who.int/publications/i/item/9789241506328 (accessed on 27 January 2020).
- von Salmuth, V.; Brennan, E.; Kerac, M.; McGrath, M.; Frison, S.; Lelijveld, N. Maternal-focused interventions to improve infant growth and nutritional status in low-middle income countries: A systematic review of reviews. PLoS ONE 2021, 16, e0256188. [Google Scholar] [CrossRef]
- WHO. WHO Guideline on the Prevention and Management of Wasting and Nutritional Oedema (Acute Malnutrition) in Infants and children under 5 years. Available online: https://app.magicapp.org/#/guideline/noPQkE/section/n32P8W (accessed on 12 December 2023).
- Sohrabi, C.; Franchi, T.; Mathew, G.; Kerwan, A.; Nicola, M.; Griffin, M.; Agha, M.; Agha, R. PRISMA 2020 statement: What’s new and the importance of reporting guidelines. Int. J. Surg. 2021, 88, 105918. [Google Scholar] [CrossRef] [PubMed]
- Drugs, C.A.f.; Health, T.i. Strings Attached: CADTH’s Database Search Filters. Available online: https://searchfilters.cadth.ca/ (accessed on 10 August 2023).
- Thomas, J.; Graziosi, S.; Brunton, J.; Ghouze, Z.; O’Driscoll, P.; Bond, M. EPPI-Reviewer: Advanced software for systematic reviews, maps and evidence synthesis. EPPI-Cent. Software. Lond. UCL Soc. Res. Inst. 2020. [Google Scholar]
- Collaboration, C. Data Collection Form for Intervention Reviews for RCTs Only [Internet]. Available online: https://dplp.cochrane.org/data-extraction-forms (accessed on 10 August 2023).
- RevMan 5. Available online: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman/revman-5-download (accessed on 10 July 2023).
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Elie Akl, R.M. Nancy Santesso Wojtek Wiercioch, and Other Members of the GRADE Working Group Introduction to GRADE Handbook: Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach. Available online: https://gdt.gradepro.org/app/handbook/handbook.html (accessed on 15 September 2023).
- Ahmadi, S.; Kazemi, F.; Masoumi Seyedeh, Z.; Parsa, P.; Roshanaei, G. Intervention based on BASNEF model increases exclusive breastfeeding in preterm infants in Iran: A randomized controlled trial. Int. Breastfeed. J. 2016, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Masoumi, S.Z.; Kazemi, F.; Ahmadi, S. Investigating the effects of instructing mothers using BASNEF model on continuing exclusive breastfeeding of late- preterm infants. J. Compr. Pediatr. 2017, 8, e59243. [Google Scholar] [CrossRef]
- World Health Organization. WHO Recommendations for Care of the Preterm or Low Birth Weight Infant. Available online: https://www.who.int/publications/i/item/9789240058262 (accessed on 15 September 2023).
- Agrasada, G.V.; Gustafsson, J.; Kylberg, E.; Ewald, U. Postnatal peer counselling on exclusive breastfeeding of low-birthweight infants: A randomized, controlled trial. Acta Paediatr. 2005, 94, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Edraki, M.; Moravej, H.; Rambod, M. Effect of home visit training program on growth and development of preterm infants: A double blind randomized controlled trial. Int. J. Community Based Nurs. Midwifery 2015, 3, 12. [Google Scholar]
- Eun Hye, J.; Hyeon Ok, J. Effects of an Infant Care Education Program for Mothers of Late-preterm Infants on Parenting Confidence, Breastfeeding Rates, and Infants’ Growth and Readmission Rates. Child Health Nurs. Res. 2020, 26, 11–22. [Google Scholar] [CrossRef]
- Gholami, S.; Farahani Azam, S.; Karahroudy Fatemeh, A.; Moghadam, F.; Boromandnia, N.; Mojen leila, k. The effect of telenursing on the rate of newborn readmission. J. Neonatal Nurs. 2021, 28, 26–30. [Google Scholar] [CrossRef]
- Gun Ja, J.; Yeon Ran, H. Effects of a Breastfeeding Support Program on the Prevalence of Exclusive Breastfeeding and Growth in Late Preterm Infants. Child Health Nurs. Res. 2020, 26, 90–97. [Google Scholar] [CrossRef]
- Moudi, Z.; Molashahi, B.; Imani, M.; Ansari, H. Effects of a feasible supportive care program on breastfeeding behaviors and neonatal outcomes among the late preterm newborns in the south east of Iran. J. Neonatal Nurs. 2017, 23, 238–241. [Google Scholar] [CrossRef]
- Thakur, S.K.; Roy, S.K.; Paul, K.; Khanam, M.; Khatun, W.; Sarker, D. Effect of nutrition education on exclusive breastfeeding for nutritional outcome of low birth weight babies. Eur. J. Clin. Nutr. 2012, 66, 376–381. [Google Scholar] [CrossRef] [PubMed]
- de Figueiredo, C.S.; Palhares, D.B.; Melnikov, P.; Moura, A.J.; dos Santos, S.C. Zinc and copper concentrations in human preterm milk. Biol. Trace Elem. Res. 2010, 136, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Marc, I.; Piedboeuf, B.; Lacaze-Masmonteil, T.; Fraser, W.; Mâsse, B.; Mohamed, I.; Qureshi, M.; Afifi, J.; Lemyre, B.; Caouette, G.; et al. Effect of Maternal Docosahexaenoic Acid Supplementation on Bronchopulmonary Dysplasia-Free Survival in Breastfed Preterm Infants: A Randomized Clinical Trial. JAMA 2020, 324, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Seiiedi-Biarag, L.; Mirghafourvand, M.; Esmaeilpour, K.; Hasanpour, S. A randomized controlled clinical trial of the effect of supportive counseling on mental health in Iranian mothers of premature infants. BMC Pregnancy Childbirth 2021, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Menke, B.M.; Hass, J.; Diener, C.; Poschl, J. Family-centered music therapy—Empowering premature infants and their primary caregivers through music: Results of a pilot study. PLoS ONE 2021, 16, e0250071. [Google Scholar] [CrossRef]
- Andrews Kathryn, G.; Martin Michelle, W.; Shenberger, E.; Pereira, S.; Fink, G.; McConnell, M. Financial Support to Medicaid-Eligible Mothers Increases Caregiving for Preterm Infants. Matern. Child Health J. 2020, 24, 587–600. [Google Scholar] [CrossRef]
- Kerac, M.; Mwangome, M.; McGrath, M.; Haider, R.; Berkley, J.A. Management of acute malnutrition in infants aged under 6 months (MAMI): Current issues and future directions in policy and research. Food Nutr. Bull. 2015, 36 (Suppl. 1), S30–S34. [Google Scholar] [CrossRef]
- Hoehn, C.; Lelijveld, N.; Mwangome, M.; Berkley, J.A.; McGrath, M.; Kerac, M. Anthropometric Criteria for Identifying Infants Under 6 Months of Age at Risk of Morbidity and Mortality: A Systematic Review. Clin. Med. Insights Pediatr. 2021, 15, 117955652110499. [Google Scholar] [CrossRef]
- Kerac, M.; Frison, S.; Connell, N.; Page, B.; McGrath, M. Informing the management of acute malnutrition in infants aged under 6 months (MAMI): Risk factor analysis using nationally-representative demographic & health survey secondary data. PeerJ 2019, 6, e5848. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, U.N.; Yiwombe, M.; Chisepo, P.; Cole, T.J.; Heikens, G.T.; Kerac, M. Interpretation of World Health Organization growth charts for assessing infant malnutrition: A randomised controlled trial. J. Paediatr Child Health 2014, 50, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Lassi, Z.S.; Rind, F.; Irfan, O.; Hadi, R.; Das, J.K.; Bhutta, Z.A. Impact of infant and young child feeding (IYCF) nutrition interventions on breastfeeding practices, growth and mortality in low-and middle-income countries: Systematic review. Nutrients 2020, 12, 722. [Google Scholar] [CrossRef] [PubMed]
- Rana, R.; McGrath, M.; Sharma, E.; Gupta, P.; Kerac, M. Effectiveness of breastfeeding support packages in low-and middle-income countries for infants under six months: A systematic review. Nutrients 2021, 13, 681. [Google Scholar] [CrossRef]
- McFadden, A.; Siebelt, L.; Marshall, J.L.; Gavine, A.; Girard, L.-C.; Symon, A.; MacGillivray, S. Counselling interventions to enable women to initiate and continue breastfeeding: A systematic review and meta-analysis. Int. Breastfeed. J. 2019, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.K.; Balogun, O.O.; Ota, E.; Takahashi, K.; Mori, R. Supplementation with multiple micronutrients for breastfeeding women for improving outcomes for the mother and baby. Cochrane Database Syst. Rev. 2016, 2, CD010647. [Google Scholar] [CrossRef] [PubMed]
- Little, M.T.; Roelen, K.; Lange, B.C.; Steinert, J.I.; Yakubovich, A.R.; Cluver, L.; Humphreys, D.K. Effectiveness of cash-plus programmes on early childhood outcomes compared to cash transfers alone: A systematic review and meta-analysis in low-and middle-income countries. PLoS Med. 2021, 18, e1003698. [Google Scholar] [CrossRef]
- Mohd Shukri, N.H.; Wells, J.C.K.; Fewtrell, M. The effectiveness of interventions using relaxation therapy to improve breastfeeding outcomes: A systematic review. Matern. Child Nutr. 2018, 14, e12563. [Google Scholar] [CrossRef]
- Lelijveld, N.; Kerac, M.; McGrath, M.; Mwangome, M.; Berkley, J.A. A Review of Methods to Detect Cases of Severely Malnourished Infants Less than 6 Months for Their Admission into Therapeutic Care. Available online: http://www.ennonline.net/mamicasedetectionreview (accessed on 22 September 2017).
- Grey, K.; Brennan, E.; Kerac, M.; McGrath, M. The MAMI Care Pathway Package: A resource to support the management of small and nutritionally at-risk infants under six months of age and their mothers (MAMI). South Sudan Med. J. 2021, 14, 94–97. [Google Scholar] [CrossRef]
- Neocleous, M.; Hepworth, K.; Cavallera, V.; Gladstone, M. Training packages for the use of child development tools in low/middle-income countries: A review. Arch. Dis. Child 2023, 108, 103–107. [Google Scholar] [CrossRef]
- Kerac, M.; McGrath, M.; Connell, N.; Kompala, C.; Moore, W.H.; Bailey, J.; Bandsma, R.; Berkley, J.A.; Briend, A.; Collins, S.; et al. ‘Severe malnutrition’: Thinking deeply, communicating simply. BMJ Glob. Health 2020, 5, e003023. [Google Scholar] [CrossRef]

| Author (Year) | Country | Study Design (Sample Size) | Population | Eligible Outcomes Reported |
|---|---|---|---|---|
| Breastfeeding counselling or education | ||||
| Ahmadi (2016) [24] | Iran | RCT (124) | Mother–infant (GA 34–37 wks, BW 2000–2500 g) | Anthropometric |
| Agrasada (2005) [27] | Philippines | RCT (204) | Mother–infant (GA 37–42 wks, LBW) | Anthropometric |
| Edraki (2015) [28] | Iran | RCT (60) | Mother–infant (GA < 37 wks, BW < 2500 g) | Anthropometric |
| Eun Hye (2020) [29] | South Korea | NRCT (56) | Mother–infant (GA 34–37 wks) | Anthropometric, Readmission |
| Gholami (2021) [30] | Iran | NRCT (288) | Mother–infant (preterm) | Readmission |
| Gun Ja (2020) [31] | South Korea | NRCT (40) | Mother–infant (GA 34–37 wks) | Anthropometric |
| Moudi (2017) [32] | Iran | NRCT (84) | Mother–infant (GA 34–37 wks) | Anthropometric |
| Thakur (2012) [33] | Bangladesh | RCT (184) | Mother–infant (BW < 2500 g) | Anthropometric |
| Maternal nutrition supplementation | ||||
| de Figueiredo (2010) [34] | Brazil | NRCT (38) | Mother–infant (GA ≤ 34 wk) | Anthropometric |
| Marc (2020) [35] | Canada | RCT (528) | Mother–infant (GA 23–28 wks) | Anthropometric, Mortality |
| Mental health | ||||
| Seiiedi-Biarag (2021) [36] | Iran | RCT (66) | Mother–infant (GA 28–33 wks) | Anthropometric |
| Relaxation therapy | ||||
| Menke (2021) [37] | Germany | RCT (50) | Family-infant (GA ≤ 30 wks) | Anthropometric |
| Cash transfer | ||||
| Andrews (2020) [38] | USA | RCT (53) | Mother–infant (preterm) | Anthropometric, Child development, Readmission |
| Certainty Assessment | No. of Patients | Effect | Certainty | Importance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Education | Standard Care | Relative (95% CI) | Absolute (95% CI) | ||
| Anthropometric recovery (no data) [Critical] | |||||||||||
| Child development (no data) [Critical] | |||||||||||
| Weight (g) at 1 month (Anthropometric outcome) | |||||||||||
| 3 [24,28,33] | randomised trials | serious a | serious b | serious c | not serious | 184 | 184 | - | MD 220.82 higher (155.76 higher to 285.88 higher) | ⨁◯◯◯ Very low | Important |
| Weight (g) at 2 months (Anthropometric outcome) | |||||||||||
| 3 [24,28,33] | randomised trials | serious a | serious d | serious c | not serious | 184 | 184 | - | MD 367.3 higher (296.05 higher to 438.56 higher) | ⨁◯◯◯ Very low | Important |
| Length (cm) at 1 month (Anthropometric outcome) | |||||||||||
| 2 [28,33] | randomised trials | serious e | serious f | serious g | not serious | 122 | 122 | - | MD 0.66 higher (0.24 higher to 1.07 higher) | ⨁◯◯◯ Very low | Important |
| Mortality (no data) [Important] | |||||||||||
| Readmission (no data) [Important] | |||||||||||
| Relapse (no data) [Important] | |||||||||||
| Non-response (no data) [Important] | |||||||||||
| Certainty Assessment | No. of Patients | Effect | Certainty | Importance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Nutrition Supplementation | Standard Care | Relative (95% CI) | Absolute (95% CI) | ||
| Anthropometric recovery (no data) [Critical] | |||||||||||
| Child development (no data) [Critical] | |||||||||||
| Weight (g) at 36 weeks PMA (Anthropometric outcomes) | |||||||||||
| 1 [35] | randomised trials | not serious | not serious | serious a | serious b | 246 | 222 | - | MD 18.7 lower (89.8 lower to 52.4 higher) | ⨁⨁◯◯ Low | Important |
| Mortality by 36 weeks PMA | |||||||||||
| 1 [35] | randomised trials | not serious | not serious | serious a | serious c | 16/268 (6.0%) | 26/255 (10.2%) | RR 0.61 (0.33 to 1.13) | 40 fewer per 1000 (from 68 fewer to 13 more) | ⨁⨁◯◯ Low | Important |
| Readmission (no data) [Important] | |||||||||||
| Relapse (no data) [Important] | |||||||||||
| Non-response (no data) [Important] | |||||||||||
| Certainty Assessment | No. of Patients | Effect | Certainty | Importance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Mental Health Interventions | Standard Care | Relative (95% CI) | Absolute (95% CI) | ||
| Anthropometric recovery (no data) [Critical] | |||||||||||
| Child development (no data) [Critical] | |||||||||||
| Weight at 2 months (Anthropometric outcomes) | |||||||||||
| 1 [36] | randomised trials | not serious | not serious | serious a | very serious b | 32 | 30 | - | MD 0.3 higher (355.14 lower to 355.74 higher) | ⨁◯◯◯ Very low | Important |
| Length at 2 months (Anthropometric outcomes) | |||||||||||
| 1 [36] | randomised trials | not serious | not serious | serious a | serious c | 32 | 30 | - | MD 0.3 lower (2.22 lower to 1.62 higher) | ⨁⨁◯◯ Low | Important |
| Head circumference at 2 months (Anthropometric outcomes) | |||||||||||
| 1 [36] | randomised trials | not serious | not serious | serious a | serious d | 32 | 30 | - | MD 0.3 higher (0.96 lower to 1.56 higher) | ⨁⨁◯◯ Low | Important |
| Mortality (no data) [Important] | |||||||||||
| Readmission (no data) [Important] | |||||||||||
| Relapse (no data) [Important] | |||||||||||
| Non-response (no data) [Important] | |||||||||||
| Certainty Assessment | No. of Patients | Effect | Certainty | Importance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Relaxation Therapy | Standard Care | Relative (95% CI) | Absolute (95% CI) | ||
| Anthropometric recovery (no data) [Critical] | |||||||||||
| Child development (no data) [Critical] | |||||||||||
| Weight at 3 months (Anthropometric outcomes) | |||||||||||
| 1 [37] | randomised trials | serious a | not serious | serious b | very serious c | 24 | 26 | - | MD 42.06 lower (242.25 lower to 158.13 higher) | ⨁◯◯◯ Very low | Important |
| Length at 3 months (Anthropometric outcomes) | |||||||||||
| 1 [37] | randomised trials | serious a | not serious | serious b | serious d | 24 | 26 | - | MD 0.4 higher (0.93 lower to 1.73 higher) | ⨁◯◯◯ Very low | Important |
| Head circumference at 3 months (Anthropometric outcomes) | |||||||||||
| 1 [37] | randomised trials | serious a | not serious | serious b | Serious e | 24 | 26 | - | MD 0.07 higher (0.63 lower to 0.77 higher) | ⨁◯◯◯ Very low | Important |
| Mortality (no data) [Important] | |||||||||||
| Readmission (no data) [Important] | |||||||||||
| Relapse (no data) [Important] | |||||||||||
| Non-response (no data) [Important] | |||||||||||
| Certainty Assessment | No. of Patients | Effect | Certainty | Importance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Cash Transfer | Standard Care | Relative (95% CI) | Absolute (95% CI) | ||
| Anthropometric recovery (no data) [Critical] | |||||||||||
| Child development score at 3 months | |||||||||||
| 1 [38] | randomised trials | very serious a | not serious | serious b | not serious | 0 | 0 | - | MD 1.05 lower (1.62 lower to 0.48 lower) | ⨁◯◯◯ Very low | Critical |
| Change in weight z-score from birth to 3 months post-discharge (Anthropometric outcomes) | |||||||||||
| 1 [38] | randomised trials | very serious a | not serious | serious b | serious c | 0 | 0 | - | MD 0.58 higher (0.23 lower to 1.39 higher) | ⨁◯◯◯ Very low | Important |
| Change in head circumference z- score from birth to 3 months post-discharge (Anthropometric outcomes) | |||||||||||
| 1 [38] | randomised trials | very serious a | not serious | serious b | serious d | 0 | 0 | - | MD 0.51 lower (2.54 lower to 1.52 higher) | ⨁◯◯◯ Very low | Important |
| Mortality (no data) | |||||||||||
| Readmission by 3 months | |||||||||||
| 1 [38] | randomised trials | very serious a | not serious | serious b | very serious e | 0 | 0 | not estimable | 220 more per 1000 (from 70 fewer to 510 more) | ⨁◯◯◯ Very low | Important |
| Relapse (no data) [Important] | |||||||||||
| Non-response (no data) [Important] | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rana, R.; Sirwani, B.; Mohandas, S.; Kirubakaran, R.; Puthussery, S.; Lelijveld, N.; Kerac, M. Effectiveness of Postnatal Maternal or Caregiver Interventions on Outcomes among Infants under Six Months with Growth Faltering: A Systematic Review. Nutrients 2024, 16, 837. https://doi.org/10.3390/nu16060837
Rana R, Sirwani B, Mohandas S, Kirubakaran R, Puthussery S, Lelijveld N, Kerac M. Effectiveness of Postnatal Maternal or Caregiver Interventions on Outcomes among Infants under Six Months with Growth Faltering: A Systematic Review. Nutrients. 2024; 16(6):837. https://doi.org/10.3390/nu16060837
Chicago/Turabian StyleRana, Ritu, Barkha Sirwani, Saranya Mohandas, Richard Kirubakaran, Shuby Puthussery, Natasha Lelijveld, and Marko Kerac. 2024. "Effectiveness of Postnatal Maternal or Caregiver Interventions on Outcomes among Infants under Six Months with Growth Faltering: A Systematic Review" Nutrients 16, no. 6: 837. https://doi.org/10.3390/nu16060837
APA StyleRana, R., Sirwani, B., Mohandas, S., Kirubakaran, R., Puthussery, S., Lelijveld, N., & Kerac, M. (2024). Effectiveness of Postnatal Maternal or Caregiver Interventions on Outcomes among Infants under Six Months with Growth Faltering: A Systematic Review. Nutrients, 16(6), 837. https://doi.org/10.3390/nu16060837