Effect of the rs2821557 Polymorphism of the Human Kv1.3 Gene on Olfactory Function and BMI in Different Age Groups
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Olfactory Sensitivity Screening
2.3. Subject Genotyping Analysis
2.4. Data Analysis
3. Results
3.1. Kv1.3 Genotype and Olfactory Scores
3.2. Olfactory Scores and BMI Status
3.3. Kv1.3 Genotype and BMI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schubert, C.R.; Cruickshanks, K.J.; Nondahl, D.M.; Klein, B.E.; Klein, R.; Fischer, M.E. Association of exercise with lower long-term risk of olfactory impairment in older adults. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Hummel, T.; Nordin, S. Olfactory disorders and their consequences for quality of life. Acta Oto-Laryngol. 2005, 125, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Miwa, T.; Furukawa, M.; Tsukatani, T.; Costanzo, R.; DiNardo, L.; Reiter, E. Impact of Olfactory Impairment on Quality of Life and Disability. Arch. Otolaryngol. Head Neck Surg. 2001, 127, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.V.; Reiter, E.R.; DiNardo, L.J.; Costanzo, R.M. Hazardous events associated with impaired olfactory function. Arch. Otolaryngol. Head Neck Surg. 2004, 130, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Connor, E.E.; Zhou, Y.; Liu, G.E. The essence of appetite: Does olfactory receptor variation play a role? J. Anim. Sci. 2018, 96, 1551–1558. [Google Scholar] [CrossRef]
- Mastinu, M.; Melis, M.; Yousaf, N.Y.; Barbarossa, I.T.; Tepper, B.J. Emotional responses to taste and smell stimuli: Self-reports, physiological measures, and a potential role for individual and genetic factors. J. Food Sci. 2023, 88, A65–A90. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, R.J. An initial evaluation of the functions of human olfaction. Chem. Senses 2010, 35, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Aschenbrenner, K.; Hummel, C.; Teszmer, K.; Krone, F.; Ishimaru, T.; Seo, H.S.; Hummel, T. The influence of olfactory loss on dietary behaviors. Laryngoscope 2008, 118, 135–144. [Google Scholar] [CrossRef]
- Duffy, V.B.; Backstrand, J.R.; Ferris, A.M. Olfactory dysfunction and related nutritional risk in free-living, elderly women. J. Am. Diet. Assoc. 1995, 95, 879–884. [Google Scholar] [CrossRef]
- Egecioglu, E.; Skibicka, K.P.; Hansson, C.; Alvarez-Crespo, M.; Friberg, P.A.; Jerlhag, E.; Engel, J.A.; Dickson, S.L. Hedonic and incentive signals for body weight control. Rev. Endocr. Metab. Disord. 2011, 12, 141–151. [Google Scholar] [CrossRef]
- Manesse, C.; Ferdenzi, C.; Sabri, M.; Bessy, M.; Rouby, C.; Faure, F.; Bellil, D.; Jomain, S.; Landis, B.N.; Hugentobler, M.; et al. Dysosmia-Associated Changes in Eating Behavior. Chemosens. Percept. 2017, 10, 104–113. [Google Scholar] [CrossRef]
- Postma, E.; Graaf, C.; Boesveldt, S. Food preferences and intake in a population of Dutch individuals with self-reported smell loss: An online survey. Food Qual. Prefer. 2019, 79, 103771. [Google Scholar] [CrossRef]
- Velluzzi, F.; Deledda, A.; Lombardo, M.; Fosci, M.; Crnjar, R.; Grossi, E.; Sollai, G. Application of Artificial Neural Networks (ANN) to Elucidate the Connections among Smell, Obesity with Related Metabolic Alterations, and Eating Habit in Patients with Weight Excess. Metabolites 2023, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Bremner, E.A.; Mainland, J.D.; Khan, R.M.; Sobel, N. The prevalence of androstenone anosmia. Chem. Senses 2003, 28, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Crnjar, R.; Solari, P.; Sollai, G. The Human Nose as a Chemical Sensor in the Perception of Coffee Aroma: Individual Variability. Chemosensors 2023, 11, 248. [Google Scholar] [CrossRef]
- Croy, I.; Nordin, S.; Hummel, T. Olfactory Disorders and Quality of Life—An Updated Review. Chem. Senses 2014, 39, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Feldmesser, E.; Bercovich, D.; Avidan, N.; Halbertal, S.; Haim, L.; Gross-Isseroff, R.; Goshen, S.; Lancet, D. Mutations in olfactory signal transduction genes are not a major cause of human congenital general anosmia. Chem. Senses 2007, 32, 21–30. [Google Scholar] [CrossRef]
- Jafek, B.W.; Gordon, A.S.; Moran, D.T.; Eller, P.M. Congenital anosmia. Ear Nose Throat J. 1990, 69, 331–337. [Google Scholar]
- Silva Teixeira, C.S.; Cerqueira, N.M.; Silva Ferreira, A.C. Unravelling the Olfactory Sense: From the Gene to Odor Perception. Chem. Senses 2016, 41, 105–121. [Google Scholar] [CrossRef]
- Sollai, G.; Tomassini Barbarossa, I.; Usai, P.; Hummel, T.; Crnjar, R. Association between human olfactory performance and ability to detect single compounds in complex chemical mixtures. Physiol. Behav. 2020, 217, 112820. [Google Scholar] [CrossRef]
- Cain, W.S.; Gent, J.F. Olfactory sensitivity: Reliability, generality, and association with aging. J. Exp. Psychol. Hum. Percept. Perform. 1991, 17, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Hasin-Brumshtein, Y.; Lancet, D.; Olender, T. Human olfaction: From genomic variation to phenotypic diversity. Trends Genet. 2009, 25, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Zhuang, H.; Chi, Q.; Vosshall, L.B.; Matsunami, H. Genetic variation in a human odorant receptor alters odour perception. Nature 2007, 449, 468–472. [Google Scholar] [CrossRef]
- Melis, M.; Tomassini Barbarossa, I.; Hummel, T.; Crnjar, R.; Sollai, G. Effect of the rs2890498 polymorphism of the OBPIIa gene on the human ability to smell single molecules. Behav. Brain Res. 2021, 402, 113127. [Google Scholar] [CrossRef]
- Sollai, G.; Melis, M.; Magri, S.; Usai, P.; Hummel, T.; Tomassini Barbarossa, I.; Crnjar, R. Association between the rs2590498 polymorphism of Odorant Binding Protein (OBPIIa) gene and olfactory performance in healthy subjects. Behav. Brain Res. 2019, 372, 112030. [Google Scholar] [CrossRef]
- Sollai, G.; Melis, M.; Tomassini Barbarossa, I.; Crnjar, R. A polymorphism in the human gene encoding OBPIIa affects the perceived intensity of smelled odors. Behav. Brain Res. 2022, 427, 113860. [Google Scholar] [CrossRef]
- Besser, G.; Erlacher, B.; Aydinkoc-Tuzcu, K.; Liu, D.T.; Pablik, E.; Niebauer, V.; Koenighofer, M.; Renner, B.; Mueller, C.A. Body-Mass-Index Associated Differences in Ortho- and Retronasal Olfactory Function and the Individual Significance of Olfaction in Health and Disease. J. Clin. Med. 2020, 9, 366. [Google Scholar] [CrossRef] [PubMed]
- Palouzier-Paulignan, B.; Lacroix, M.C.; Aimé, P.; Baly, C.; Caillol, M.; Congar, P.; Julliard, A.K.; Tucker, K.; Fadool, D.A. Olfaction under metabolic influences. Chem Senses 2012, 37, 769–797. [Google Scholar] [CrossRef] [PubMed]
- Poessel, M.; Freiherr, J.; Wiencke, K.; Villringer, A.; Horstmann, A. Insulin Resistance Is Associated with Reduced Food Odor Sensitivity across a Wide Range of Body Weights. Nutrients 2020, 12, 2201. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Tang, K.; Wu, J.; Xu, H.; Zhang, W.; Cao, T.; Zhou, Y.; Yu, T.; Li, A. Leptin modulates olfactory discrimination and neural activity in the olfactory bulb. Acta Physiol. 2019, 227, e13319. [Google Scholar] [CrossRef]
- Tschöp, M.; Weyer, C.; Tataranni, P.A.; Devanarayan, V.; Ravussin, E.; Heiman, M.L. Circulating ghrelin levels are decreased in human obesity. Diabetes 2001, 50, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Franco-Lira, M.; Henríquez-Roldán, C.; Osnaya, N.; González-Maciel, A.; Reynoso-Robles, R.; Villarreal-Calderon, R.; Herritt, L.; Brooks, D.; Keefe, S.; et al. Urban air pollution: Influences on olfactory function and pathology in exposed children and young adults. Exp. Toxicol. Pathol. Off. J. Ges. Fur Toxikol. Pathol. 2010, 62, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Iannario, M.; Manisera, M.; Piccolo, D.; Zuccolotto, P. Sensory analysis in the food industry as a tool for marketing decisions. Adv. Data Anal. Classif. 2012, 6, 303–321. [Google Scholar] [CrossRef]
- Sollai, G.; Crnjar, R. Age-Related Olfactory Decline Is Associated with Levels of Exercise and Non-Exercise Physical Activities. Front. Aging Neurosci. 2021, 13, 695115. [Google Scholar] [CrossRef] [PubMed]
- Sollai, G.; Crnjar, R. Association among Olfactory Function, Lifestyle and BMI in Female and Male Elderly Subjects: A Cross-Sectional Study. Nutrients 2023, 15, 2492. [Google Scholar] [CrossRef] [PubMed]
- Sorokowska, A.; Sorokowski, P.; Frackowiak, T. Determinants of human olfactory performance: A cross-cultural study. Sci. Total Environ. 2015, 506–507, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Sorokowska, A.; Sorokowski, P.; Hummel, T. Cross-Cultural Administration of an Odor Discrimination Test. Chemosens. Percept. 2014, 7, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Croy, I.; Symmank, A.; Schellong, J.; Hummel, C.; Gerber, J.; Joraschky, P.; Hummel, T. Olfaction as a marker for depression in humans. J. Affect. Disord. 2014, 160, 80–86. [Google Scholar] [CrossRef]
- Graves, A.B.; Bowen, J.D.; Rajaram, L.; McCormick, W.C.; McCurry, S.M.; Schellenberg, G.D.; Larson, E.B. Impaired olfaction as a marker for cognitive decline: Interaction with apolipoprotein E ε4 status. Neurology 1999, 53, 1480–1487. [Google Scholar] [CrossRef]
- Pastor, A.; Fernández-Aranda, F.; Fitó, M.; Jiménez-Murcia, S.; Botella, C.; Fernández-Real, J.M.; Frühbeck, G.; Tinahones, F.J.; Fagundo, A.B.; Rodriguez, J.; et al. A Lower Olfactory Capacity Is Related to Higher Circulating Concentrations of Endocannabinoid 2-Arachidonoylglycerol and Higher Body Mass Index in Women. PLoS ONE 2016, 11, e0148734. [Google Scholar] [CrossRef]
- Patel, Z.M.; DelGaudio, J.M.; Wise, S.K. Higher Body Mass Index Is Associated with Subjective Olfactory Dysfunction. Behav. Neurol. 2015, 2015, 675635. [Google Scholar] [CrossRef]
- Perricone, C.; Shoenfeld, N.; Agmon-Levin, N.; de Carolis, C.; Perricone, R.; Shoenfeld, Y. Smell and autoimmunity: A comprehensive review. Clin. Rev. Allergy Immunol. 2013, 45, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.M.; Wroblewski, K.E.; Kern, D.W.; Schumm, L.P.; McClintock, M.K. Olfactory dysfunction predicts 5-year mortality in older adults. PLoS ONE 2014, 9, e107541. [Google Scholar] [CrossRef]
- Ross, G.W.; Petrovitch, H.; Abbott, R.D.; Tanner, C.M.; Popper, J.; Masaki, K.; Launer, L.; White, L.R. Association of olfactory dysfunction with risk for future Parkinson’s disease. Ann. Neurol. 2008, 63, 167–173. [Google Scholar] [CrossRef]
- Sollai, G.; Melis, M.; Mastinu, M.; Paduano, D.; Chicco, F.; Magri, S.; Usai, P.; Hummel, T.; Barbarossa, I.T.; Crnjar, R. Olfactory Function in Patients with Inflammatory Bowel Disease (IBD) Is Associated with Their Body Mass Index and Polymorphism in the Odor Binding-Protein (OBPIIa) Gene. Nutrients 2021, 13, 703. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, S.; Proft, F.; Schulze-Koops, H.; Hundt, W.; Heinrich, P.; Schulz, S.; Gruenke, M. Gustatory and olfactory function in rheumatoid arthritis. Scand. J. Rheumatol. 2011, 40, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, S.; Reindl, W.; Dempfle, A.; Schuster, A.; Wolf, P.; Hundt, W.; Huber, W. Smell and taste in inflammatory bowel disease. PLoS ONE 2013, 8, e73454. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, S.; Reindl, W.; Kessel, C.; Ott, R.; Zahnert, T.; Hundt, W.; Heinrich, P.; Saur, D.; Huber, W. Olfactory and gustatory function in irritable bowel syndrome. Eur. Arch. Otorhinolaryngol. 2010, 267, 1081–1087. [Google Scholar] [CrossRef]
- Velluzzi, F.; Anedda, J.; Pisanu, S.; Dell’Antonia, M.; Deledda, A.; Boi, A.; Ferreli, C.; Atzori, L. Mediterranean Diet, Lifestyle and Quality of Life in Sardinian Patients Affected with Hidradenitis Suppurativa. J. Public Health Res. 2022, 11, 2706. [Google Scholar] [CrossRef]
- Walliczek-Dworschak, U.; Wendler, J.; Khan, T.; Aringer, M.; Hähner, A.; Hummel, T. Chemosensory function is decreased in rheumatoid arthritis. Eur. Arch. Otorhinolaryngol. 2020, 277, 1675–1680. [Google Scholar] [CrossRef]
- Wilson, R.S.; Arnold, S.E.; Schneider, J.A.; Boyle, P.A.; Buchman, A.S.; Bennett, D.A. Olfactory impairment in presymptomatic Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2009, 1170, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.S.; Schneider, J.A.; Arnold, S.E.; Tang, Y.; Boyle, P.A.; Bennett, D.A. Olfactory Identification and Incidence of Mild Cognitive Impairment in Older Age. Arch. Gen. Psychiatry 2007, 64, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Öberg, C.; Larsson, M.; Bäckman, L. Differential sex effects in olfactory functioning: The role of verbal processing. J. Int. Neuropsychol. Soc. 2002, 8, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Sorokowski, P.; Karwowski, M.; Misiak, M.; Marczak, M.K.; Dziekan, M.; Hummel, T.; Sorokowska, A. Sex Differences in Human Olfaction: A Meta-Analysis. Front. Psychol. 2019, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Attems, J.; Walker, L.; Jellinger, K.A. Olfaction and Aging: A Mini-Review. Gerontology 2015, 61, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Cain, W.S.; Stevens, J.C. Uniformity of olfactory loss in aging. Ann. N. Y. Acad. Sci. 1989, 561, 29–38. [Google Scholar] [CrossRef]
- Doty, R.L.; Shaman, P.; Applebaum, S.L.; Giberson, R.; Siksorski, L.; Rosenberg, L. Smell identification ability: Changes with age. Science 1984, 226, 1441–1443. [Google Scholar] [CrossRef]
- Min, H.J.; Kim, S.M.; Han, D.H.; Kim, K.S. The sniffing bead system, an olfactory dysfunction screening tool for geriatric subjects: A cross-sectional study. BMC Geriatr. 2021, 21, 54. [Google Scholar] [CrossRef]
- Schubert, C.R.; Fischer, M.E.; Pinto, A.A.; Klein, B.E.K.; Klein, R.; Tweed, T.S.; Cruickshanks, K.J. Sensory Impairments and Risk of Mortality in Older Adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017, 72, 710–715. [Google Scholar] [CrossRef]
- Fadool, D.A.; Tucker, K.; Perkins, R.; Fasciani, G.; Thompson, R.N.; Parsons, A.D.; Overton, J.M.; Koni, P.A.; Flavell, R.A.; Kaczmarek, L.K. Kv1.3 channel gene-targeted deletion produces “Super-Smeller Mice” with altered glomeruli, interacting scaffolding proteins, and biophysics. Neuron 2004, 41, 389–404. [Google Scholar] [CrossRef]
- Guthoff, M.; Tschritter, O.; Berg, D.; Liepelt, I.; Schulte, C.; Machicao, F.; Haering, H.U.; Fritsche, A. Effect of genetic variation in Kv1.3 on olfactory function. Diabetes/Metab. Res. Rev. 2009, 25, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.; Tomassini Barbarossa, I.; Crnjar, R.; Sollai, G. Olfactory Sensitivity Is Associated with Body Mass Index and Polymorphism in the Voltage-Gated Potassium Channels Kv1.3. Nutrients 2022, 14, 4986. [Google Scholar] [CrossRef]
- Tschritter, O.; Machicao, F.; Stefan, N.; Schäfer, S.; Weigert, C.; Staiger, H.; Spieth, C.; Häring, H.U.; Fritsche, A. A new variant in the human Kv1.3 gene is associated with low insulin sensitivity and impaired glucose tolerance. J. Clin. Endocrinol. Metab. 2006, 91, 654–658. [Google Scholar] [CrossRef]
- Tucker, K.; Cavallin, M.A.; Jean-Baptiste, P.; Biju, K.C.; Overton, J.M.; Pedarzani, P.; Fadool, D.A. The Olfactory Bulb: A Metabolic Sensor of Brain Insulin and Glucose Concentrations via a Voltage-Gated Potassium Channel. Results Probl. Cell Differ. 2010, 52, 147–157. [Google Scholar] [PubMed]
- Tucker, K.; Overton, J.M.; Fadool, D.A. Kv1.3 gene-targeted deletion alters longevity and reduces adiposity by increasing locomotion and metabolism in melanocortin-4 receptor-null mice. Int. J. Obes. 2008, 32, 1222–1232. [Google Scholar] [CrossRef] [PubMed]
- Tucker, K.; Overton, J.M.; Fadool, D.A. Diet-induced obesity resistance of Kv1.3−/− mice is olfactory bulb dependent. J. Neuroendocrinol. 2012, 24, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Koni, P.A.; Wang, P.; Li, G.; Kaczmarek, L.; Wu, Y.; Li, Y.; Flavell, R.A.; Desir, G.V. The voltage-gated potassium channel Kv1.3 regulates energy homeostasis and body weight. Hum. Mol. Genet. 2003, 12, 551–559. [Google Scholar] [CrossRef]
- Xu, J.; Wang, P.; Li, Y.; Li, G.; Kaczmarek, L.K.; Wu, Y.; Koni, P.A.; Flavell, R.A.; Desir, G.V. The voltage-gated potassium channel Kv1.3 regulates peripheral insulin sensitivity. Proc. Natl. Acad. Sci. USA 2004, 101, 3112–3117. [Google Scholar] [CrossRef]
- Boesveldt, S.; de Graaf, K. The Differential Role of Smell and Taste for Eating Behavior. Perception 2017, 46, 307–319. [Google Scholar] [CrossRef]
- Hoffman, H.J.; Ishii, E.K.; MacTurk, R.H. Age-related changes in the prevalence of smell/taste problems among the United States adult population. Results of the 1994 disability supplement to the National Health Interview Survey (NHIS). Ann. N. Y. Acad. Sci. 1998, 855, 716–722. [Google Scholar] [CrossRef]
- Hoffman, H.J.; Rawal, S.; Li, C.M.; Duffy, V.B. New chemosensory component in the U.S. National Health and Nutrition Examination Survey (NHANES): First-year results for measured olfactory dysfunction. Rev. Endocr. Metab. Disord. 2016, 17, 221–240. [Google Scholar] [CrossRef]
- Pence, T.S.; Reiter, E.R.; DiNardo, L.J.; Costanzo, R.M. Risk factors for hazardous events in olfactory-impaired patients. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Rawal, S.; Hoffman, H.J.; Bainbridge, K.E.; Huedo-Medina, T.B.; Duffy, V.B. Prevalence and Risk Factors of Self-Reported Smell and Taste Alterations: Results from the 2011–2012 US National Health and Nutrition Examination Survey (NHANES). Chem. Senses 2016, 41, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, B.; Russell, J.; Sue, C.M.; Flood, V.M.; Burlutsky, G.; Mitchell, P. Olfactory impairment in older adults is associated with poorer diet quality over 5 years. Eur. J. Nutr. 2016, 55, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Boesveldt, S.; Bobowski, N.; McCrickerd, K.; Maître, I.; Sulmont-Rossé, C.; Forde, C.G. The changing role of the senses in food choice and food intake across the lifespan. Food Qual. Prefer. 2018, 68, 80–89. [Google Scholar] [CrossRef]
- Boesveldt, S.; Parma, V. The importance of the olfactory system in human well-being, through nutrition and social behavior. Cell Tissue Res. 2021, 383, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Coutts, D.; Wang, T.; Cakmak, Y.O. Systematic review of olfactory shifts related to obesity. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2019, 20, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Hummel, T.; Kobal, G.; Gudziol, H.; Mackay-Sim, A. Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: An upgrade based on a group of more than 3,000 subjects. Eur. Arch. Otorhinolaryngol. 2007, 264, 237–243. [Google Scholar] [CrossRef]
- Velluzzi, F.; Deledda, A.; Onida, M.; Loviselli, A.; Crnjar, R.; Sollai, G. Relationship between Olfactory Function and BMI in Normal Weight Healthy Subjects and Patients with Overweight or Obesity. Nutrients 2022, 14, 1262. [Google Scholar] [CrossRef]
- Hummel, T.; Sekinger, B.; Wolf, S.R.; Pauli, E.; Kobal, G. ‘Sniffi’ sticks’: Olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem. Senses 1997, 22, 39–52. [Google Scholar] [CrossRef]
- Rousset, F. genepop’007: A complete re-implementation of the genepop software for Windows and Linux. Mol. Ecol. Resour. 2008, 8, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Schubert, C.R.; Carmichael, L.L.; Murphy, C.; Klein, B.E.K.; Klein, R.; Cruickshanks, K.J. Olfaction and the 5-Year Incidence of Cognitive Impairment in an Epidemiological Study of Older Adults. J. Am. Geriatr. Soc. 2008, 56, 1517–1521. [Google Scholar] [CrossRef] [PubMed]
- Seubert, J.; Laukka, E.J.; Rizzuto, D.; Hummel, T.; Fratiglioni, L.; Bäckman, L.; Larsson, M. Prevalence and Correlates of Olfactory Dysfunction in Old Age: A Population-Based Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017, 72, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Schubert, C.R.; Cruickshanks, K.J.; Fischer, M.E.; Huang, G.H.; Klein, B.E.; Klein, R.; Pankow, J.S.; Nondahl, D.M. Olfactory impairment in an adult population: The Beaver Dam Offspring Study. Chem. Senses 2012, 37, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Dintica, C.S.; Marseglia, A.; Rizzuto, D.; Wang, R.; Seubert, J.; Arfanakis, K.; Bennett, D.A.; Xu, W. Impaired olfaction is associated with cognitive decline and neurodegeneration in the brain. Neurology 2019, 92, e700–e709. [Google Scholar] [CrossRef] [PubMed]
- Hedner, M.; Larsson, M.; Arnold, N.; Zucco, G.M.; Hummel, T. Cognitive factors in odor detection, odor discrimination, and odor identification tasks. J. Clin. Exp. Neuropsychol. 2010, 32, 1062–1067. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M.; Finkel, D.; Pedersen, N.L. Odor identification: Influences of age, gender, cognition, and personality. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2000, 55, P304–P310. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.R. The neurobiology of food intake in an obesogenic environment. Proc. Nutr. Soc. 2012, 71, 478–487. [Google Scholar] [CrossRef]
- Bolhuis, D.P.; Lakemond, C.M.; de Wijk, R.A.; Luning, P.A.; de Graaf, C. Effect of salt intensity in soup on ad libitum intake and on subsequent food choice. Appetite 2012, 58, 48–55. [Google Scholar] [CrossRef]
- Gaillet-Torrent, M.; Sulmont-Rossé, C.; Issanchou, S.; Chabanet, C.; Chambaron, S. Impact of a non-attentively perceived odour on subsequent food choices. Appetite 2014, 76, 17–22. [Google Scholar] [CrossRef]
- Lopez-Gonzalez, D.; Partida-Gaytán, A.; Wells, J.C.; Reyes-Delpech, P.; Avila-Rosano, F.; Ortiz-Obregon, M.; Gomez-Mendoza, F.; Diaz-Escobar, L.; Clark, P. Obesogenic Lifestyle and Its Influence on Adiposity in Children and Adolescents, Evidence from Mexico. Nutrients 2020, 12, 819. [Google Scholar] [CrossRef] [PubMed]
- Ramaekers, M.G.; Boesveldt, S.; Lakemond, C.M.; van Boekel, M.A.; Luning, P.A. Odors: Appetizing or satiating? Development of appetite during odor exposure over time. Int. J. Obes. 2014, 38, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Ruijschop, R.M.; Boelrijk, A.E.; de Ru, J.A.; de Graaf, C.; Westerterp-Plantenga, M.S. Effects of retro-nasal aroma release on satiation. Br. J. Nutr. 2008, 99, 1140–1148. [Google Scholar] [CrossRef]
- Stroebele, N.; De Castro, J.M. Effect of ambience on food intake and food choice. Nutrition 2004, 20, 821–838. [Google Scholar] [CrossRef] [PubMed]
- Hardikar, S.; Wallroth, R.; Villringer, A.; Ohla, K. Shorter-lived neural taste representations in obese compared to lean individuals. Sci. Rep. 2018, 8, 11027. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.S.; Hajnal, A.; Czaja, K.; Di Lorenzo, P.M. Taste Responses in the Nucleus of the Solitary Tract of Awake Obese Rats Are Blunted Compared with Those in Lean Rats. Front. Integr. Neurosci. 2019, 13, 35. [Google Scholar] [CrossRef]


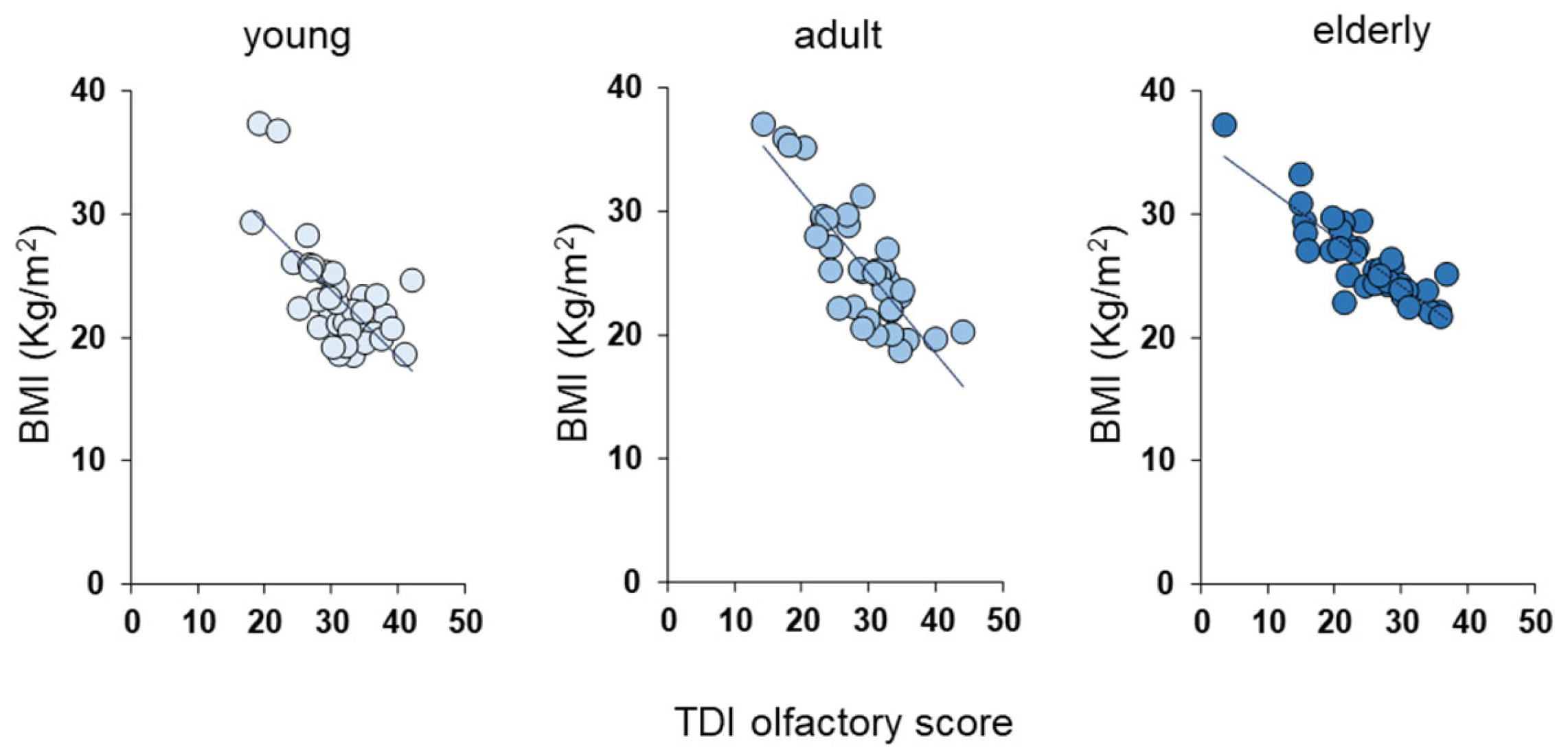
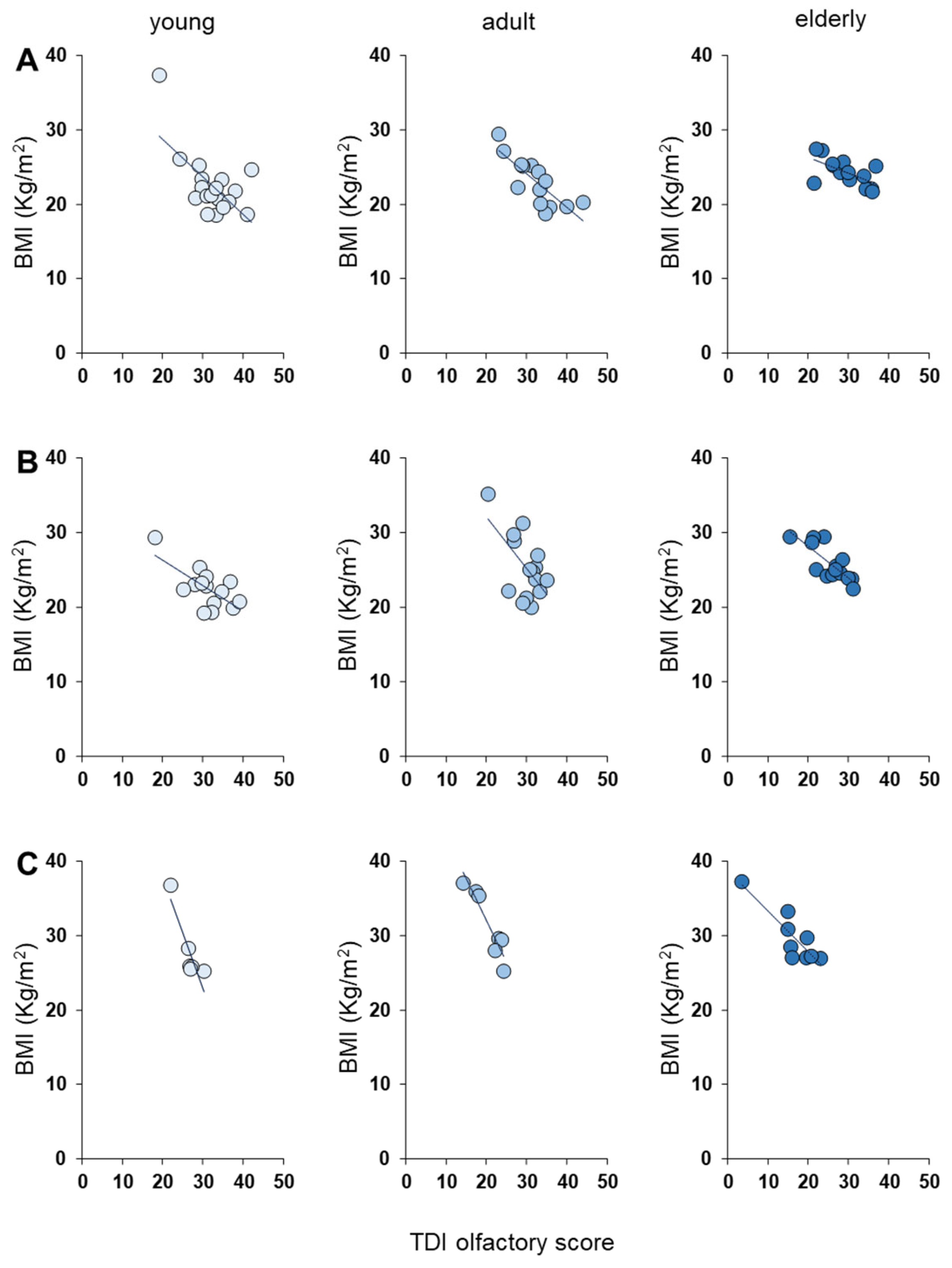
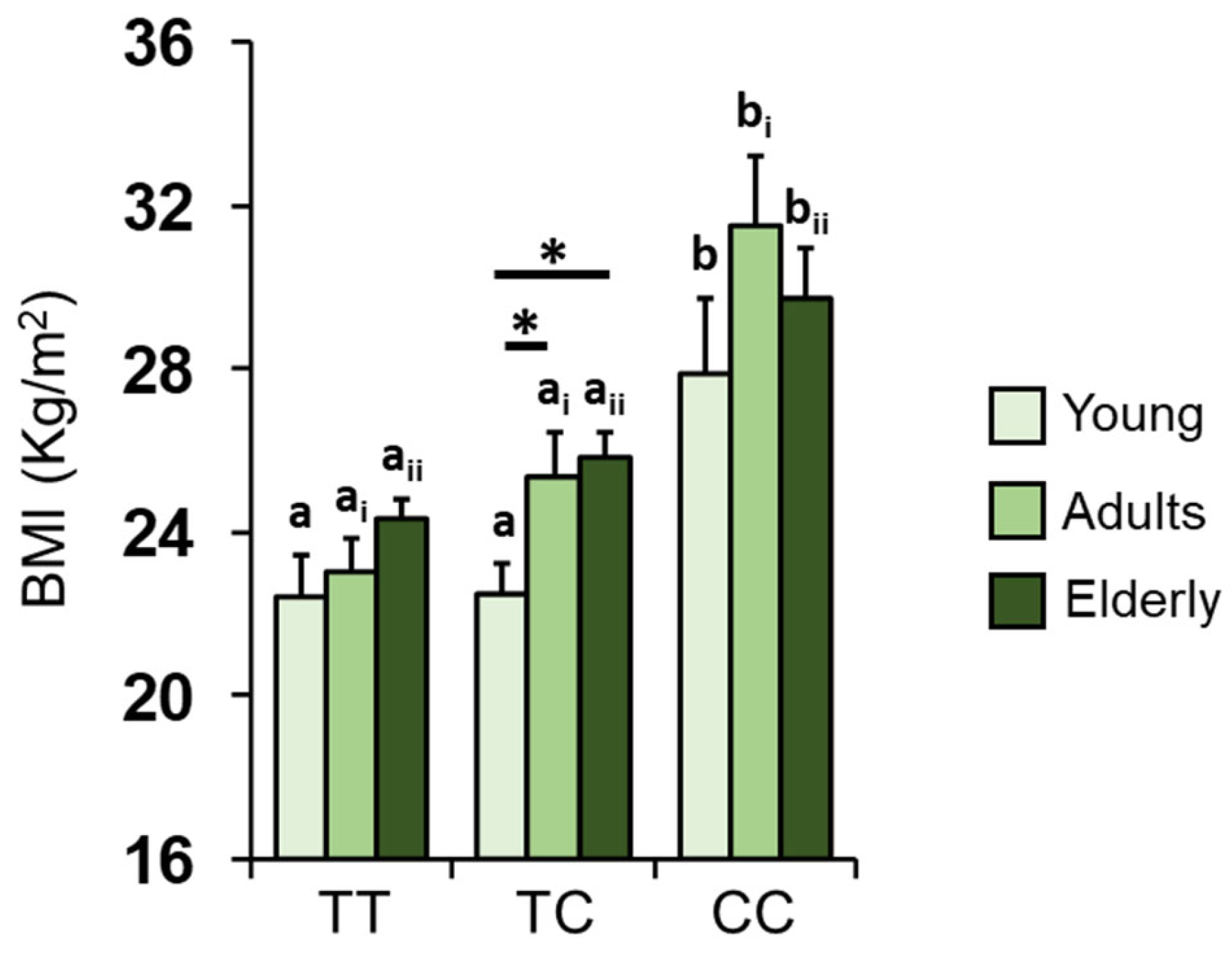
| Age Group | Olfactory Function Based on Genotype |
|---|---|
| Young (16–35 years) | TT = TC > CC |
| Adults (36–55 years) | TT = TC > CC |
| Elderly (>55 years) | TT > TC > CC |
| Genotype | Olfactory Function Based on Age |
|---|---|
| TT | Young = Adults = Elderly |
| TC | Young = Adults > Elderly |
| CC | Young > Adults = Elderly |
| Age Group 16–35 years | Normosmic n (%) | Hyposmic n (%) | p-Value a |
|---|---|---|---|
| Genotype | 0.029 | ||
| TT | 11 (61.11) | 8 (38.10) | |
| TC | 7 (38.89) | 7 (33.33) | |
| CC | 0 (0) | 6 (28.57) | |
| Allele | 0.019 | ||
| T | 29 (80.56) | 23 (54.76) | |
| C | 7 (19.44) | 19 (45.24) | |
| a p-value derived from Fisher’s Exact Test. Genotypes: TT n = 19; TC n = 14; CC n = 6. | |||
| Age Group 36–55 years | Normosmic n (%) | Hyposmic n (%) | p-Value a |
| Genotype | 0.013 | ||
| TT | 9 (52.94) | 5 (26.32) | |
| TC | 8 (47.06) | 7 (36.84) | |
| CC | 0 (0) | 7 (36.84) | |
| Allele | 0.009 | ||
| T | 26 (76.47) | 17 (44.74) | |
| C | 8 (23.53) | 21 (55.26) | |
| a p-value derived from Fisher’s Exact Test. Genotypes: TT n = 14; TC n = 15; CC n = 7. | |||
| Age Group >55 years | Normosmic n (%) | Hyposmic n (%) | p-Value a |
| Genotype | 0.011 | ||
| TT | 7 (63.64) | 7 (26.92) | |
| TC | 4 (36.36) | 10 (38.46) | |
| CC | 0 (0) | 9 (34.62) | |
| Allele | 0.005 | ||
| T | 18 (81.82) | 24 (46.15) | |
| C | 4 (18.18) | 28 (53.85) | |
| a p-value derived from Fisher’s Exact Test. Genotypes: TT n = 14; TC n = 14; CC n = 9. | |||
| Variable | x2 | p-Value |
|---|---|---|
| BMI | 46.72 | <0.0001 |
| Kv1.3 genotype | 26.04 | <0.0001 |
| Age | 20.40 | <0.0001 |
| Age—Kv1.3 genotype | 16.77 | 0.0021 |
| Age Group 16–35 years | NW n (%) | OW n (%) | p-Value a |
|---|---|---|---|
| Genotype | 0.003 | ||
| TT | 16 (57.14) | 3 (27.27) | |
| TC | 12 (42.86) | 2 (18.18) | |
| CC | 0 (0) | 6 (54.55) | |
| Allele | 0.001 | ||
| T | 44 (78.57) | 8 (36.36) | |
| C | 12 (21.43) | 14 (63.64) | |
| a p-value derived from Fisher’s Exact Test. Genotypes: TT n = 19; TC n = 14; CC n = 6. | |||
| Age Group 36–55 years | NW n (%) | OW n (%) | p-Value a |
| Genotype | 0.005 | ||
| TT | 9 (64.29) | 5 (22.73) | |
| TC | 5 (35.71) | 10 (45.45) | |
| CC | 0 (0) | 7 (31.82) | |
| Allele | 0.003 | ||
| T C | 23 (82.14) 5 (17.86) | 20 (45.45) 24 (54.55) | |
| a p-value derived from Fisher’s Exact Test. Genotypes: TT n = 14; TC n = 15; CC n = 7. | |||
| Age Group >55 years | Normosmic n (%) | Hyposmic n (%) | p-Value a |
| Genotype | 0.009 | ||
| TT | 8 (53.33) | 6 (27.27) | |
| TC | 7 (46.67) | 7 (31.82) | |
| CC | 0 (0) | 9 (40.91) | |
| Allele | 0.005 | ||
| T | 23 (76.67) | 19 (43.18) | |
| C | 7 (23.33) | 25 (56.82) | |
| a p-value derived from Fisher’s Exact Test. Genotypes: TT n = 14; TC n = 14; CC n = 9. | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melis, M.; Mastinu, M.; Sollai, G. Effect of the rs2821557 Polymorphism of the Human Kv1.3 Gene on Olfactory Function and BMI in Different Age Groups. Nutrients 2024, 16, 821. https://doi.org/10.3390/nu16060821
Melis M, Mastinu M, Sollai G. Effect of the rs2821557 Polymorphism of the Human Kv1.3 Gene on Olfactory Function and BMI in Different Age Groups. Nutrients. 2024; 16(6):821. https://doi.org/10.3390/nu16060821
Chicago/Turabian StyleMelis, Melania, Mariano Mastinu, and Giorgia Sollai. 2024. "Effect of the rs2821557 Polymorphism of the Human Kv1.3 Gene on Olfactory Function and BMI in Different Age Groups" Nutrients 16, no. 6: 821. https://doi.org/10.3390/nu16060821
APA StyleMelis, M., Mastinu, M., & Sollai, G. (2024). Effect of the rs2821557 Polymorphism of the Human Kv1.3 Gene on Olfactory Function and BMI in Different Age Groups. Nutrients, 16(6), 821. https://doi.org/10.3390/nu16060821








