Botanical Impurities in the Supply Chain: A New Allergenic Risk Exacerbated by Geopolitical Challenges
Abstract
1. Introduction
2. Sensitization Rates of Vegetable Contaminants Potential Allergens
2.1. Cereals Containing Gluten
2.2. Peanuts
2.3. Soybeans, Lupin
2.4. Celery
2.5. Sesame Seeds
2.6. Mushrooms
2.7. Yams (Dioscorea spp.)
2.8. Buckwheat (Fagopyrum esculentum L.)
2.9. Tomatoes
2.10. Sunflower Seeds and Products Thereof
3. Characterization of Vegetable Allergens: Unveiling Molecular Complexity
3.1. Prolamin Superfamily
3.1.1. Cereal Prolamins
3.1.2. Bifunctional Inhibitors
- Hor v 15, a monomeric α-amylase inhibitor from barley.
- Tri a 28, a dimeric α-amylase inhibitor, and Tri a 29, a tetrameric α-amylase inhibitor, both from wheat.
3.1.3. 2S Albumins
- Ara h 2 and Ara h 6 from peanuts.
- Ber e 1 from Brazil nuts.
- Cor a 14 from hazelnuts.
- Jug r 1 from English walnuts.
- Ses I 1 from sesame seeds.
- Sin a 1 from yellow mustard.
3.1.4. Non-Specific Lipid Transfer Proteins
- Mal d 3 from apples.
- Pru p 3 from peaches.
- Cor a 8 from hazelnuts.
- Jug r 3 from walnuts.
3.2. Profilin-Like Superfamily
- Cit s 2 from oranges.
- Cuc m 2 from melons.
- Mus a 1 from bananas.
3.3. Cupin Superfamily
3.3.1. Vicilins
- Ara h 1 from peanuts.
- Gly m 5 from soybeans.
- Jug r 2 from walnuts.
- Ses I 3 from sesame seeds.
3.3.2. Legumins
- Ara h 3 from peanuts.
- Gly m 6 from soybeans.
- Ber e 2 from Brazil nuts.
- Fag e 1 from buckwheat.
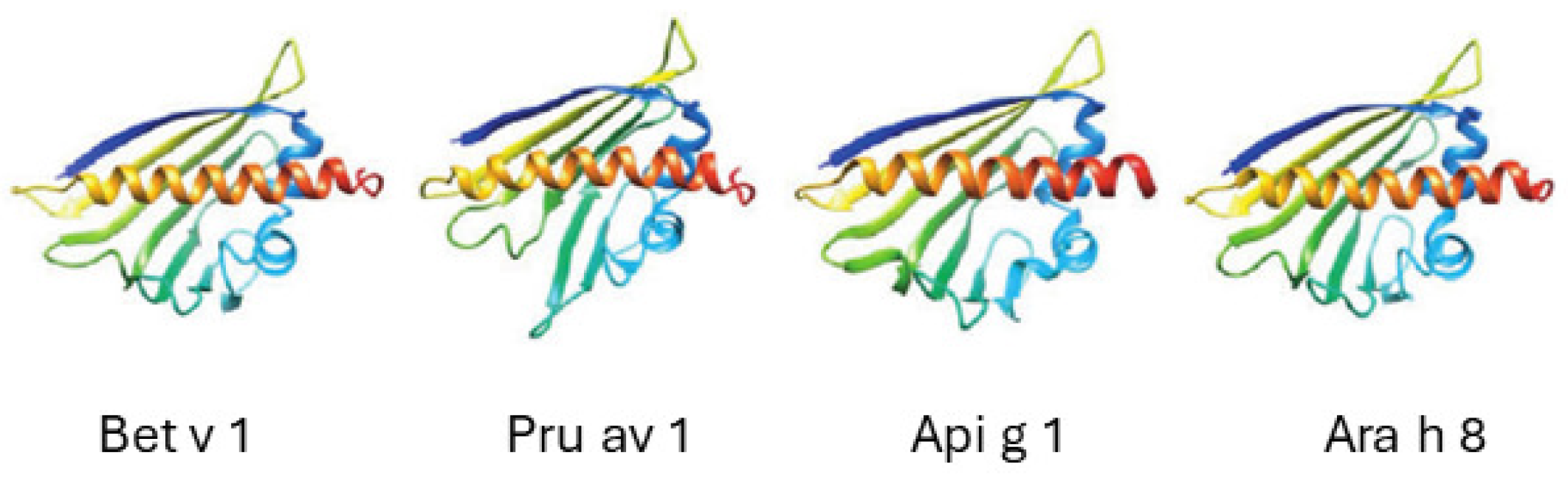
3.4. Bet v 1-Like Superfamily
The PR-10 Proteins
3.5. Cereals Containing Gluten
3.6. Peanuts
3.7. Soybeans, Lupin
3.8. Sesame Seeds
3.9. Mushrooms
3.10. Yams (Dioscorea spp.)
3.11. Buckwheat (Fagopyrum esculentum L.)
3.12. Celery and Tomatoes
3.13. Sunflower Seeds and Products Thereof
4. The Regulatory Context
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Polat, O.; Doğan Başar, B.; Torun, E.; Ekşi, İ.H. Dynamic Interlinkages between Geopolitical Stress and Agricultural Commodity Market: Novel Findings in the Wake of the Russian Ukrainian Conflict. Borsa Istanb. Rev. 2023, 23, S74–S83. [Google Scholar] [CrossRef]
- Jagtap, S.; Trollman, H.; Trollman, F.; Garcia-Garcia, G.; Parra-López, C.; Duong, L.; Martindale, W.; Munekata, P.E.S.; Lorenzo, J.M.; Hdaifeh, A.; et al. The Russia-Ukraine Conflict: Its Implications for the Global Food Supply Chains. Foods 2022, 11, 2098. [Google Scholar] [CrossRef] [PubMed]
- Ben Hassen, T.; El Bilali, H. Impacts of the Russia-Ukraine War on Global Food Security: Towards More Sustainable and Resilient Food Systems? Foods 2022, 11, 2301. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.; Zhao, W.; Symochko, L.; Inacio, M.; Bogunovic, I.; Barcelo, D. The Russian-Ukrainian Armed Conflict Will Push Back the Sustainable Development Goals. Geogr. Sustain. 2022, 3, 277–287. [Google Scholar] [CrossRef]
- Muraro, A.; de Silva, D.; Halken, S.; Worm, M.; Khaleva, E.; Arasi, S.; Dunn-Galvin, A.; Nwaru, B.I.; De Jong, N.W.; Rodríguez Del Río, P.; et al. Managing Food Allergy: GA2LEN Guideline 2022. World Allergy Organ. J. 2022, 15, 100687. [Google Scholar] [CrossRef]
- Fiocchi, A.; Monaci, L.; De Angelis, E.; Calandrelli, V.; Dahdah, L.; Valluzzi, R.; Urbani, S.; Mazzuca, C.; Arasi, S.; Cafarotti, A.; et al. Reactivity to Allergenic Food Contaminants: A Study on Products on the Market. Clin. Transl. Allergy 2023, 13, e12301. [Google Scholar] [CrossRef]
- Remington, B.C.; Taylor, S.L.; Marx, D.B.; Petersen, B.J.; Baumert, J.L. Soy in Wheat–Contamination Levels and Food Allergy Risk Assessment. Food Chem. Toxicol. 2013, 62, 485–491. [Google Scholar] [CrossRef]
- Taylor, S.L.; Baumert, J.L. Cross-Contamination of Foods and Implications for Food Allergic Patients. Curr. Allergy Asthma Rep. 2010, 10, 265–270. [Google Scholar] [CrossRef]
- Zurzolo, G.A.; Mathai, M.L.; Koplin, J.J.; Allen, K.J. Hidden Allergens in Foods and Implications for Labelling and Clinical Care of Food Allergic Patients. Curr. Allergy Asthma Rep. 2012, 12, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Hossny, E.; Ebisawa, M.; El-Gamal, Y.; Arasi, S.; Dahdah, L.; El-Owaidy, R.; Galvan, C.A.; Lee, B.W.; Levin, M.; Martinez, S.; et al. Challenges of Managing Food Allergy in the Developing World. World Allergy Organ. J. 2019, 12, 100089. [Google Scholar] [CrossRef] [PubMed]
- RASFF, Peanuts in Soy Lecithin from India. Risk Analysis—Gift. Available online: https://www.greatitalianfoodtrade.it/en/sicurezza/rasff-arachidi-nella-lecitina-di-soia-dallindia-analisi-del-rischio/ (accessed on 13 December 2023).
- Krejner-Bienias, A.; Grzela, K.; Kulus, M.; Grzela, T. Peanut Contamination in Food Products: A Real Danger for Allergic People? Adv. Dermatol. Allergol. 2023, 40, 625–629. [Google Scholar] [CrossRef]
- Dinardo, G.; Cafarotti, A.; Galletta, F.; Fiocchi, A.; Arasi, S. Omalizumab in Severe Asthma and Food Allergies with IgE Levels >1500 KU/L: Two-Year Evaluation. Pediatr. Allergy Immunol. 2023, 34, e14057. [Google Scholar] [CrossRef]
- Graham, F.; Fiocchi, A. The Importance of Threshold Dose-Distribution Data for Priority Allergens and the Need for Future Studies. Allergy 2022, 77, 2886–2887. [Google Scholar] [CrossRef]
- Taylor, S.L.; Hefle, S.L.; Bindslev-Jensen, C.; Bock, S.A.; Burks, A.W.; Christie, L.; Hill, D.J.; Host, A.; Hourihane, J.O.B.; Lack, G.; et al. Factors Affecting the Determination of Threshold Doses for Allergenic Foods: How Much Is Too Much? J. Allergy Clin. Immunol. 2002, 109, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Graham, F.; Benhamou, A.H.; Liu, Y.J.; Caubet, J.C.; Eigenmann, P.A. Real-Life Evaluation of Tolerance to Foods with Precautionary Allergen Labeling in Children with IgE-Mediated Food Allergy. Allergy 2023, 78, 2558–2561. [Google Scholar] [CrossRef] [PubMed]
- Arasi, S.; Nurmatov, U.; Dunn-Galvin, A.; Roberts, G.; Turner, P.J.; Shinder, S.B.; Gupta, R.; Eigenmann, P.; Nowak-Wegrzyn, A.; Ansotegui, I.J.; et al. WAO Consensus on DEfinition of Food Allergy SEverity (DEFASE). World Allergy Organ. J. 2023, 16, 100753. [Google Scholar] [CrossRef]
- Lyons, S.A.; Clausen, M.; Knulst, A.C.; Ballmer-Weber, B.K.; Fernandez-Rivas, M.; Barreales, L.; Bieli, C.; Dubakiene, R.; Fernandez-Perez, C.; Jedrzejczak-Czechowicz, M.; et al. Prevalence of Food Sensitization and Food Allergy in Children Across Europe. J. Allergy Clin. Immunol. Pract. 2020, 8, 2736–2746.e9. [Google Scholar] [CrossRef] [PubMed]
- Cianferoni, A. Wheat Allergy: Diagnosis and Management. J. Asthma Allergy 2016, 9, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Östblom, E.; Wickman, M.; Van Hage, M.; Lilja, G. Reported Symptoms of Food Hypersensitivity and Sensitization to Common Foods in 4-Year-Old Children. Acta Paediatr. 2008, 97, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Matricardi, P.M.; Bockelbrink, A.; Beyer, K.; Keil, T.; Niggemann, B.; Grüber, C.; Wahn, U.; Lau, S. Primary versus Secondary Immunoglobulin E Sensitization to Soy and Wheat in the Multi-Centre Allergy Study Cohort. Clin. Exp. Allergy 2008, 38, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Vierk, K.A.; Koehler, K.M.; Fein, S.B.; Street, D.A. Prevalence of Self-Reported Food Allergy in American Adults and Use of Food Labels. J. Allergy Clin. Immunol. 2007, 119, 1504–1510. [Google Scholar] [CrossRef]
- Keet, C.A.; Matsui, E.C.; Dhillon, G.; Lenehan, P.; Paterakis, M.; Wood, R.A. The Natural History of Wheat Allergy. Ann. Allergy Asthma Immunol. 2009, 102, 410–415. [Google Scholar] [CrossRef]
- Hemmer, W.; Sesztak-Greinecker, G.; Wöhrl, S.; Wantke, F. Food Allergy to Millet and Cross-Reactivity with Rice, Corn and Other Cereals. Allergol. Int. 2017, 66, 490–492. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Sampson, H.A. Peanut Allergy: Emerging Concepts and Approaches for an Apparent Epidemic. J. Allergy Clin. Immunol. 2007, 120, 491–503. [Google Scholar] [CrossRef]
- Baseggio Conrado, A.; Patel, N.; Turner, P.J. Global Patterns in Anaphylaxis Due to Specific Foods: A Systematic Review. J. Allergy Clin. Immunol. 2021, 148, 1515–1525.e3. [Google Scholar] [CrossRef] [PubMed]
- Katz, Y.; Gutierrez-Castrellon, P.; González, M.G.; Rivas, R.; Lee, B.W.; Alarcon, P. A Comprehensive Review of Sensitization and Allergy to Soy-Based Products. Clin. Rev. Allergy Immunol. 2014, 46, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Ballmer-Weber, B.K.; Vieths, S. Soy Allergy in Perspective. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Dramburg, S.; Hilger, C.; Santos, A.F.; de las Vecillas, L.; Aalberse, R.C.; Acevedo, N.; Aglas, L.; Altmann, F.; Arruda, K.L.; Asero, R.; et al. EAACI Molecular Allergology User’s Guide 2.0. Pediatr. Allergy Immunol. 2023, 34 (Suppl. 28), e13854. [Google Scholar] [CrossRef] [PubMed]
- Giampietro, P.G.; Ragno, V.; Daniele, S.; Cantani, A.; Ferrara, M.; Businco, L. Soy Hypersensitivity in Children with Food Allergy. Ann. Allergy 1992, 69, 143–146. [Google Scholar] [PubMed]
- Bock, S.A.; Muoz-Furlong, A.; Sampson, H.A. Fatalities Due to Anaphylactic Reactions to Foods. J. Allergy Clin. Immunol. 2001, 107, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Kayode, O.S.; Siew, L.Q.C.; Pillai, P.; Haque, R.; Rutkowski, K.; Caballero, M.R. Mushroom Allergy: Case Series. J. Allergy Clin. Immunol. Pract. 2020, 8, 375–379. [Google Scholar] [CrossRef]
- Xu, Y.-Y.; Yin, J. Identification of a Thermal Stable Allergen in Yam (Dioscorea Opposita) to Cause Anaphylaxis. Asia Pac. Allergy 2018, 8, e4. [Google Scholar] [CrossRef] [PubMed]
- Norbäck, D.; Wieslander, G. A Review on Epidemiological and Clinical Studies on Buckwheat Allergy. Plants 2021, 10, 607. [Google Scholar] [CrossRef] [PubMed]
- Larramendi, C.H.; Ferrer, A.; Huertas, A.J.; García-Abujeta, J.L.; Andreu, C.; Tella, R.; Cerdà, M.T.; Bartra, J.; Lavín, J.R.; Pagán, J.A.; et al. Sensitization to Tomato Peel and Pulp Extracts in the Mediterranean Coast of Spain: Prevalence and Co-Sensitization with Aeroallergens. Clin. Exp. Allergy 2008, 38, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The Protein Families Database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y.; Matsunaga, T.; Fukuyama, K.; Miyazaki, T.; Morimoto, T. Tertiary and Quaternary Structures of 0.19 Alpha-Amylase Inhibitor from Wheat Kernel Determined by X-Ray Analysis at 2.06 A Resolution. Biochemistry 1997, 36, 13503–13511. [Google Scholar] [CrossRef] [PubMed]
- Brant, A. Baker’s Asthma. Curr. Opin. Allergy Clin. Immunol. 2007, 7, 152–155. [Google Scholar] [CrossRef]
- Egger, M.; Hauser, M.; Mari, A.; Ferreira, F.; Gadermaier, G. The Role of Lipid Transfer Proteins in Allergic Diseases. Curr. Allergy Asthma Rep. 2010, 10, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Indolfi, C.; Dinardo, G.; Klain, A.; Contieri, M.; Umano, G.R.; Decimo, F.; Abbadessa, S.; Vitulano, C.; Ciprandi, G.; Miraglia Del Giudice, M. Sensitization to NsLTP: A Retrospective Study in An Italian Pediatric Population over the Last Decade. J. Immunol. Res. 2023, 2023, 4053799. [Google Scholar] [CrossRef]
- Asero, R.; Pravettoni, V. Anaphylaxis to Plant-Foods and Pollen Allergens in Patients with Lipid Transfer Protein Syndrome. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 379–385. [Google Scholar] [CrossRef]
- Kiguchi, T.; Yamamoto-Hanada, K.; Saito-Abe, M.; Sato, M.; Irahara, M.; Ogita, H.; Miyagi, Y.; Inuzuka, Y.; Toyokuni, K.; Nishimura, K.; et al. Pollen-Food Allergy Syndrome and Component Sensitization in Adolescents: A Japanese Population-Based Study. PLoS ONE 2021, 16, e0249649. [Google Scholar] [CrossRef] [PubMed]
- Radauer, C.; Breiteneder, H. Evolutionary Biology of Plant Food Allergens. J. Allergy Clin. Immunol. 2007, 120, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, M.; Skypala, I.J.; Caubet, J.C.; Du Toit, G.; Nowak-Wegrzyn, A. Diagnosis and Management of Pollen Food Allergy Syndrome to Nuts. J. Allergy Clin. Immunol. Pr. 2024. epub ahead of print. [Google Scholar] [CrossRef]
- Maleki, S.J.; Chung, S.Y.; Champagne, E.T.; Raufman, J.P. The Effects of Roasting on the Allergenic Properties of Peanut Proteins. J. Allergy Clin. Immunol. 2000, 106, 763–768. [Google Scholar] [CrossRef]
- Beyer, K.; Morrow, E.; Li, X.M.; Bardina, L.; Bannon, G.A.; Burks, A.W.; Sampson, H.A. Effects of Cooking Methods on Peanut Allergenicity. J. Allergy Clin. Immunol. 2001, 107, 1077–1081. [Google Scholar] [CrossRef]
- Jones, R.; Stark, D.; Sussman, G.; Yunginger, J. 961 Recovery of Peanut Allergens from Ventilation Filters of Commercial Airliners. J. Allergy Clin. Immunol. 1996, 97, 423. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Furlong, T.J.; DeSimone, J.; Sampson, H.A. Self-Reported Allergic Reactions to Peanut on Commercial Airliners. J. Allergy Clin. Immunol. 1999, 104, 186–189. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Furlong, T.J.; DeSimone, J.; Sampson, H.A. The US Peanut and Tree Nut Allergy Registry: Characteristics of Reactions in Schools and Day Care. J. Pediatr. 2001, 138, 560–565. [Google Scholar] [CrossRef]
- Franck, P.; Moneret Vautrin, D.A.; Dousset, B.; Kanny, G.; Nabet, P.; Guénard-Bilbaut, L.; Parisot, L. The Allergenicity of Soybean-Based Products Is Modified by Food Technologies. Int. Arch. Allergy Immunol. 2002, 128, 212–219. [Google Scholar] [CrossRef]
- Codina, R.; Oehling, A.G.; Lockey, R.F. Neoallergens in Heated Soybean Hull. Int. Arch. Allergy Immunol. 1998, 117, 120–125. [Google Scholar] [CrossRef]
- Eisner, P.; Weisz, U.; Osen, R.; Mittermaier, S. Innovative Nahrungsmittel. In Biologische Transformation; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Bernhisel-Broadbent, J.; Sampson, H.A. Cross-Allergenicity in the Legume Botanical Family in Children with Food Hypersensitivity. J. Allergy Clin. Immunol. 1989, 83 Pt 1, 435–440. [Google Scholar] [CrossRef] [PubMed]
- L’Hocine, L.; Boye, J.I. Allergenicity of Soybean: New Developments in Identification of Allergenic Proteins, Cross-Reactivities and Hypoallergenization Technologies. Crit. Rev. Food Sci. Nutr. 2007, 47, 127–143. [Google Scholar] [CrossRef] [PubMed]
- WHO/IUIS Allergen Nomenclature Home Page. Available online: http://allergen.org/ (accessed on 27 July 2022).
- Walker, M.J.; Gowland, M.H.; Points, J. Managing Food Allergens in the U.K. Retail Supply Chain. J. AOAC Int. 2018, 101, 45–55. [Google Scholar] [CrossRef]
- Regulation—2021/382—EN—EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2021/382/oj (accessed on 17 February 2024).
- Codex Alimentarius Commission: 24–26 September 2020 and 12 October 2020. Available online: https://www.fao.org/newsroom/detail/Codex-Alimentarius-Commission-24-26-September-2020-and-12-October-2020/en (accessed on 17 February 2024).
- Guidance on Food Allergen Management for Food Manufacturers Version 2; FoodDrinkEurope: Brussels, Belgium, 2022.
- Dinardo, G.; Fierro, V.; Del Giudice, M.M.; Urbani, S.; Fiocchi, A. Food-Labeling Issues for Severe Food-Allergic Consumers. Curr. Opin. Allergy Clin. Immunol. 2023, 23, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Directive 2005/29/EC of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32005L0029 (accessed on 14 December 2023).
- Regulation (EC) No 178/2002 of the European Parliament and of the Council. Available online: https://www.legislation.gov.uk/eur/2002/178/contents (accessed on 14 December 2023).
- Miraglia del Giudice, M.; Dinardo, G.; Klain, A.; D’Addio, E.; Bencivenga, C.L.; Decimo, F.; Indolfi, C. Anaphylaxis after Shrimp Intake in a European Pediatric Population: Role of Molecular Diagnostics and Implications for Novel Foods. Children 2023, 10, 1583. [Google Scholar] [CrossRef] [PubMed]
- European Union. Official Journal of the European Union, L 304, 22 November 2011, CELEX1. EU: European Union 22 November 2011. Available online: https://eur-lex.europa.eu/legal-content/NL/TXT/?uri=OJ:L:2011:304:TOC (accessed on 14 December 2023).
- Food Allergen Labeling and Consumer Protection Act of 2004 (FALCPA)|FDA. Available online: https://www.fda.gov/food/food-allergensgluten-free-guidance-documents-regulatory-information/food-allergen-labeling-and-consumer-protection-act-2004-falcpa (accessed on 28 January 2023).
- An Update on Sesame Allergen Labeling on Food Packages|FDA. Available online: https://www.fda.gov/news-events/fda-voices/update-sesame-allergen-labeling-food-packages (accessed on 14 December 2023).
- Fiocchi, A.; Risso, D.; DunnGalvin, A.; González Díaz, S.N.; Monaci, L.; Fierro, V.; Ansotegui, I.J. Food Labeling Issues for Severe Food Allergic Patients. World Allergy Organ. J. 2021, 14, 100598. [Google Scholar] [CrossRef]
- FoodDrinkEurope Position: Precautionary Allergen Labelling—FoodDrinkEurope: FoodDrinkEurope. Available online: https://www.fooddrinkeurope.eu/resource/guidelines-on-precautionary-allergen-labelling/ (accessed on 14 December 2023).
- Turner, P.J.; Patel, N.; Ballmer-Weber, B.K.; Baumert, J.L.; Blom, W.M.; Brooke-Taylor, S.; Brough, H.; Campbell, D.E.; Chen, H.; Chinthrajah, R.S.; et al. Peanut Can Be Used as a Reference Allergen for Hazard Characterization in Food Allergen Risk Management: A Rapid Evidence Assessment and Meta-Analysis. J. Allergy Clin. Immunol. Pr. 2022, 10, 59–70. [Google Scholar] [CrossRef]
- Risk Assessment of Food Allergens: Part 3: Review and Establish Precautionary Labelling in Foods of the Priority Allergens: Meeting Report. Available online: https://www.who.int/publications/i/item/9789240072510 (accessed on 14 December 2023).
- Risk Assessment of Food Allergens: Part 2: Review and Establish Threshold Levels in Foods for the Priority Allergens: Meeting Report. Available online: https://www.who.int/publications/i/item/9789240065420 (accessed on 14 February 2023).
- Allen, K.J.; Turner, P.J.; Pawankar, R.; Taylor, S.; Sicherer, S.; Lack, G.; Rosario, N.; Ebisawa, M.; Wong, G.; Mills, E.N.C.; et al. Precautionary Labelling of Foods for Allergen Content: Are We Ready for a Global Framework? World Allergy Organ. J. 2014, 7, 10. [Google Scholar] [CrossRef]

| Prevalence (95% CI) of Food Sensitization to: | Reykjavik | Zurich |
|---|---|---|
| Wheat | 3.11 (2.39–3.82) | 14.44 (12.98–15.90) |
| Hazelnut | 1.87 (1.31–2.43) | 14.35 (12.89–15.81) |
| Tomato | 2.55 (1.90–3.23) | 13.27 (11.86–14.68) |
| Peach | 2.49 (1.84–3.13) | 13.21 (11.80–14.62) |
| Celery | 2.42 (1.79–3.06) | 13.09 (11.69–14.49) |
| Carrot | 2.11 (1.52–2.71) | 12.46 (11.09–13.83) |
| Sesame seed | 2.86 (2.17–3.55) | 12.10 (10.74–13.45) |
| Apple | 2.05 (1.46–2.64) | 11.95 (10.60–13.30) |
| Peanut | 2.31 (1.69–2.93) | 10.06 (8.81–11.31) |
| Walnut | 1.37 (0.89–1.85) | 9.52 (8.30–10.74) |
| Buckwheat | 1.37 (0.89–1.85) | 8.89 (7.70–10.07) |
| Sunflower seed | 1.37 (0.89–1.85) | 8.89 (7.70–10.07) |
| Poppy seed | 0.75 (0.39–1.10) | 8.50 (7.34–9.66) |
| Corn | 1.80 (1.25–2.35) | 8.35 (7.20–9.50) |
| Lentils | 2.11 (1.52–2.71) | 7.99 (6.86–9.11) |
| Soybean | 1.19 (0.74–1.63) | 7.72 (6.61–8.83) |
| Mustard seed | 0.37 (0.12–0.63) | 4.92 (4.02–5.82) |
| Superfamily | Family | Allergen Sources |
|---|---|---|
| Prolamin | Cereal prolamins | Grains of cereal grasses |
| Bifunctional inhibitors | Grains of cereal grasses | |
| 2S albumins | Tree nuts, peanuts, legumes, seeds | |
| Non-specific lipid Transfer proteins | Fruits, tree nuts, peanuts, vegetables | |
| Profilin-like | Profilin | Fruits, vegetables |
| Cupin | Vicilins | Tree nuts, peanuts, legumes, seeds |
| Legumins | Tree nuts, peanuts, legumes, seeds | |
| Bet v 1-like | PR-10 | Fruits, vegetables, legumes, tree nuts |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinardo, G.; Dahdah, L.; Cafarotti, A.; Arasi, S.; Fierro, V.; Pecora, V.; Mazzuca, C.; Urbani, S.; Artesani, M.C.; Riccardi, C.; et al. Botanical Impurities in the Supply Chain: A New Allergenic Risk Exacerbated by Geopolitical Challenges. Nutrients 2024, 16, 628. https://doi.org/10.3390/nu16050628
Dinardo G, Dahdah L, Cafarotti A, Arasi S, Fierro V, Pecora V, Mazzuca C, Urbani S, Artesani MC, Riccardi C, et al. Botanical Impurities in the Supply Chain: A New Allergenic Risk Exacerbated by Geopolitical Challenges. Nutrients. 2024; 16(5):628. https://doi.org/10.3390/nu16050628
Chicago/Turabian StyleDinardo, Giulio, Lamia Dahdah, Arianna Cafarotti, Stefania Arasi, Vincenzo Fierro, Valentina Pecora, Carmen Mazzuca, Sara Urbani, Maria Cristina Artesani, Carla Riccardi, and et al. 2024. "Botanical Impurities in the Supply Chain: A New Allergenic Risk Exacerbated by Geopolitical Challenges" Nutrients 16, no. 5: 628. https://doi.org/10.3390/nu16050628
APA StyleDinardo, G., Dahdah, L., Cafarotti, A., Arasi, S., Fierro, V., Pecora, V., Mazzuca, C., Urbani, S., Artesani, M. C., Riccardi, C., Valluzzi, R. L., Indolfi, C., Miraglia del Giudice, M., & Fiocchi, A. (2024). Botanical Impurities in the Supply Chain: A New Allergenic Risk Exacerbated by Geopolitical Challenges. Nutrients, 16(5), 628. https://doi.org/10.3390/nu16050628






