Effect of Dietary Supplementation with Omega-3 Fatty Acid on the Generation of Regulatory T Lymphocytes and on Antioxidant Parameters and Markers of Oxidative Stress in the Liver Tissue of IL−10 Knockout Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diet
2.2. Culture of Mesenteric Lymph Nodes and Determination of IL-6
2.3. Determination of Liver Protein
2.4. Determination of Hepatic Reduced Glutathione (GSH)
2.5. Determination of Hepatic Vitamin E
2.6. Determination of Hepatic Glutathione Peroxidase (GPx)
2.7. Determination of Hepatic Malondialdehyde (MDA)
2.8. Determination of the Weight of Muscle and Liver Tissues
2.9. Histopathological Analysis
2.10. Immunophenotyping of Mesenteric Lymph Node Cells
2.11. Statistical Analysis
3. Results
3.1. Effects of PUFAs on Body Weight Gain and Energy Intake
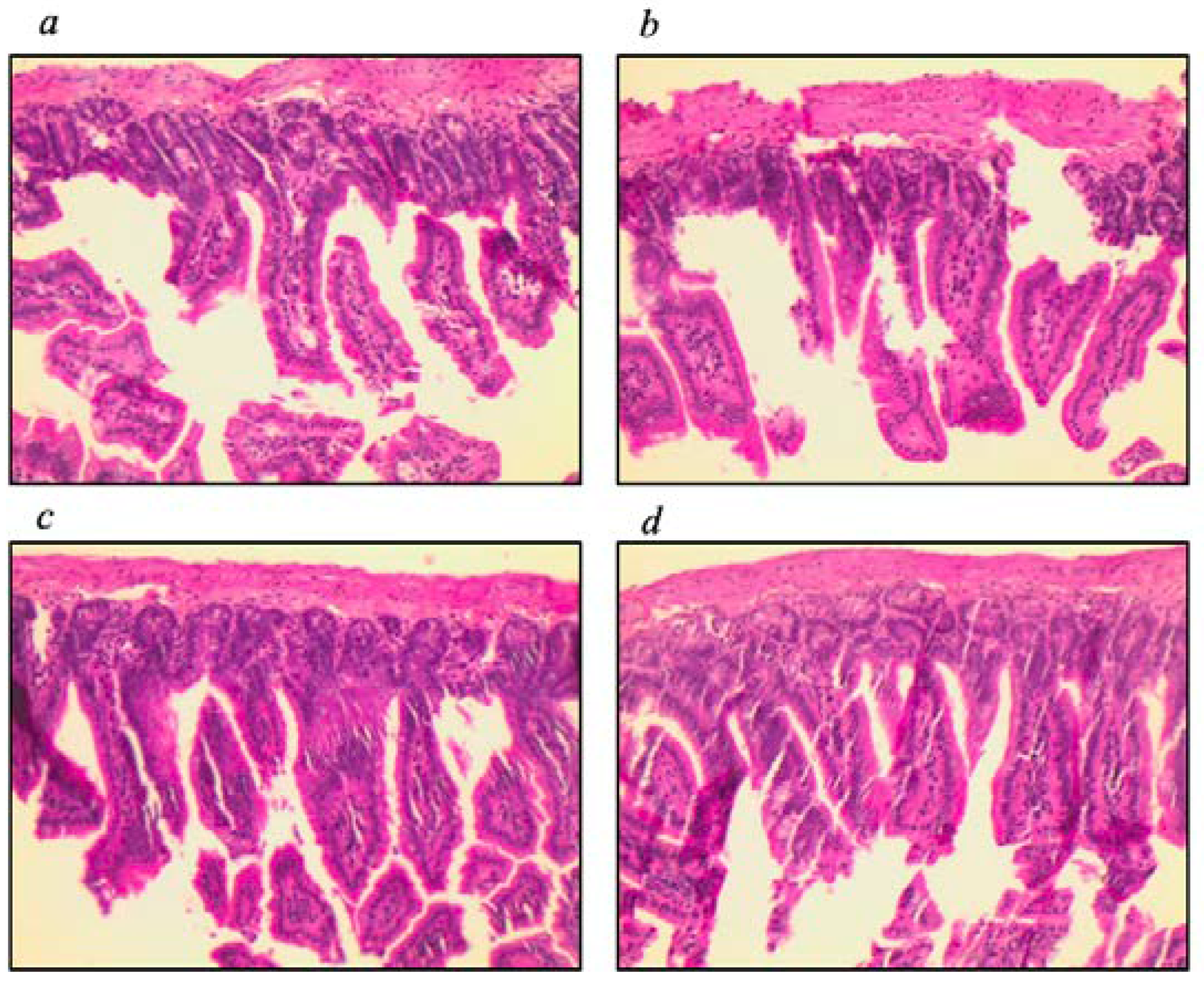
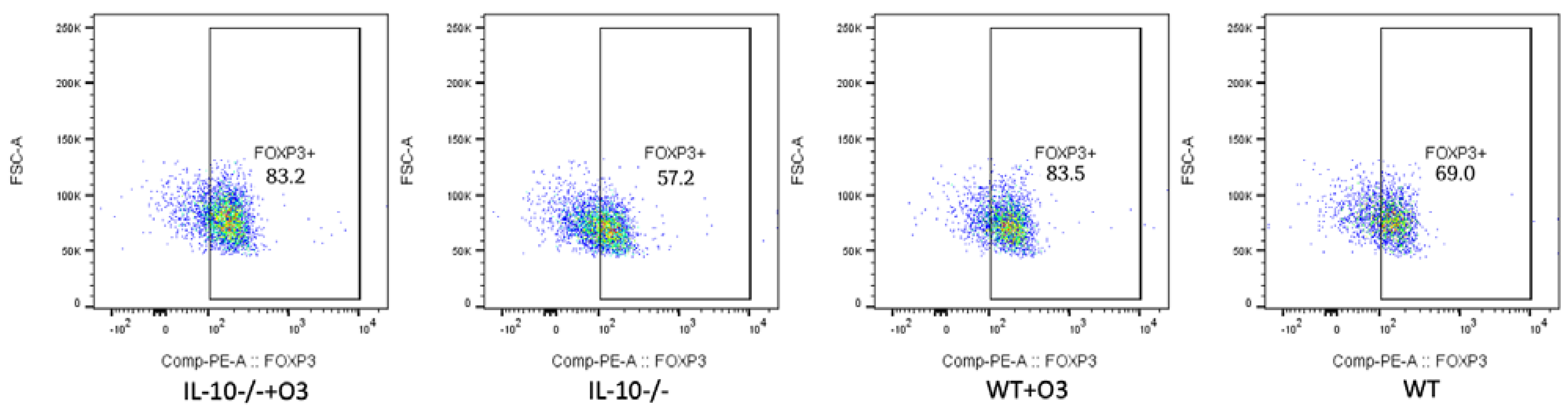
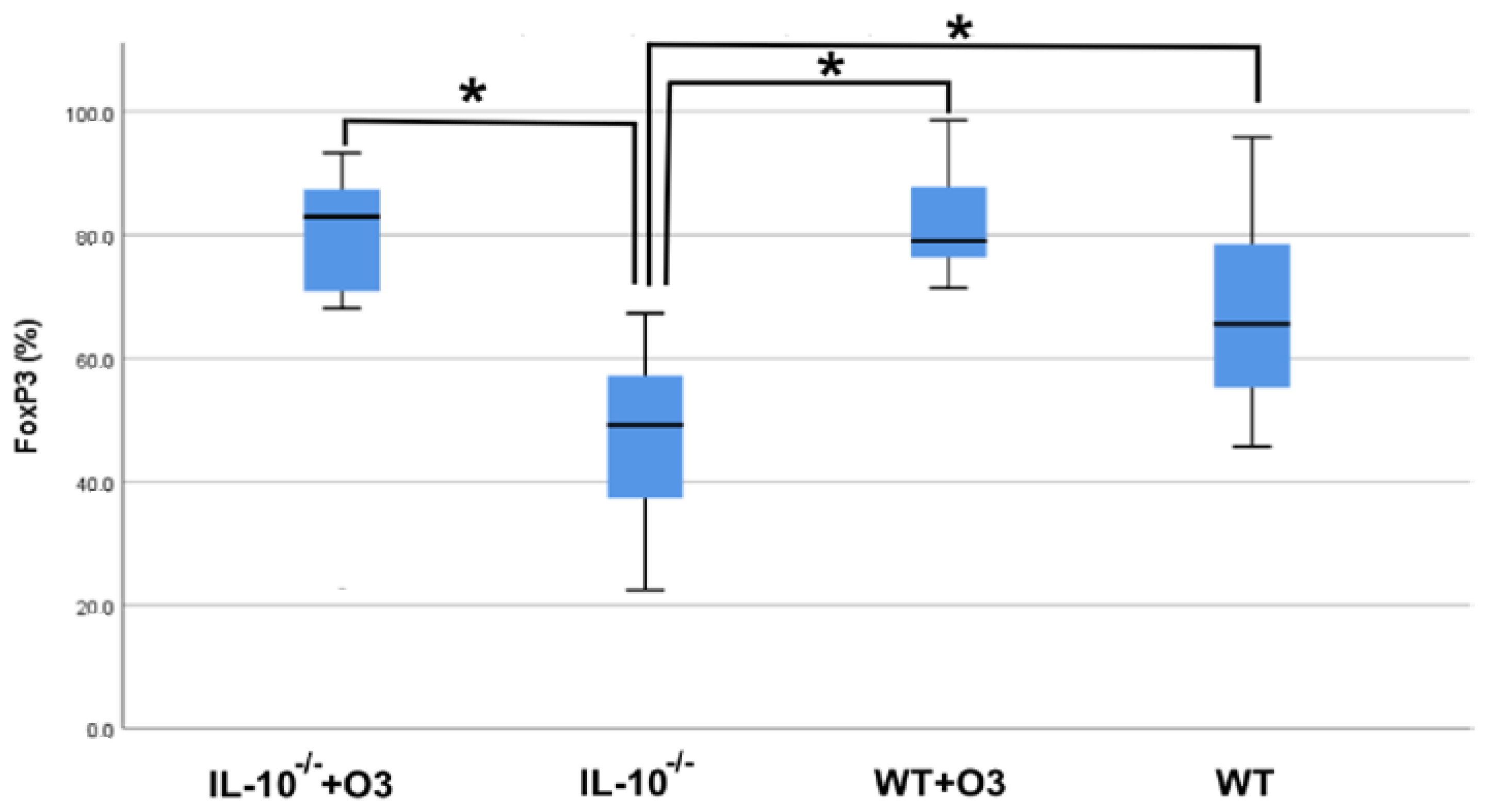
3.2. Assessment of Intestinal Tissue and Immunophenotyping of Cells from the Mesenteric Lymph Node
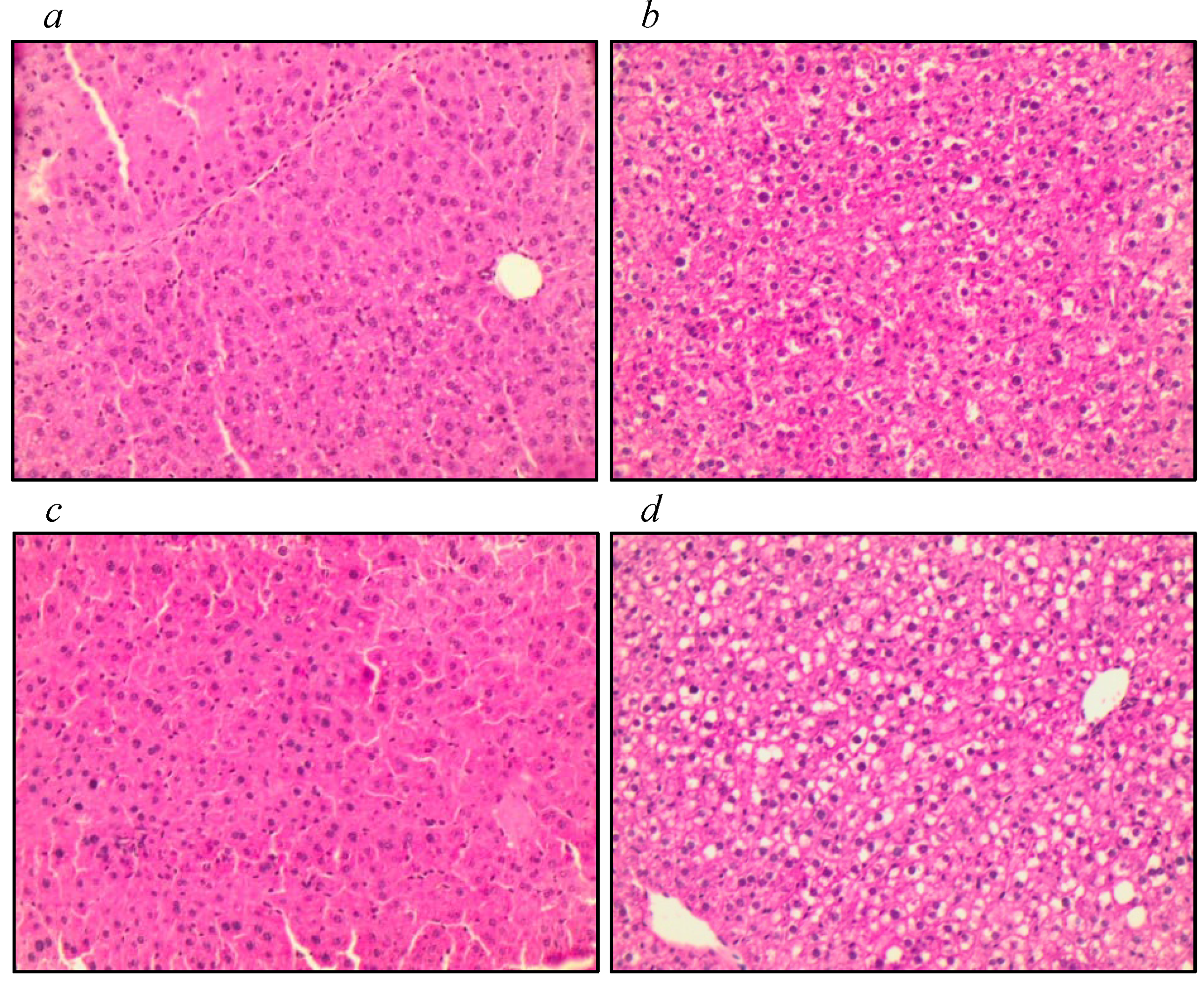
3.3. Assessment of Liver Tissue, Antioxidant Function, and Markers of Oxidative Stress
3.4. Tissue Assessment: Anterior Tibialis, Long Digital Extensor, Soleus, and Gastrocnemius Muscles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santoro, A.; Bientinesi, E.; Monti, D. Immunosenescence and Inflammaging in the Aging Process: Age-Related Diseases or Longevity? Ageing Res. Rev. 2021, 71, 101422. [Google Scholar] [CrossRef] [PubMed]
- Willis, C.M.; Nicaise, A.M.; Krzak, G.; Ionescu, R.B.; Pappa, V.; D’Angelo, A.; Agarwal, R.; Repollés-de-Dalmau, M.; Peruzzotti-Jametti, L.; Pluchino, S. Soluble Factors Influencing the Neural Stem Cell Niche in Brain Physiology, Inflammation, and Aging. Exp. Neurol. 2022, 355, 114124. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Martucci, M.; Conte, M.; Capri, M.; Franceschi, C.; Salvioli, S. Inflammaging, Hormesis and the Rationale for Anti-Aging Strategies. Ageing Res. Rev. 2020, 64, 101142. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-Aging. An Evolutionary Perspective on Immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Bosco, N.; Bourdet-Sicard, R.; Capuron, L.; Delzenne, N.; Doré, J.; Franceschi, C.; Lehtinen, M.J.; Recker, T.; Salvioli, S.; et al. Health Relevance of the Modification of Low Grade Inflammation in Ageing (Inflammageing) and the Role of Nutrition. Ageing Res. Rev. 2017, 40, 95–119. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Gonzalez-Freire, M.; Fabbri, E.; Simonsick, E.; Tanaka, T.; Moore, Z.; Salimi, S.; Sierra, F.; de Cabo, R. Measuring Biological Aging in Humans: A Quest. Aging Cell 2020, 19, e13080. [Google Scholar] [CrossRef]
- Sharma, V.; Mehdi, M.M. Oxidative Stress, Inflammation and Hormesis: The Role of Dietary and Lifestyle Modifications on Aging. Neurochem. Int. 2023, 164, 105490. [Google Scholar] [CrossRef]
- Fredman, G.; Tabas, I. Boosting Inflammation Resolution in Atherosclerosis: The Next Frontier for Therapy. Am. J. Pathol. 2017, 187, 1211–1221. [Google Scholar] [CrossRef]
- Sorokin, A.V.; Arnardottir, H.; Svirydava, M.; Ng, Q.; Baumer, Y.; Berg, A.; Pantoja, C.J.; Florida, E.M.; Teague, H.L.; Yang, Z.H.; et al. Comparison of the Dietary Omega-3 Fatty Acids Impact on Murine Psoriasis-like Skin Inflammation and Associated Lipid Dysfunction. J. Nutr. Biochem. 2023, 117, 109348. [Google Scholar] [CrossRef]
- Drobnic, F.; Rueda, F.; Pons, V.; Banquells, M.; Cordobilla, B.; Domingo, J.C. Erythrocyte Omega-3 Fatty Acid Content in Elite Athletes in Response to Omega-3 Supplementation: A Dose-Response Pilot Study. J. Lipids 2017, 2017, 1472719. [Google Scholar] [CrossRef] [PubMed]
- Żebrowska, A.; Hall, B.; Stolecka-Warzecha, A.; Stanula, A.; Sadowska-Krępa, E. The Effect of Omega-3 Fatty Acid Supplementation on Serum Adipocytokines, Lipid Profile and Biochemical Markers of Inflammation in Recreational Runners. Nutrients 2021, 13, 456. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Muñoz, D.; Niven, J.; Kucuk, S.; Cucchi, D.; Certo, M.; Jones, S.W.; Fischer, D.P.; Mauro, C.; Nicolaou, A. Omega-3 Polyunsaturated Fatty Acids Reverse the Impact of Western Diets on Regulatory T Cell Responses through Averting Ceramide-Mediated Pathways. Biochem. Pharmacol. 2022, 204, 115211. [Google Scholar] [CrossRef] [PubMed]
- McBurney, M.I.; Tintle, N.L.; Harris, W.S. The Omega-3 Index Is Inversely Associated with the Neutrophil-Lymphocyte Ratio in Adults’. Prostaglandins Leukot. Essent. Fat. Acids 2022, 177, 102397. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 Fatty Acids and Inflammatory Processes: From Molecules to Man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef]
- Kavyani, Z.; Musazadeh, V.; Fathi, S.; Hossein Faghfouri, A.; Dehghan, P.; Sarmadi, B. Efficacy of the Omega-3 Fatty Acids Supplementation on Inflammatory Biomarkers: An Umbrella Meta-Analysis. Int. Immunopharmacol. 2022, 111, 109104. [Google Scholar] [CrossRef]
- Yang, J.; Fernández-Galilea, M.; Martínez-Fernández, L.; González-Muniesa, P.; Pérez-Chávez, A.; Martínez, J.A.; Moreno-Aliaga, M.J. Oxidative Stress and Non-Alcoholic Fatty Liver Disease: Effects of Omega-3 Fatty Acid Supplementation. Nutrients 2019, 11, 872. [Google Scholar] [CrossRef] [PubMed]
- Akki, A.; Yang, H.; Gupta, A.; Chacko, V.P.; Yano, T.; Leppo, M.K.; Steenbergen, C.; Walston, J.; Weiss, R.G. Skeletal Muscle ATP Kinetics Are Impaired in Frail Mice. Age 2014, 36, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Ko, F.; Abadir, P.; Marx, R.; Westbrook, R.; Cooke, C.; Yang, H.; Walston, J. Impaired Mitochondrial Degradation by Autophagy in the Skeletal Muscle of the Aged Female Interleukin 10 Null Mouse. Exp. Gerontol. 2016, 73, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Walston, J.; Fedarko, N.; Yang, H.; Leng, S.; Beamer, B.; Espinoza, S.; Lipton, A.; Zheng, H.; Becker, K. The Physical and Biological Characterization of a Frail Mouse Model. J. Gerontol. A Biol. Med. Sci. 2008, 63, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, S.; Liu, J.; Liang, B.; Wang, X.; Wang, H.; Li, W.; Tong, Q.; Yi, J.; Zhao, L.; et al. Longitudinal Characteristics of Lymphocyte Responses and Cytokine Profiles in the Peripheral Blood of SARS-CoV-2 Infected Patients. EBioMedicine 2020, 55, 102763. [Google Scholar] [CrossRef]
- Meftahi, G.H.; Jangravi, Z.; Sahraei, H.; Bahari, Z. The Possible Pathophysiology Mechanism of Cytokine Storm in Elderly Adults with COVID-19 Infection: The Contribution of “Inflame-Aging. Inflamm. Res. 2020, 69, 825–839. [Google Scholar] [CrossRef]
- Pan, L.; Xie, W.; Fu, X.; Lu, W.; Jin, H.; Lai, J.; Zhang, A.; Yu, Y.; Li, Y.; Xiao, W. Inflammation and Sarcopenia: A Focus on Circulating Inflammatory Cytokines. Exp. Gerontol. 2021, 154, 111544. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of Total, Protein-Bound, and Nonprotein Sulfhydryl Groups in Tissue with Ellman’s Reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, J.; Fortis, I.; Blachier, S.; Kia, D.; Favier, A. Simultaneous determination of retinol,-tocopherol and fl-carotene in serum by isocratic high-performance liquid chromatography. J. Chromatogr. 1991, 572, 103–116. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the Quantitative and Qualitative Characterization of Erythrocyte Glutathione Peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Erdelmeier, I.; Gérard-Monnier, D.; Régnard, K.; Moze-Henry, N.; Yadan, J.C.; Chaudière, J. Reactions of 1-Methyl-2-Phenylindole with Malondialdehyde and 4-Hydroxyalkenals. Analytical Applications to a Colorimetric Assay of Lipid Peroxidation. Chem. Res. Toxicol. 1998, 11, 1176–1183. [Google Scholar] [CrossRef]
- Prado, R.Q.; Bertolini, T.B.; Piñeros, A.R.; Gembre, A.F.; S G Ramos, S.G.; Silva, C.L.; Borges, M.C.; Bonato, V.L.D. Attenuation of experimental asthma by mycobacterial protein combined with CpG requires a TLR9-dependent IFN-γ-CCR2 signalling circuit. Clin Exp Allergy. 2015, 45, 1459–1471. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and Validation of a Histological Scoring System for Nonalcoholic Fatty Liver Disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Perry, C.; Chung, J.Y.; Ylaya, K.; Choi, C.H.; Simpson, A.; Matsumoto, K.T.; Smith, W.A.; Hewitt, S.M. A Buffered Alcohol-Based Fixative for Histomorphologic and Molecular Applications. J. Histochem. Cytochem. 2016, 64, 425–440. [Google Scholar] [CrossRef]
- Rizvi, S.; Raza, S.T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The Role of Vitamin E in Human Health a Some Diseases. Sultan Qaboos Univ. Med. J. 2014, 14, e157–e165. [Google Scholar] [PubMed]
- Pei, J.; Pan, X.; Wei, G.; Hua, Y. Research Progress of Glutathione Peroxidase Family (GPX) in Redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef] [PubMed]
- Bidu, C.; Escoula, Q.; Bellenger, S.; Spor, A.; Galan, M.; Geissler, A.; Bouchot, A.; Dardevet, D.; Morio-Liondor, B.; Cani, P.D.; et al. The Transplantation of V3 PUFA-Altered Gut Microbiota of Fat-1 Mice to Wild-Type Littermates Prevents Obesity and Associated Metabolic Disorders. Diabetes 2018, 67, 1512–1523. [Google Scholar] [CrossRef]
- Saito, P.; Melo, C.P.B.; Martinez, R.M.; Fattori, V.; Cezar, T.L.C.; Pinto, I.C.; Bussmann, A.J.C.; Vignoli, J.A.; Georgetti, S.R.; Baracat, M.M.; et al. The Lipid Mediator Resolvin D1 Reduces the Skin Inflammation and Oxidative Stress Induced by UV Irradiation in Hairless Mice. Front. Pharmacol. 2018, 9, 1242–1257. [Google Scholar] [CrossRef]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2020, 10, 1607–1687. [Google Scholar] [CrossRef]
- Orbe-Orihuela, Y.C.; Lagunas-Martínez, A.; Bahena-Román, M.; Madrid-Marina, V.; Torres-Poveda, K.; Flores-Alfaro, E.; Méndez-Padrón, A.; Díaz-Benítez, C.E.; Peralta-Zaragoza, O.; Antúnez-Ortiz, D.; et al. High Relative Abundance of Firmicutes and Increased TNF-α Levels Correlate with Obesity in Children. Salud Publica Mex. 2018, 60, 5–11. [Google Scholar] [CrossRef]
- Reyes-Farias, M.; Fos-Domenech, J.; Serra, D.; Herrero, L.; Sánchez-Infantes, D. White Adipose Tissue Dysfunction in Obesity and Aging. Biochem. Pharmacol. 2021, 192, 114723. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Delproposto, J.B.; Westcott, D.J.; Saltiel, A.R. Phenotypic Switching of Adipose Tissue Macrophages with Obesity Is Generated by Spatiotemporal Differences in Macrophage Subtypes. Diabetes 2008, 57, 3239–3246. [Google Scholar] [CrossRef]
- Marcon, R.; Bento, A.F.; Dutra, R.C.; Bicca, M.A.; Leite, D.F.P.; Calixto, J.B. Maresin 1, a Proresolving Lipid Mediator Derived from Omega-3 Polyunsaturated Fatty Acids, Exerts Protective Actions in Murine Models of Colitis. J. Immunol. 2013, 191, 4288–4298. [Google Scholar] [CrossRef]
- Sun, M.; Pang, L.; Ju, X.; Sun, H.; Yu, J.; Zhao, H.; Yao, W.; Wei, M. Attenuating Effects of Omega-3 Fatty Acids (Omegaven) on Irradiation-Induced Intestinal Injury in Mice. Food Chem. Toxicol. 2014, 64, 275–280. [Google Scholar] [CrossRef]
- Kang, S.; Huang, J.; Lee, B.K.; Jung, Y.S.; Im, E.; Koh, J.M.; Im, D.S. Omega-3 Polyunsaturated Fatty Acids Protect Human Hepatoma Cells from Developing Steatosis through FFA4 (GPR120). Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 105–116. [Google Scholar] [CrossRef]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.Q.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 Is an Omega-3 Fatty Acid Receptor Mediating Potent Anti-Inflammatory and Insulin-Sensitizing Effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef]
- Castro, G.S.F.; dos Santos, R.A.; Portari, G.V.; Jordão, A.A.; Vannucchi, H. Omega-3 Improves Glucose Tolerance but Increases Lipid Peroxidation and DNA Damage in Hepatocytes of Fructose-Fed Rats. Appl. Physiol. Nutr. Metab. 2012, 37, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Soares Gonçalves Velasque, M.J.; Branchini, G.; Catarina, A.V.; Bettoni, L.; Fernandes, R.S.; Ferreira da Silva, A.; Dorneles, G.P.; Martins da Silva, I.; Santos, M.A.; Sumienski, J.; et al. Fish Oil - Omega-3 Exerts Protective Effect in Oxidative Stress and Liver Dysfunctions Resulting from Experimental Sepsis. J. Clin. Exp. Hepatol. 2022, 13, 64–74. [Google Scholar] [CrossRef] [PubMed]
- El-Mowafy, A.M.; Abdel-Aziz, A.A.; Abdel-Dayem, M.A. Novel Protection by Omega-3-FAs (DHA or EPA) against Carbamazepine’s Liver-Injury: Differential Suppression of Oxidative-Stress and Inflammatory Markers, and the Influence on Carbamazepine-Clearance. Clin. Nutr. Open Sci. 2022, 42, 14–26. [Google Scholar] [CrossRef]
- Park, Y.; Harris, W.S. Omega-3 Fatty Acid Supplementation Accelerates Chylomicron Triglyceride Clearance. J. Lipid Res. 2003, 44, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.C.; Ristow, M.; Viña, J. Antioxidant Supplements in Exercise: Worse than Useless? Am. J. Physiol. Endocrinol. Metab. 2012, 302, 476–477. [Google Scholar] [CrossRef] [PubMed]
- Merry, T.L.; Ristow, M. Do Antioxidant Supplements Interfere with Skeletal Muscle Adaptation to Exercise Training? J. Physiol. 2016, 594, 5135–5147. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, M.G.; Kerksick, C.M.; Lamprecht, M.; McAnulty, S.R. Does Vitamin C and e Supplementation Impair the Favorable Adaptations of Regular Exercise? Oxid. Med. Cell. Longev. 2012, 2012, 707941. [Google Scholar] [CrossRef] [PubMed]
- Sala, D.; Sacco, A. Signal Transducer and Activator of Transcription 3 Signaling as a Potential Target to Treat Muscle Wasting Diseases. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 171–176. [Google Scholar] [CrossRef]
- Gingras, A.A.; White, P.J.; Chouinard, P.Y.; Julien, P.; Davis, T.A.; Dombrowski, L.; Couture, Y.; Dubreuil, P.; Myre, A.; Bergeron, K.; et al. Long-Chain Omega-3 Fatty Acids Regulate Bovine Whole-Body Protein Metabolism by Promoting Muscle Insulin Signalling to the Akt-MTOR-S6K1 Pathway and Insulin Sensitivity. J. Physiol. 2007, 579, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Hemmings, B.A.; Restuccia, D.F. PI3K-PKB/Akt Pathway. Cold Spring Harb. Perspect. Biol. 2012, 4. [Google Scholar] [CrossRef]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Omega-3 Polyunsaturated Fatty Acids Augment the Muscle Protein Anabolic Response to Hyperinsulinaemia-Hyperaminoacidaemia in Healthy Young and Middle-Aged Men and Women. Clin. Sci. 2011, 121, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Deldicque, L.; Cani, P.D.; Philp, A.; Raymackers, J.-M.; Meakin, P.J.; Ashford, M.L.J.; Delzenne, N.M.; Francaux, M.; Baar, K. The Unfolded Protein Response Is Activated in Skeletal Muscle by High-Fat Feeding: Potential Role in the Downregulation of Protein Synthesis. Am. J. Physiol.—Endocrinol. Metab. 2010, 299, 695–705. [Google Scholar] [CrossRef] [PubMed]


| (a) | ||||||||
| Variables | IL−10−/−+O3 | IL−10−/− | WT | WT+O3 | ||||
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | |
| Starting weight | 22.15 | 21.3–22.5 | 22.0 | 21.4–22.8 | 19.3 | 18.7–19.7 | 19.5 | 18.5–20.2 |
| Final weight | 29.65 | 27.4–30.5 | 26.4 | 24.9–27.6 | 25.5 | 24–26.4 | 23.2 | 21.5–23.8 |
| Weight delta | 7.45 | 5.5–8.7 | 4.3 | 3.5–4.9 | 6.5 | 4.9–8.2 | 3.9 | 2.2–4.7 |
| (b) | ||||||||
| Bonferroni post-hoc test p-Value | ||||||||
| IL−10−/− vs. Il-10−/−+O3 | IL−10−/− vs. WT | IL−10−/− vs. WT+03 | IL−10−/−+O3 vs. WT | IL−10−/−+O3 vs. WT+03 | WT vs. WT+O3 | |||
| Starting weight | >0.999 | 0.001 * | 0.001 * | <0.001 * | <0.001 * | >0.999 | ||
| Final weight | 0.251 | 0.004 * | >0.999 | <0.001 * | 0.013 * | 0.036 * | ||
| Weight delta | 0.027 * | >0.999 | >0.999 | <0.001 * | >0.999 | 0.006 * | ||
| (a) | |||||||||
| Variables | IL−10−/−+O3 | IL−10−/− | WT+O3 | WT | Kruskal-Wallis p-Value | ||||
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | ||
| Scores | 1.0 | 0–1.0 | 3.0 | 1.50–3.0 | 1.0 | 0–10 | 2.0 | 0–3.0 | 0.009 * |
| (b) | |||||||||
| Bonferroni post-hoc test p-Value | |||||||||
| IL−10−/− vs. Il-10−/−+O3 | IL−10−/− vs. WT | IL−10−/− vs. WT-03 | IL−10−/−+O3 vs. WT | IL−10−/−+O3 vs. WT+03 | WT vs. WT+O3 | ||||
| Scores | 0.017 * | 0.260 | 0.017 * | >0.999 | >0.999 | >0.999 | |||
| (a) | ||||||||||||||
| Variables | IL−10−/−+O3 | IL−10−/− | WT+O3 | WT | Kruskal-Wallis p-Value | |||||||||
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | |||||||
| Liver weight | 1.2 | 1.1–1.3 | 1.2 | 1.2–1.3 | 1.1 | 1.1–1.2 | 1.2 | 1.1–1.2 | 0.208 | |||||
| GSH hepatic | 201.7 | 167.4–269.6 | 216.2 | 171.2–271.1 | 270.0 | 232.7–278.7 | 231.0 | 197.6–320.0 | 0.236 | |||||
| GPx hepatic | 224.7 | 148.9–239.3 | 237.5 | 219.4–263.5 | 217.8 | 201.1–240.2 | 223.0 | 207.3–236.6 | 0.257 | |||||
| MDA hepatic | 25.0 | 24.0–29.0 | 17.7 | 15.6–24.8 | 21.3 | 20.6–24.8 | 21.0 | 19.4–23.2 | 0.025 * | |||||
| Vit E hepatic | 208.2 | 167.4–277.5 | 259.0 | 185.6–336.1 | 374.9 | 302.8–397.1 | 378.5 | 294.7–481.7 | <0.001 * | |||||
| (b) | ||||||||||||||
| Bonferroni Post-hoc test p-Value | ||||||||||||||
| IL−10−/− vs. IL−10−/−+O3 | IL−10−/− vs. WT | IL−10−/− vs. WT+03 | IL−10−/−+O3 vs. WT | IL−10−/−+O3 vs. WT+03 | WT vs. WT+O3 | |||||||||
| MDA hepatic | 0.030 * | >0.999 | >0.999 | 0.085 | 0.236 | >0.999 | ||||||||
| VIT E hepatic | 0.951 | 0.139 | 0.211 | 0.001 * | 0.002* | >0.999 | ||||||||
| Groups | Chi-Square p-Value | Bonferroni Post-Hoc Test | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL−10−/−+O3 | IL−10−/− | WT+O3 | WT | IL−10−/− vs. WT | WT vs. WT+O3 | |||||||
| n | % | n | % | n | % | n | % | |||||
| Muscle | Slender | 9 | 64.3% | 11 | 84.6% | 15 | 88.2% | 2 | 15.4% | <0.001 * | 0.002 * | <0.001 * |
| Normal | 5 | 35.7% | 2 | 15.4% | 2 | 11.8% | 11 | 84.6% | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campanari, D.D.; Cipriano, U.G.; Fraga-Silva, T.F.d.C.; Ramalho, L.N.Z.; Ovidio, P.P.; Jordão Júnior, A.A.; Bonato, V.L.D.; Ferriolli, E. Effect of Dietary Supplementation with Omega-3 Fatty Acid on the Generation of Regulatory T Lymphocytes and on Antioxidant Parameters and Markers of Oxidative Stress in the Liver Tissue of IL−10 Knockout Mice. Nutrients 2024, 16, 634. https://doi.org/10.3390/nu16050634
Campanari DD, Cipriano UG, Fraga-Silva TFdC, Ramalho LNZ, Ovidio PP, Jordão Júnior AA, Bonato VLD, Ferriolli E. Effect of Dietary Supplementation with Omega-3 Fatty Acid on the Generation of Regulatory T Lymphocytes and on Antioxidant Parameters and Markers of Oxidative Stress in the Liver Tissue of IL−10 Knockout Mice. Nutrients. 2024; 16(5):634. https://doi.org/10.3390/nu16050634
Chicago/Turabian StyleCampanari, Daniela Dalpubel, Ualter Guilherme Cipriano, Thais Fernanda de Campos Fraga-Silva, Leandra Náira Zambelli Ramalho, Paula Payão Ovidio, Alceu Afonso Jordão Júnior, Vânia Luiza Deperon Bonato, and Eduardo Ferriolli. 2024. "Effect of Dietary Supplementation with Omega-3 Fatty Acid on the Generation of Regulatory T Lymphocytes and on Antioxidant Parameters and Markers of Oxidative Stress in the Liver Tissue of IL−10 Knockout Mice" Nutrients 16, no. 5: 634. https://doi.org/10.3390/nu16050634
APA StyleCampanari, D. D., Cipriano, U. G., Fraga-Silva, T. F. d. C., Ramalho, L. N. Z., Ovidio, P. P., Jordão Júnior, A. A., Bonato, V. L. D., & Ferriolli, E. (2024). Effect of Dietary Supplementation with Omega-3 Fatty Acid on the Generation of Regulatory T Lymphocytes and on Antioxidant Parameters and Markers of Oxidative Stress in the Liver Tissue of IL−10 Knockout Mice. Nutrients, 16(5), 634. https://doi.org/10.3390/nu16050634






