Adherence to Mediterranean Diet, Dietary Salt Intake, and Susceptibility to Nephrolithiasis: A Case–Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Populations
2.2. Data Collection
2.3. MD Adherence
2.4. Salt Habits
2.5. Combined Impact of MD Adhesion and Salt Intake on NL Susceptibility
2.6. NL-Related Metabolic Risk Factors
2.7. Statistical Analysis
3. Results
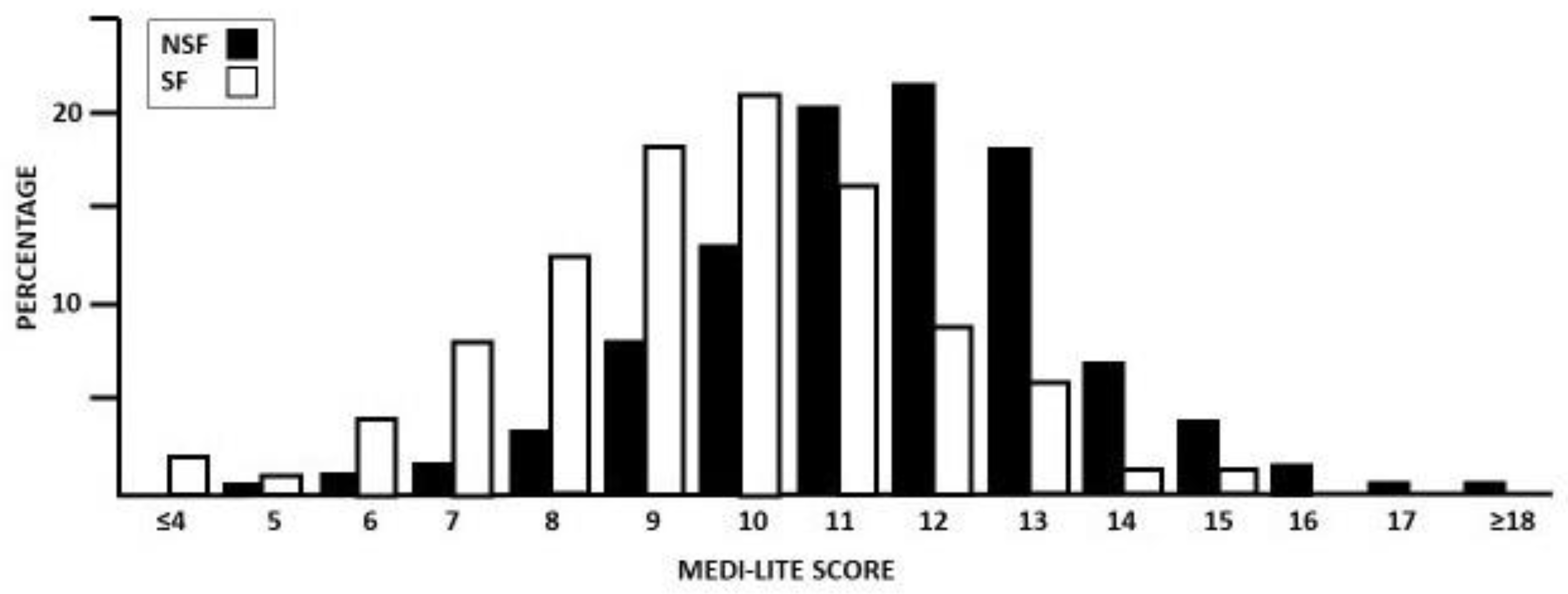
3.1. MEDI-LITE Score
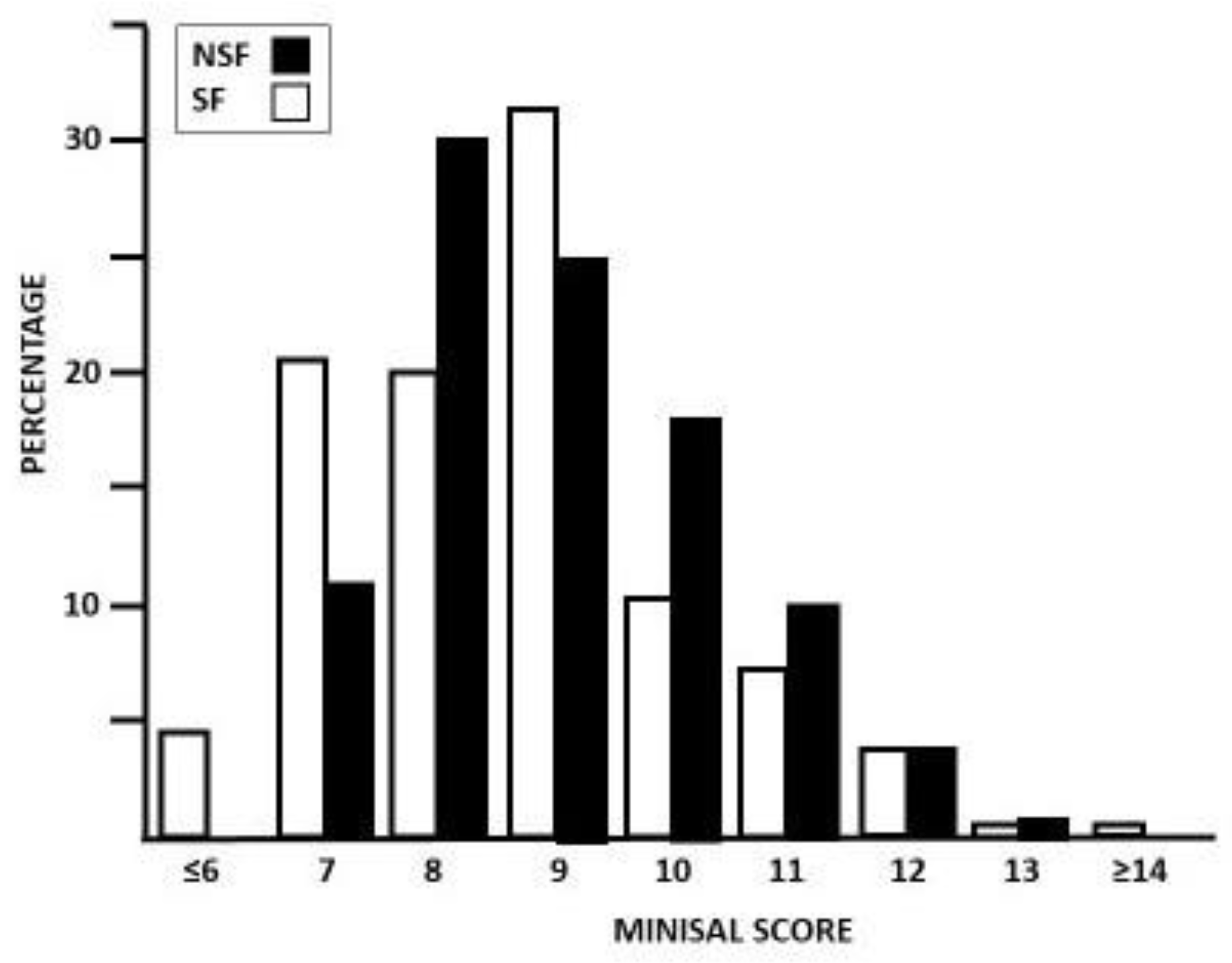
3.2. MINISAL Score
3.3. Combined MD Adhesion and Salt Consumption
3.4. Correlation between MEDI-LITE Score, MINISAL Score, and NL Metabolic Risk Factors
4. Discussion
5. Practical Application
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ziemba, J.B.; Matlaga, B.R. Epidemiology and economics of nephrolithiasis. Investig. Clin. Urol. 2017, 58, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Turney, B.W.; Reynard, J.M.; Noble, J.G.; Keoghane, S.R. Trends in urological stone disease. BJU Int. 2012, 109, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Romero, V.; Akpinar, H.; Assimos, D.G. Kidney stones: A global picture of prevalence, incidence, and associated risk factors. Rev. Urol. 2010, 12, e86–e96. [Google Scholar]
- Chewcharat, A.; Curhan, G. Trends in the prevalence of kidney stones in the United States from 2007 to 2016. Urolithiasis 2021, 49, 27–39. [Google Scholar] [CrossRef]
- Wang, K.; Ge, J.; Han, W.; Wang, D.; Zhao, Y.; Shen, Y.; Chen, J.; Chen, D.; Wu, J.; Shen, N.; et al. Risk factors for kidney stone disease recurrence: A comprehensive meta-analysis. BMC Urol. 2022, 22, 62. [Google Scholar] [CrossRef]
- Shoag, J.; Halpern, J.; Goldfarb, D.S.; Eisner, B.H. Risk of chronic and end stage kidney disease in patients with nephrolithiasis. J. Urol. 2014, 192, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
- Dhondup, T.; Kittanamongkolchai, W.; Vaughan, L.E.; Mehta, R.A.; Chhina, J.K.; Enders, F.T.; Hickson, L.J.; Lieske, J.C.; Rule, A.D. Risk of ESRD and Mortality in Kidney and Bladder Stone Formers. Am. J. Kidney Dis. 2018, 72, 790–797. [Google Scholar] [CrossRef]
- Jungers, P.; Joly, D.; Barbey, F.; Choukroun, G.; Daudon, M. ESRD caused by nephrolithiasis: Prevalence, mechanisms, and prevention. Am. J. Kidney Dis. 2004, 44, 799–805. [Google Scholar] [CrossRef]
- Rule, A.D.; Bergstralh, E.J.; Melton, L.J., 3rd; Li, X.; Weaver, A.L.; Lieske, J.C. Kidney stones and the risk for chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2009, 4, 804–811. [Google Scholar] [CrossRef]
- Chuang, T.F.; Hung, H.C.; Li, S.F.; Lee, M.W.; Pai, J.Y.; Hung, C.T. Risk of chronic kidney disease in patients with kidney stones-a nationwide cohort study. BMC Nephrol. 2020, 21, 292. [Google Scholar] [CrossRef]
- Rule, A.D.; Lieske, J.C.; Pais, V.M., Jr. Management of Kidney Stones in 2020. JAMA 2020, 323, 1961–1962. [Google Scholar] [CrossRef] [PubMed]
- Turney, B.W.; Appleby, P.N.; Reynard, J.M.; Noble, J.G.; Key, T.J.; Allen, N.E. Diet and risk of kidney stones in the Oxford cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC). Eur. J. Epidemiol. 2014, 29, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Siener, R. Nutrition and Kidney Stone Disease. Nutrients 2021, 13, 1917. [Google Scholar] [CrossRef] [PubMed]
- Meschi, T.; Maggiore, U.; Fiaccadori, E.; Schianchi, T.; Bosi, S.; Adorni, G.; Ridolo, E.; Guerra, A.; Allegri, F.; Novarini, A.; et al. The effect of fruits and vegetables on urinary stone risk factors. Kidney Int. 2004, 66, 2402–2410. [Google Scholar] [CrossRef]
- Gottlieb, S. High protein diet brings risk of kidney stones. BMJ 2002, 325, 408. [Google Scholar] [CrossRef]
- Tracy, C.R.; Best, S.; Bagrodia, A.; Poindexter, J.R.; Adams-Huet, B.; Sakhaee, K.; Maalouf, N.; Pak, C.Y.; Pearle, M.S. Animal protein and the risk of kidney stones: A comparative metabolic study of animal protein sources. J. Urol. 2014, 192, 137–141. [Google Scholar] [CrossRef]
- Lin, B.B.; Lin, M.E.; Huang, R.H.; Hong, Y.K.; Lin, B.L.; He, X.J. Dietary and lifestyle factors for primary prevention of nephrolithiasis: A systematic review and meta-analysis. BMC Nephrol. 2020, 21, 267. [Google Scholar] [CrossRef]
- Leone, A.; Fernández-Montero, A.; de la Fuente-Arrillaga, C.; Martínez-González, M.Á.; Bertoli, S.; Battezzati, A.; Bes-Rastrollo, M. Adherence to the Mediterranean Dietary Pattern and Incidence of Nephrolithiasis in the Seguimiento Universidad de Navarra Follow-up (SUN) Cohort. Am. J. Kidney Dis. 2017, 70, 778–786. [Google Scholar] [CrossRef]
- Maddahi, N.; Aghamir, S.M.K.; Moddaresi, S.S.; Mirzaei, K.; Alizadeh, S.; Yekaninejad, M.S. The association of Dietary Approaches to Stop Hypertension-style diet with urinary risk factors of kidney stones formation in men with nephrolithiasis. Clin. Nutr. ESPEN 2020, 39, 173–179. [Google Scholar] [CrossRef]
- Rodrigues, F.G.; Lima, T.M.; Zambrano, L.; Heilberg, I.P. Dietary pattern analysis among stone formers: Resemblance to a DASH-style diet. Braz. J. Nephrol. 2020, 42, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.N.; Fung, T.T.; Curhan, G.C. DASH-style diet associates with reduced risk for kidney stones. J. Am. Soc. Nephrol. 2009, 20, 2253–2259. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.A.; Mohebbi, N. Urinary pH and stone formation. J. Nephrol. 2010, 16, S165–S169. [Google Scholar]
- Welch, A.A.; Mulligan, A.; Bingham, S.A.; Khaw, K.T. Urine pH is an indicator of dietary acid-base load, fruit and vegetables and meat intakes: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk population study. Br. J. Nutr. 2008, 99, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Adomako, E.A.; Li, X.; Sakhaee, K.; Moe, O.W.; Maalouf, N.M. Urine pH and Citrate as Predictors of Calcium Phosphate Stone Formation. Kidney360 2023, 4, 1123–1129. [Google Scholar] [CrossRef]
- Rodriguez, A.; Curhan, G.C.; Gambaro, G.; Taylor, E.N.; Ferraro, P.M. Mediterranean diet adherence and risk of incident kidney stones. Am. J. Clin. Nutr. 2020, 111, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Bedford, J.L.; Barr, S.I. Higher urinary sodium, a proxy for intake, is associated with increased calcium excretion and lower hip bone density in healthy young women with lower calcium intakes. Nutrients 2011, 3, 951–961. [Google Scholar] [CrossRef]
- Coe, F.L.; Worcester, E.M.; Evan, A.P. Idiopathic hypercalciuria and formation of calcium renal stones. Nat. Rev. Nephrol. 2016, 12, 519–533. [Google Scholar] [CrossRef]
- Klein, J.; Netsch, C.; Sievert, K.D.; Miernik, A.; Westphal, J.; Leyh, H.; Herrmann, T.R.W.; Olbert, P.; Häcker, A.; Bachmann, A.; et al. Extracorporeal shock wave lithotripsy. Der Urologe 2018, 57, 463–473. [Google Scholar] [CrossRef]
- Jiang, P.; Xie, L.; Arada, R.; Patel, R.M.; Landman, J.; Clayman, R.V. Qualitative Review of Clinical Guidelines for Medical and Surgical Management of Urolithiasis: Consensus and Controversy 2020. J. Urol. 2021, 205, 999–1008. [Google Scholar] [CrossRef]
- Junuzovic, D.; Prstojevic, J.K.; Hasanbegovic, M.; Lepara, Z. Evaluation of Extracorporeal Shock Wave Lithotripsy (ESWL): Efficacy in Treatment of Urinary System Stones. Acta Inform. Med. 2014, 22, 309–314. [Google Scholar] [CrossRef]
- Sofi, F.; Dinu, M.; Pagliai, G.; Marcucci, R.; Casini, A. Validation of a literature-based adherence score to Mediterranean diet: The MEDI-LITE score. Int. J. Food Sci. Nutr. 2017, 68, 757–762. [Google Scholar] [CrossRef]
- D’Elia, L.; Manfredi, M.; Strazzullo, P.; Galletti, F.; MINISAL-SIIA Study Group. Validation of an easy questionnaire on the assessment of salt habit: The MINISAL-SIIA Study Program. Eur. J. Clin. Nutr. 2019, 73, 793–800. [Google Scholar] [CrossRef]
- Leaf, D.E. Calcium kidney stones. N. Engl. J. Med. 2010, 363, 2470–2471. [Google Scholar]
- AUA Website. Available online: https://www.auanet.org/guidelines-and-quality (accessed on 30 January 2024).
- Bilezikian, J.P.; Bandeira, L.; Khan, A.; Cusano, N.E. Hyperparathyroidism. Lancet 2018, 391, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B.L.; Brown, E.M.; Collins, M.T.; Jüppner, H.; Lakatos, P.; Levine, M.A.; Mannstadt, M.M.; Bilezikian, J.P.; Romanischen, A.F.; Thakker, R.V. Epidemiology and Diagnosis of Hypoparathyroidism. J. Clin. Endocrinol. Metab. 2016, 101, 2284–2299. [Google Scholar] [CrossRef] [PubMed]
- Fillée, C.; Keller, T.; Mourad, M.; Brinkmann, T.; Ketelslegers, J.M. Impact of vitamin D-related serum PTH reference values on the diagnosis of mild primary hyperparathyroidism, using bivariate calcium/PTH reference regions. Clin. Endocrinol. 2012, 76, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.S.; Biondi, B. Subclinical thyroid disease. Lancet 2012, 379, 1142–1154. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Bakris, G.L.; Borghi, C.; Chonchol, M.B.; Feldman, D.; Lanaspa, M.A.; Merriman, T.R.; Moe, O.W.; Mount, D.B.; Sanchez Lozada, L.G.; et al. Hyperuricemia, Acute and Chronic Kidney Disease, Hypertension, and Cardiovascular Disease: Report of a Scientific Workshop Organized by the National Kidney Foundation. Am. J. Kidney Dis. 2018, 71, 851–865. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, C.; Arcella, D.; Piccinelli, R.; Sette, S.; Le Donne, C.; Turrini, A. INRAN-SCAI 2005-06 Study Group. The Italian National Food Consumption Survey INRAN-SCAI 2005-06: Main results in terms of food consumption. Public Health Nutr. 2009, 12, 2504–2532. [Google Scholar] [CrossRef] [PubMed]
- Hamm, L.L.; Hering-Smith, K.S. Pathophysiology of hypocitraturic nephrolithiasis. Endocrinol. Metab. Clin. N. Am. 2002, 31, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Abate, V.; Vergatti, A.; Fiore, A.; Forte, A.; Attanasio, A.; Altavilla, N.; De Filippo, G.; Rendina, D.; D’Elia, L. Low Potassium Intake: A Common Risk Factor for Nephrolithiasis in Patients with High Blood Pressure. High. Blood Press. Cardiovasc. Prev. 2023, 30, 343–350. [Google Scholar] [CrossRef]
- Barghouthy, Y.; Corrales, M.; Somani, B. The Relationship between Modern Fad Diets and Kidney Stone Disease: A Systematic Review of Literature. Nutrients 2021, 13, 4270. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, J.W.; Qu, P.F.; Wang, K.K.; Yan, Y.; Chu, C.; Zheng, W.L.; Xu, X.J.; Lv, Y.B.; Ma, Q.; et al. Association between urinary sodium excretion and uric acid, and its interaction on the risk of prehypertension among Chinese young adults. Sci. Rep. 2018, 8, 7749. [Google Scholar] [CrossRef]
- Wang, Y.; Chu, C.; Wang, K.K.; Hu, J.W.; Yan, Y.; Lv, Y.B.; Cao, Y.M.; Zheng, W.L.; Dang, X.L.; Xu, J.T.; et al. Effect of Salt Intake on Plasma and Urinary Uric Acid Levels in Chinese Adults: An Interventional Trial. Sci. Rep. 2018, 8, 1434. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Joshi, N.K.; Jain, Y.K.; Bajpai, N.; Bhardwaj, P.; Chaturvedi, M.; Patil, M.S.; Gaidhane, A.; Quazi Syed, Z.; Saxena, D. Dietary Determinants of Renal Calculi: A Case-Control Study from a Tertiary Care Hospital of Western Rajasthan. Cureus 2022, 14, e31460. [Google Scholar] [CrossRef]
- Donfrancesco, C.; Ippolito, R.; Lo Noce, C.; Palmieri, L.; Iacone, R.; Russo, O.; Vannuzzo, D.; Galletti, F.; Galeone, D.; Giampaoli, S.; et al. Excess dietary sodium and inadequate potassium intake in Italy: Results of the MINISAL study. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 850–856. [Google Scholar] [CrossRef]
- Malavolti, M.; Naska, A.; Fairweather-Tait, S.J.; Malagoli, C.; Vescovi, L.; Marchesi, C.; Vinceti, M.; Filippini, T. Sodium and Potassium Content of Foods Consumed in an Italian Population and the Impact of Adherence to a Mediterranean Diet on Their Intake. Nutrients 2021, 13, 2681. [Google Scholar] [CrossRef]
- Rendina, D.; De Filippo, G.; De Pascale, F.; Zampa, G.; Muscariello, R.; De Palma, D.; Ippolito, R.; Strazzullo, P. The changing profile of patients with calcium nephrolithiasis and the ascendancy of overweight and obesity: A comparison of two patient series observed 25 years apart. Nephrol. Dial. Transplant. 2013, 28 (Suppl. S4), iv146–iv151. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Talati, V.M.; Soares, R.M.O.; Khambati, A.; Nadler, R.B.; Perry, K.T., Jr. Trends in urinary calculi composition from 2005 to 2015: A single tertiary center study. Urolithiasis 2020, 48, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Lieske, J.C.; Peña de la Vega, L.S.; Slezak, J.M.; Bergstralh, E.J.; Leibson, C.L.; Ho, K.L.; Gettman, M.T. Renal stone epidemiology in Rochester, Minnesota: An update. Kidney Int. 2006, 69, 760–764. [Google Scholar] [CrossRef]
- Bentley-Lewis, R.; Koruda, K.; Seely, E.W. The metabolic syndrome in women. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 696–704. [Google Scholar] [CrossRef]
- Beigh, S.H.; Jain, S. Prevalence of metabolic syndrome and gender differences. Bioinformation 2012, 8, 613–616. [Google Scholar] [CrossRef]
- Rahman, I.A.; Nusaly, I.F.; Syahrir, S.; Nusaly, H.; Mansyur, M.A. Association between metabolic syndrome components and the risk of developing nephrolithiasis: A systematic review and Bayesian metanalysis. F100Research 2021, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota-Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef] [PubMed]
- Rendina, D.; De Filippo, G.; Iannuzzo, G.; Abate, V.; Strazzullo, P.; Falchetti, A. Idiopathic Osteoporosis and Nephrolithiasis: Two Sides of the Same Coin? Int. J. Mol. Sci. 2020, 21, 8183. [Google Scholar] [CrossRef] [PubMed]
- Rendina, D.; D’Elia, L.; Evangelista, M.; De Filippo, G.; Giaquinto, A.; Abate, V.; Barone, B.; Piccinocchi, G.; Prezioso, D.; Strazzullo, P. Metabolic syndrome is associated to an increased risk of low bone mineral density in free-living women with suspected osteoporosis. J. Endocrinol. Investig. 2021, 44, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Rendina, D.; D’Elia, L.; De Filippo, G.; Abate, V.; Evangelista, M.; Giaquinto, A.; Barone, B.; Piccinocchi, G.; Prezioso, D.; Strazzullo, P. Metabolic syndrome is not associated to an increased risk of low bone mineral density in men at risk for osteoporosis. J. Endocrinol. Investig. 2022, 45, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Kopp, W. How Western Diet And Lifestyle Drive The Pandemic Of Obesity And Civilization Diseases. Diabetes Metab. Syndr. Obes. 2019, 12, 2221–2236. [Google Scholar] [CrossRef]
- D’Elia, L.; Strazzullo, P. Dietary Salt Restriction and Adherence to the Mediterranean Diet: A Single Way to Reduce Cardiovascular Risk? J. Clin. Med. 2024, 13, 486. [Google Scholar] [CrossRef]
- Prezioso, D.; Piccinocchi, G.; Abate, V.; Ancona, M.; Celia, A.; De Luca, C.; Ferrari, R.; Ferraro, P.M.; Mancon, S.; Mazzon, G.; et al. The role of the general practictioner in the management of urinary calculi. Arch. Ital. Urol. Androl. 2023, 95, 12155. [Google Scholar] [CrossRef]
| SF | NSF | |
|---|---|---|
| Number (n) | 255 | 535 |
| Male/Female (n; %) | 117; 45.9:138; 54.1 | 245; 45.8:290; 54.2 |
| Age (years) | 53.1 ± 10.4 | 55.1 ± 12.7 |
| BMI (kg/m2) | 27.7 ± 4.97 | 26.7 ± 4.98 |
| Schooling (n; %) | 167; 65.5 | 222; 41.7 a |
| Stone-Forming Patients (n = 255) | Non-Stone-Forming Patients (n = 535) | |||||
|---|---|---|---|---|---|---|
| Food Consumption | High | Medium | Low | High | Medium | Low |
| Fruit | 49; 19.3 | 95; 37.2 | 111; 43.5 | 133; 24.9 a | 252; 47.1 a | 150; 28.0 a |
| Vegetables | 20; 70.8 | 144; 56.6 | 91; 35.6 | 82; 15.3 a | 324; 60.6 a | 129; 24.1 a |
| Cereal | 34; 13.3 | 156; 61.2 | 65; 25.5 | 27; 13.5 a | 116; 58.0 a | 57; 28.5 a |
| Legumes | 53; 20.8 | 166; 65.1 | 36; 14.1 | 251; 46.9 a | 225; 42.1 a | 59; 28.5 a |
| Fish | 29; 11.4 | 140; 54.9 | 86; 33.7 | 102; 19.1 a | 327; 61.1 a | 105; 19.6 a |
| Meat | 21; 8.3 | 110; 43.1 | 124; 48.6 | 37; 6.9 a | 137; 25.6 a | 361; 67.5 a |
| Dairy Products | 30; 11.8 | 107; 42.0 | 118; 46.2 | 23; 4.3 a | 123; 23.0 a | 389; 72.7 a |
| Alcohol | 8; 3.1 | 229; 89.8 | 18; 7.1 | 1; 0.2 a | 459; 85.8 a | 75; 14.0 a |
| Olive Oil | 200; 78.4 | 40; 15.7 | 15; 5.9 | 503; 90.0 a | 3; 5.6 a | 2; 0.4 a |
| SF (n = 255) | NSF (n = 535) | |||||
|---|---|---|---|---|---|---|
| Salt Consumption | High | Medium | Low | High | Medium | Low |
| (1) How often do you use salt at table? | 54; 21.2 | 49; 19.2 | 152; 59.6 | 54; 10.1 a | 133; 24.9 a | 348; 65.0 a |
| (2) How much bread do you eat in one day? | 82; 32.2 | 172; 67.4 | 1; 0.4 | 81; 15.1 a | 413; 77.2 a | 41; 7.7 a |
| (3) How many times a week do you eat cheese and/or cold cuts? | 15; 5.9 | 139; 54.5 | 101; 39.6 | 35; 6.5 a | 207; 38.7 a | 293; 54.8 a |
| (4) Do you ever get thirsty especially after a meal? | 26; 10.2 | 63; 24.7 | 166; 65.1 | 32; 6.0 a | 201; 37.6 a | 302; 56.4 a |
| (5) When you eat out of home, does food seem usually salty, normal, or bland? | 37; 14.7 | 173; 67.8 | 45; 17.6 | 136; 25.4 a | 362; 67.7 a | 37; 6.9 a |
| Group | MEDI-LITE | MINISAL | SF (n = 255) | NSF (n = 535) | p |
|---|---|---|---|---|---|
| A | >11 | <9 | 43; 16.9 | 211; 39.4 | <0.05 |
| B | >11 | >9 | 45; 17.6 | 179; 33.5 | |
| C | <11 | <9 | 61; 23.9 | 61; 11.4 | |
| D | <11 | >9 | 106; 41.6 | 84; 15.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abate, V.; Vergatti, A.; Iaccarino Idelson, P.; Recano, C.; Brancaccio, M.; Prezioso, D.; Muscariello, R.; Nuzzo, V.; De Filippo, G.; Strazzullo, P.; et al. Adherence to Mediterranean Diet, Dietary Salt Intake, and Susceptibility to Nephrolithiasis: A Case–Control Study. Nutrients 2024, 16, 783. https://doi.org/10.3390/nu16060783
Abate V, Vergatti A, Iaccarino Idelson P, Recano C, Brancaccio M, Prezioso D, Muscariello R, Nuzzo V, De Filippo G, Strazzullo P, et al. Adherence to Mediterranean Diet, Dietary Salt Intake, and Susceptibility to Nephrolithiasis: A Case–Control Study. Nutrients. 2024; 16(6):783. https://doi.org/10.3390/nu16060783
Chicago/Turabian StyleAbate, Veronica, Anita Vergatti, Paola Iaccarino Idelson, Costantino Recano, Marzia Brancaccio, Domenico Prezioso, Riccardo Muscariello, Vincenzo Nuzzo, Gianpaolo De Filippo, Pasquale Strazzullo, and et al. 2024. "Adherence to Mediterranean Diet, Dietary Salt Intake, and Susceptibility to Nephrolithiasis: A Case–Control Study" Nutrients 16, no. 6: 783. https://doi.org/10.3390/nu16060783
APA StyleAbate, V., Vergatti, A., Iaccarino Idelson, P., Recano, C., Brancaccio, M., Prezioso, D., Muscariello, R., Nuzzo, V., De Filippo, G., Strazzullo, P., Faraonio, R., Galletti, F., Rendina, D., & D’Elia, L. (2024). Adherence to Mediterranean Diet, Dietary Salt Intake, and Susceptibility to Nephrolithiasis: A Case–Control Study. Nutrients, 16(6), 783. https://doi.org/10.3390/nu16060783






