Geriatric Nutritional Risk Index and First-Year Mortality in Incident Hemodialysis Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Demographic and Clinical Data
2.3. GNRI Calculation and Outcomes
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Patients
3.2. GNRI Distribution
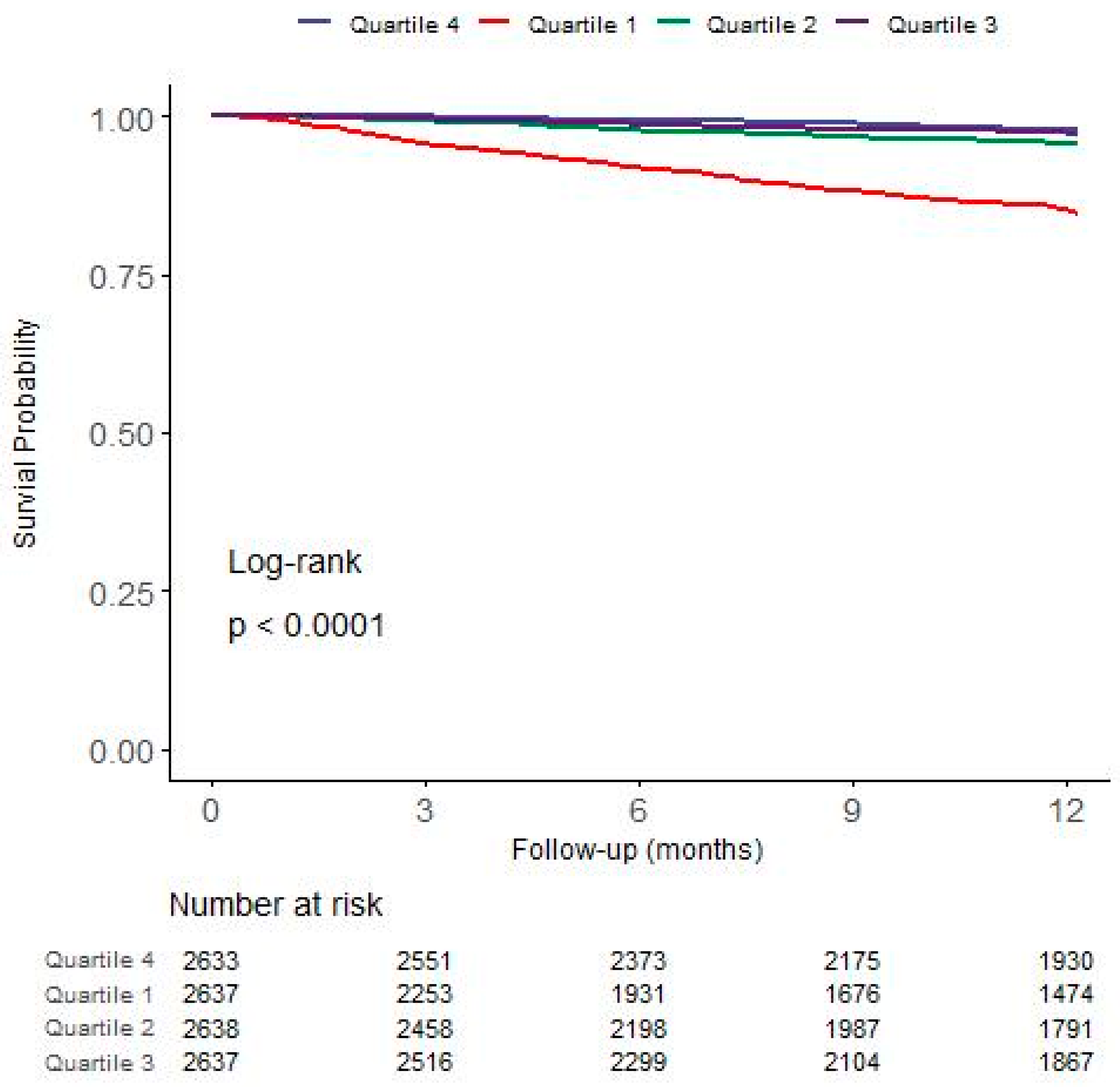
3.3. Association between GNRI and First-Year Mortality
3.4. Comparison of Predictive Values for Mortality
3.5. Subgroup Analyses for Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lukowsky, L.R.; Kheifets, L.; Arah, O.A.; Nissenson, A.R.; Kalantar-Zadeh, K. Patterns and predictors of early mortality in incident hemodialysis patients: New insights. Am. J. Nephrol. 2012, 35, 548–558. [Google Scholar] [CrossRef]
- Bradbury, B.D.; Fissell, R.B.; Albert, J.M.; Anthony, M.S.; Critchlow, C.W.; Pisoni, R.L.; Port, F.K.; Gillespie, B.W. Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clin. J. Am. Soc. Nephrol. 2007, 2, 89–99. [Google Scholar] [CrossRef]
- Heaf, J.; Heiro, M.; Petersons, A.; Vernere, B.; Povlsen, J.V.; Sørensen, A.B.; Clyne, N.; Bumblyte, I.; Zilinskiene, A.; Randers, E.; et al. First-year mortality in incident dialysis patients: Results of the Peridialysis study. BMC Nephrol. 2022, 23, 229. [Google Scholar] [CrossRef]
- Lassalle, M.; Labeeuw, M.; Frimat, L.; Villar, E.; Joyeux, V.; Couchoud, C.; Stengel, B. Age and comorbidity may explain the paradoxical association of an early dialysis start with poor survival. Kidney Int. 2010, 77, 700–707. [Google Scholar] [CrossRef]
- Molnar, M.Z.; Streja, E.; Kovesdy, C.P.; Hoshino, J.; Hatamizadeh, P.; Glassock, R.J.; Ojo, A.O.; Kalantar-Zadeh, K. Estimated glomerular filtration rate at reinitiation of dialysis and mortality in failed kidney transplant recipients. Nephrol. Dial. Transplant. 2012, 27, 2913–2921. [Google Scholar] [CrossRef]
- Cooper, B.A.; Branley, P.; Bulfone, L.; Collins, J.F.; Craig, J.C.; Fraenkel, M.B.; Harris, A.; Johnson, D.W.; Kesselhut, J.; Li, J.J.; et al. A randomized, controlled trial of early versus late initiation of dialysis. N. Engl. J. Med. 2010, 363, 609–619. [Google Scholar] [CrossRef]
- Rambod, M.; Bross, R.; Zitterkoph, J.; Benner, D.; Pithia, J.; Colman, S.; Kovesdy, C.P.; Kopple, J.D.; Kalantar-Zadeh, K. Association of Malnutrition-Inflammation Score with quality of life and mortality in hemodialysis patients: A 5-year prospective cohort study. Am. J. Kidney Dis. 2009, 53, 298–309. [Google Scholar] [CrossRef]
- De Roij Van Zuijdewijn, C.L.; ter Wee, P.M.; Chapdelaine, I.; Bots, M.L.; Blankestijn, P.J.; van den Dorpel, M.A.; Nubé, M.J.; Grooteman, M.P. A Comparison of 8 Nutrition-Related Tests to Predict Mortality in Hemodialysis Patients. J. Ren. Nutr. 2015, 25, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.-P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, I.; Ishimura, E.; Kato, Y.; Okuno, S.; Yamamoto, T.; Yamakawa, T.; Mori, K.; Inaba, M.; Nishizawa, Y. Geriatric Nutritional Risk Index, a simplified nutritional screening index, is a significant predictor of mortality in chronic dialysis patients. Nephrol. Dial. Transplant. 2010, 25, 3361–3365. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Furuya, R.; Takita, T.; Maruyama, Y.; Yamaguchi, Y.; Ohkawa, S.; Kumagai, H. Simplified nutritional screening tools for patients on maintenance hemodialysis. Am. J. Clin. Nutr. 2008, 87, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Ito, Y.; Ishii, H.; Aoyama, T.; Kamoi, D.; Kasuga, H.; Yasuda, K.; Maruyama, S.; Matsuo, S.; Murohara, T.; et al. Geriatric nutritional risk index accurately predicts cardiovascular mortality in incident hemodialysis patients. J. Cardiol. 2014, 64, 32–36. [Google Scholar] [CrossRef]
- Cho, A.; Park, S.Y.; Cha, Y.S.; Park, H.C.; Kim, D.H.; Lee, Y.K. The change in Geriatric Nutritional Risk Index is associated with mortality in patients who start hemodialysis: Korean Renal Data Registry, 2016–2018. Sci. Rep. 2022, 12, 20352. [Google Scholar] [CrossRef]
- Yajima, T.; Yajima, K.; Takahashi, H. Impact of Annual Change in Geriatric Nutritional Risk Index on Mortality in Patients Undergoing Hemodialysis. Nutrients 2020, 12, 3333. [Google Scholar] [CrossRef]
- Hong, Y.A.; Ban, T.H.; Kang, C.-Y.; Hwang, S.D.; Choi, S.R.; Lee, H.; Jung, H.-Y.; Kim, K.; Kwon, Y.E.; Kim, S.H.; et al. Trends in epidemiologic characteristics of end-stage renal disease from 2019 Korean Renal Data System (KORDS). Kidney Res. Clin. Pract. 2021, 40, 52–61. [Google Scholar] [CrossRef]
- Jin, D.C.; Yun, S.R.; Lee, S.W.; Han, S.W.; Kim, W.; Park, J.; Kim, Y.K. Lessons from 30 years’ data of Korean end-stage renal disease registry, 1985–2015. Kidney Res. Clin. Pract. 2015, 34, 132–139. [Google Scholar] [CrossRef]
- Pablo, A.M.; Izaga, M.A.; Alday, L.A. Assessment of nutritional status on hospital admission: Nutritional scores. Eur. J. Clin. Nutr. 2003, 57, 824–831. [Google Scholar] [CrossRef]
- Shah, B.; Sucher, K.; Hollenbeck, C.B. Comparison of ideal body weight equations and published height-weight tables with body mass index tables for healthy adults in the United States. Nutr. Clin. Pract. 2006, 21, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Jerin, L.; Ladavac, R.; Kuzmanovic, G.; Dodic, D.; Griparic, D. Subjective general assessment of nutritional status in patients with chronic renal failure and regular hemodialysis. Acta Medica Croat. 2003, 57, 23–28. [Google Scholar]
- Cooper, B.A.; Bartlett, L.H.; Aslani, A.; Allen, B.J.; Ibels, L.S.; Pollock, C.A. Validity of subjective global assessment as a nutritional marker in end-stage renal disease. Am. J. Kidney Dis. 2002, 40, 126–132. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Kopple, J.D.; Block, G.; Humphreys, M.H. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am. J. Kidney Dis. 2001, 38, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Kopple, J.D.; Humphreys, M.H.; Block, G. Comparing outcome predictability of markers of malnutrition-inflammation complex syndrome in haemodialysis patients. Nephrol. Dial. Transplant. 2004, 19, 1507–1519. [Google Scholar] [CrossRef] [PubMed]
- Beberashvili, I.; Azar, A.; Sinuani, I.; Kadoshi, H.; Shapiro, G.; Feldman, L.; Averbukh, Z.; Weissgarten, J. Comparison analysis of nutritional scores for serial monitoring of nutritional status in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2013, 8, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Inoue, K.; Shimizu, K.; Hiraga, K.; Takahashi, E.; Otaki, K.; Yoshikawa, T.; Furuta, K.; Tokunaga, C.; Sakakibara, T.; et al. Comparison of Nutritional Risk Scores for Predicting Mortality in Japanese Chronic Hemodialysis Patients. J. Ren. Nutr. 2017, 27, 201–206. [Google Scholar] [CrossRef]
- Hakim, R.M.; Levin, N. Malnutrition in hemodialysis patients. Am. J. Kidney Dis. 1993, 21, 125–137. [Google Scholar] [CrossRef]
- Bergstrom, J. Nutrition and mortality in hemodialysis. J. Am. Soc. Nephrol. 1995, 6, 1329–1341. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Cano, N.J.; Franch, H.; Fouque, D.; Himmelfarb, J.; Kalantar-Zadeh, K.; Kuhlmann, M.K.; Stenvinkel, P.; TerWee, P.; Teta, D.; et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: A consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013, 84, 1096–1107. [Google Scholar] [CrossRef]
- Gracia-Iguacel, C.; González-Parra, E.; Pérez-Gómez, M.V.; Mahíllo, I.; Egido, J.; Ortiz, A.; Carrero, J.J. Prevalence of protein-energy wasting syndrome and its association with mortality in haemodialysis patients in a centre in Spain. Nefrologia 2013, 33, 495–505. [Google Scholar]
- Sarav, M.; Kovesdy, C.P. Protein Energy Wasting in Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2018, 13, 1558–1560. [Google Scholar] [CrossRef]
- Foley, R.N.; Chen, S.C.; Solid, C.A.; Gilbertson, D.T.; Collins, A.J. Early mortality in patients starting dialysis appears to go unregistered. Kidney Int. 2014, 86, 392–398. [Google Scholar] [CrossRef]
- Robinson, B.M.; Zhang, J.; Morgenstern, H.; Bradbury, B.D.; Ng, L.J.; McCullough, K.P.; Gillespie, B.W.; Hakim, R.; Rayner, H.; Fort, J.; et al. Worldwide, mortality risk is high soon after initiation of hemodialysis. Kidney Int. 2014, 85, 158–165. [Google Scholar] [CrossRef]
- Chan, K.E.; Maddux, F.W.; Tolkoff-Rubin, N.; Karumanchi, S.A.; Thadhani, R.; Hakim, R.M. Early outcomes among those initiating chronic dialysis in the United States. Clin. J. Am. Soc. Nephrol. 2011, 6, 2642–2649. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Kovesdy, C.P.; Streja, E.; Rhee, C.M.; Soohoo, M.; Chen, J.L.; Molnar, M.Z.; Obi, Y.; Gillen, D.; Nguyen, D.V.; et al. Transition of care from pre-dialysis prelude to renal replacement therapy: The blueprints of emerging research in advanced chronic kidney disease. Nephrol. Dial. Transplant. 2017, 32 (Suppl. S2), ii91–ii98. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, S. Risk factors for mortality in patients undergoing hemodialysis: A systematic review and meta-analysis. Int. J. Cardiol. 2017, 238, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Maduell, F.; Moreso, F.; Mora-Macià, J.; Pons, M.; Ramos, R.; Carreras, J.; Soler, J.; Torres, F. ESHOL study reanalysis: All-cause mortality considered by competing risks and time-dependent covariates for renal transplantation. Nefrologia 2016, 36, 156–163. [Google Scholar] [CrossRef]
- Lukowsky, L.R.; Kheifets, L.; Arah, O.A.; Nissenson, A.R.; Kalantar-Zadeh, K. Nutritional predictors of early mortality in incident hemodialysis patients. Int. Urol. Nephrol. 2014, 46, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P.; Kalantar-Zadeh, K. Why is protein-energy wasting associated with mortality in chronic kidney disease? Semin. Nephrol. 2009, 29, 3–14. [Google Scholar] [CrossRef] [PubMed]
- de Mutsert, R.; Grootendorst, D.C.; Indemans, F.; Boeschoten, E.W.; Krediet, R.T.; Dekker, F.W. Association between serum albumin and mortality in dialysis patients is partly explained by inflammation, and not by malnutrition. J. Ren. Nutr. 2009, 19, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Don, B.R.; Kaysen, G. Serum albumin: Relationship to inflammation and nutrition. Semin. Dial. 2004, 17, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Jialin, W.; Yi, Z.; Weijie, Y. Relationship between body mass index and mortality in hemodialysis patients: A meta-analysis. Nephron Clin. Pract. 2012, 121, c102–c111. [Google Scholar] [CrossRef]

| Total (n = 10,545) | Quartile 1 56.7–91.7 (n = 2637) | Quartile 2 91.8–97.1 (n = 2638) | Quartile 3 97.2–101.2 (n = 2637) | Quartile 4 101.3–129.6 (n = 2633) | p-Value | |
|---|---|---|---|---|---|---|
| Age at first HD, year | 63.9 ± 13.7 | 68.0 ± 13.4 | 65.1 ± 13.3 | 63.1 ± 13.1 | 59.5 ± 13.4 | <0.001 |
| Women | 3919 (37.2) | 1077 (40.8) | 1081 (41.0) | 974 (36.9) | 787 (29.9) | <0.001 |
| Main cause of ESRD | <0.001 | |||||
| Diabetes | 5587 (53.0) | 1317 (49.9) | 1418 (53.8) | 1461 (55.4) | 1391 (52.8) | |
| Hypertension | 2143 (20.3) | 514 (19.5) | 552 (21.0) | 525 (19.9) | 550 (20.9) | |
| Glomerulonephritis | 735 (7.0) | 177 (6.7) | 175 (6.8) | 181 (6.9) | 198 (7.5) | |
| Others | 2080 (19.7) | 629 (23.9) | 480 (18.5) | 470 (17.8) | 494 (18.8) | |
| SBP (mmHg) | 143.2 ± 19.5 | 141.5 ± 20.6 | 143.2 ± 20.0 | 144.0 ± 19.1 | 144.0 ± 18.4 | <0.001 |
| DBP (mmHg) | 76.0 ± 12.4 | 74.6 ± 12.9 | 75.8 ± 12.5 | 75.9 ± 11.9 | 77.7 ± 12.1 | <0.001 |
| Body mass index (kg/m2) | 22.8 ± 3.7 | 20.6 ± 3.4 | 22.8 ± 3.6 | 23.8 ± 3.4 | 24.2 ± 3.4 | <0.001 |
| Kt/V | 1.48 ± 0.28 | 1.49 ± 0.30 | 1.50 ± 0.29 | 1.47 ± 0.28 | 1.44 ± 0.26 | <0.001 |
| Comorbid conditions | ||||||
| Coronary artery disease | 1012 (9.6) | 254 (9.6) | 265 (10.0) | 247 (9.4) | 246 (9.3) | 0.808 |
| Heart failure | 513 (4.9) | 185 (7.0) | 113 (4.3) | 118 (4.5) | 97 (3.7) | <0.001 |
| Arrhythmia | 302 (2.9) | 92 (3.5) | 78 (3.0) | 77 (2.9) | 55 (2.1) | 0.023 |
| Cerebrovascular disease | 848 (8.0) | 2789 (10.5) | 206 (7.8) | 189 (7.2) | 175 (6.6) | <0.001 |
| COPD | 74 (0.7) | 28 (1.1) | 16 (0.6) | 18 (0.7) | 12 (0.5) | 0.056 |
| Liver disease | 415 (3.9) | 146 (5.5) | 108 (4.1) | 78 (3.0) | 83 (3.2) | <0.001 |
| GI disease | 1116 (10.6) | 309 (11.7) | 277 (10.5) | 270 (10.2) | 260 (9.9) | 0.149 |
| Vascular access | <0.001 | |||||
| Fistula | 7017 (66.5) | 1250 (47.4) | 1727 (65.5) | 1962 (74.4) | 2078 (78.9) | |
| Graft | 1665 (15.8) | 445 (16.9) | 451 (17.1) | 404 (15.3) | 365 (13.9) | |
| Catheter | 1863 (17.7) | 942 (35.7) | 460 (17.4) | 271 (10.3) | 190 (7.2) | |
| Hemoglobin, g/dL | 10.4 ± 1.2 | 9.9 ± 1.4 | 10.4 ± 1.2 | 10.5 ± 1.0 | 10.7± 1.0 | <0.001 |
| Albumin, g/dL | 3.8 ± 0.5 | 3.2 ± 0.4 | 3.7 ± 0.2 | 4.0 ± 0.1 | 4.3 ± 0.2 | <0.001 |
| Calcium, mg/dL | 8.5 ± 0.8 | 8.3 ± 0.9 | 8.5 ± 0. | 8.6 ± 0.7 | 8.8 ± 0.8 | <0.001 |
| Phosphorus, mg/dL | 4.7 ± 1.4 | 4.2 ± 1.4 | 4.7 ± 1.4 | 4.8 ± 1.4 | 5.2 ± 1.4 | <0.001 |
| Intact PTH, pg/mL | 197.0 ± 176.3 | 179.4 ± 167.6 | 204.3 ± 199.5 | 200.0 ± 172.6 | 204.4 ± 162.2 | <0.001 |
| Cholesterol, mg/dL | 139.7 ± 38.4 | 139.6 ± 41.2 | 139.9 ± 37.5 | 140.0 ± 37.2 | 139.4 ± 37.7 | 0.956 |
| Univariate | p-Value | Multivariate | p-Value | |
|---|---|---|---|---|
| All-cause | ||||
| GNRI quartile | ||||
| Quartile 1 | 7.74 (5.76–10.36) | <0.001 | 2.56 (2.13–3.09) | <0.001 |
| Quartile 2 | 2.08 (1.48–2.91) | <0.001 | 1.33 (1.06–1.68) | 0.016 |
| Quartile 3 | 1.28 (0.89–1.86) | 0.18 | 1.00 (0.76–1.32) | 0.997 |
| Quartile 4 | Reference | Reference | ||
| Cardiovascular | ||||
| GNRI quartile | ||||
| Quartile 1 | 6.22 (4.07–9.50) | <0.001 | 2.29 (1.71–3.08) | <0.001 |
| Quartile 2 | 1.51 (0.91–2.50) | 0.11 | 1.05 (0.72–1.53) | 0.804 |
| Quartile 3 | 0.92 (0.52–1.61) | 0.77 | 0.75 (0.48–1.17) | 0.205 |
| Quartile 4 | Reference | Reference |
| Models | AUC (95% Confidence Intervals) | p-Value |
|---|---|---|
| All-cause | ||
| Baseline | 70.8 (68.4–73.2) | |
| +BMI | 72.2 (69.8–74.5) | 0.002 |
| +Albumin | 76.2 (73.9–78.4) | <0.001 |
| +GNRI | 77.1 (74.8–79.2) | <0.001 |
| BMI vs. GNRI | <0.001 | |
| Albumin vs. GNRI | 0.009 | |
| Cardiovascular | ||
| Baseline | 70.1 (66.4–73.8) | |
| +BMI | 70.6 (66.9–74.4) | 0.518 |
| +Albumin | 75.1 (71.4–78.7) | <0.001 |
| +GNRI | 75.7 (72.2–79.0) | <0.001 |
| BMI vs. GNRI | <0.001 | |
| Albumin vs. GNRI | 0.132 |
| Hazard Ratio (95% Confidence Interval) * | ||
|---|---|---|
| Quartile 1 (vs. Quartile 4) | p-Value for Interaction | |
| Age | ||
| <65 years | 1.91 (1.29–2.84) | 0.409 |
| ≥65 years | 2.50 (1.99– 3.14) | |
| Sex | ||
| Woman | 1.67 (1.17–2.40) | 0.659 |
| Man | 3.03 (2.39–3.84) | |
| Diabetes | ||
| Yes | 2.00 (1.54–2.59) | 0.076 |
| No | 3.48 (2.56–4.72) | |
| History of CVD | ||
| Yes | 3.59 (2.42–5.32) | 0.185 |
| No | 2.20 (1.74–2.77) | |
| Vascular access | ||
| AVF | 2.59 (1.93–3.48) | 0.711 |
| AVG or catheter | 2.41 (1.81–3.23) | |
| Hemoglobin | ||
| <10 mg/dL | 2.23 (1.62–3.09) | 0.722 |
| ≥10 mg/dL | 2.60 (2.00–3.37) | |
| Phosphorus | ||
| <5 mg/dL | 3.03 (2.39–3.83) | 0.342 |
| ≥5 mg/dL | 1.79 (1.18–2.72) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, G.H.; Choi, H.B.; Park, H.C.; Kim, D.H.; Lee, Y.-K.; Cho, A. Geriatric Nutritional Risk Index and First-Year Mortality in Incident Hemodialysis Patients. Nutrients 2024, 16, 652. https://doi.org/10.3390/nu16050652
Song GH, Choi HB, Park HC, Kim DH, Lee Y-K, Cho A. Geriatric Nutritional Risk Index and First-Year Mortality in Incident Hemodialysis Patients. Nutrients. 2024; 16(5):652. https://doi.org/10.3390/nu16050652
Chicago/Turabian StyleSong, Gi Hyun, Han Byul Choi, Hayne Cho Park, Do Hyoung Kim, Young-Ki Lee, and AJin Cho. 2024. "Geriatric Nutritional Risk Index and First-Year Mortality in Incident Hemodialysis Patients" Nutrients 16, no. 5: 652. https://doi.org/10.3390/nu16050652
APA StyleSong, G. H., Choi, H. B., Park, H. C., Kim, D. H., Lee, Y.-K., & Cho, A. (2024). Geriatric Nutritional Risk Index and First-Year Mortality in Incident Hemodialysis Patients. Nutrients, 16(5), 652. https://doi.org/10.3390/nu16050652






