Twenty-Four-Hour Urinary Sugars Biomarker in a Vending Machine Intake Paradigm in a Diverse Population
Abstract
1. Introduction
2. Subjects and Methods
2.1. Study Recruitment and Data Collection
2.2. Ad libitum Dietary Intake Assessment
2.3. 24 h Urine Collection and Urinary Sugars Biomarker Assessment
2.4. Statistical Analysis
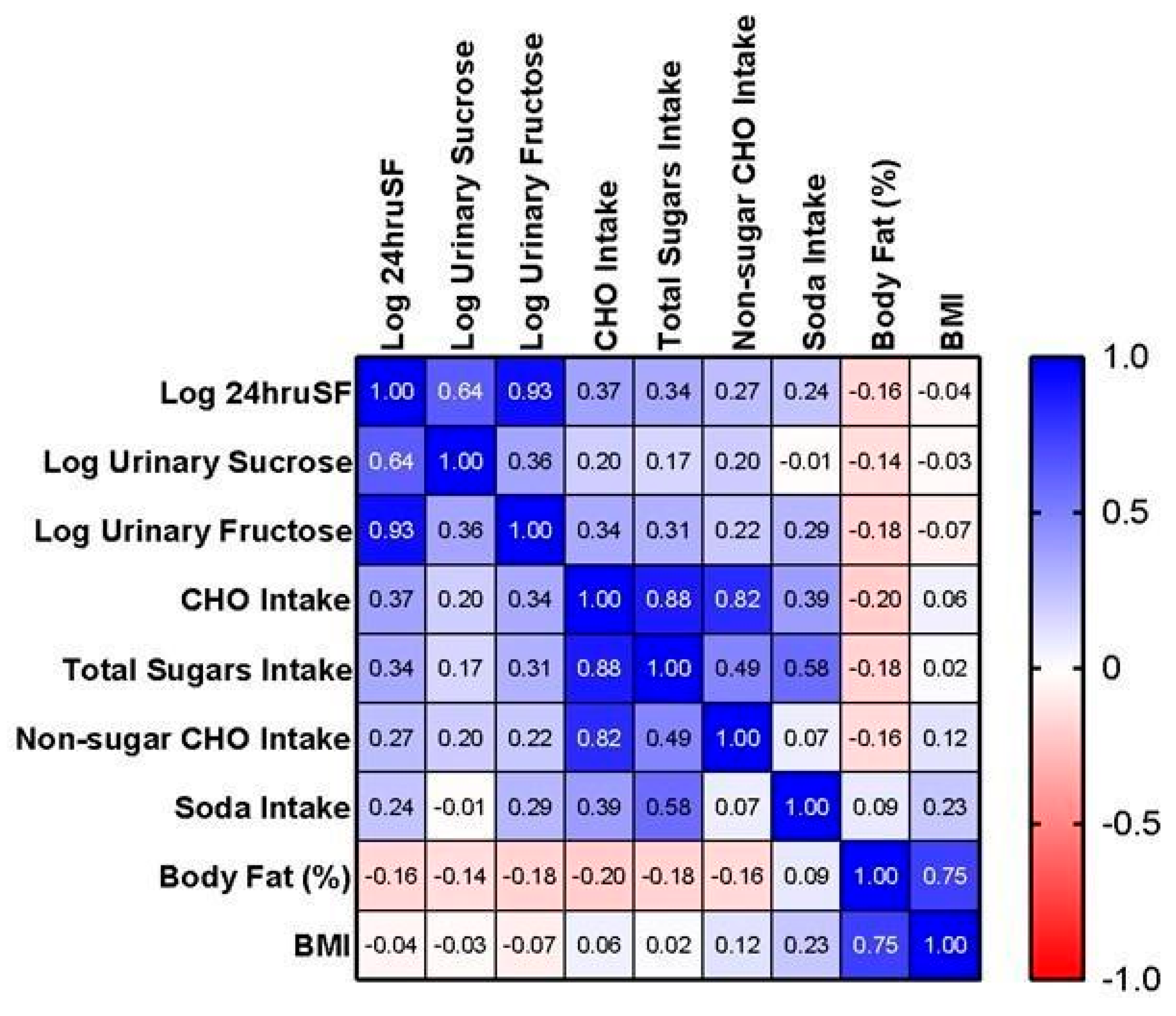
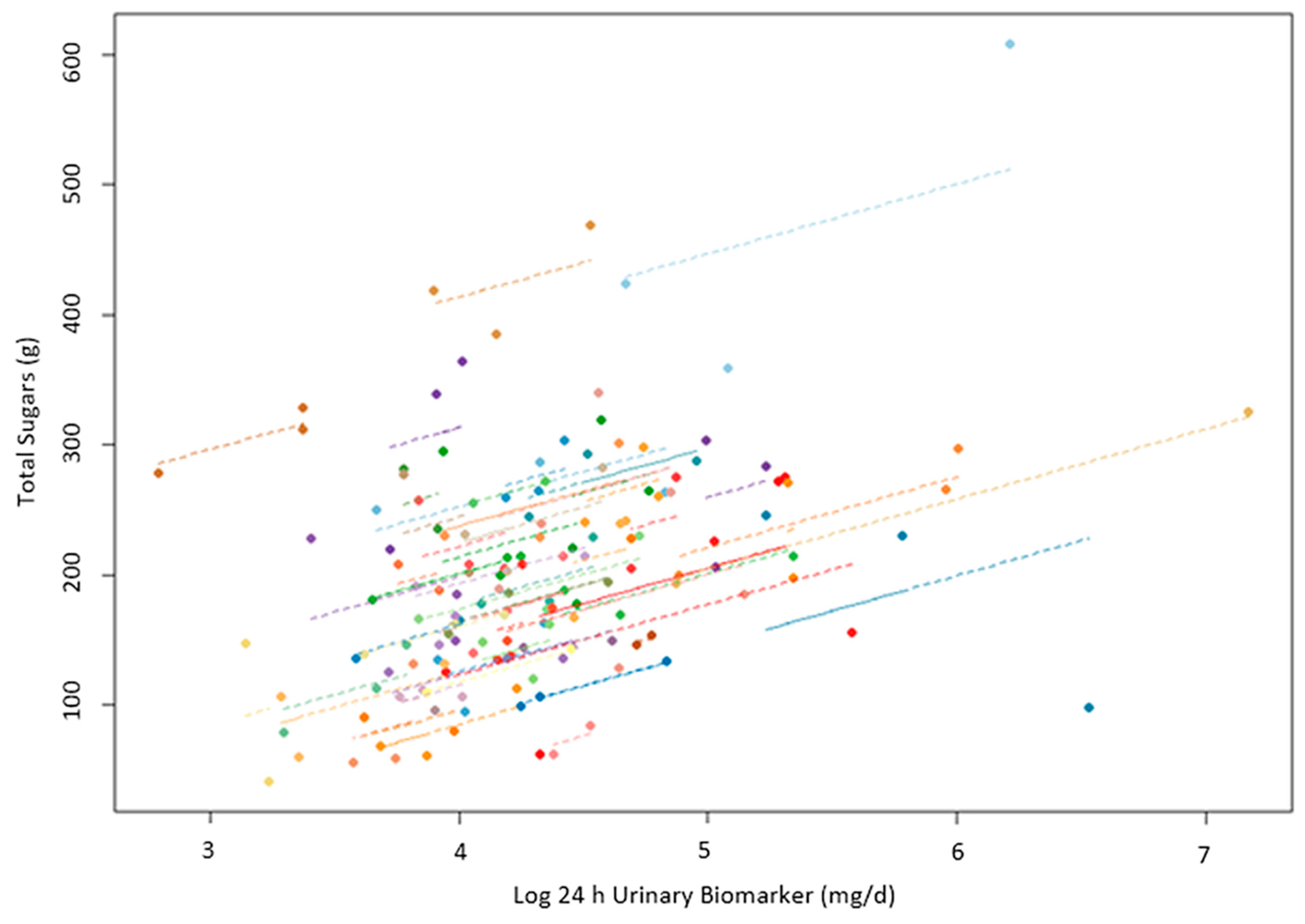
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stanhope, K.L. Sugar consumption, metabolic disease and obesity: The state of the controversy. Crit. Rev. Clin. Lab. Sci. 2016, 53, 52–67. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Z.; Chen, B.; Li, J.; Yuan, X.; Li, J.; Wang, W.; Dai, T.; Chen, H.; Wang, Y.; et al. Dietary sugar consumption and health: Umbrella review. BMJ 2023, 381, e071609. [Google Scholar] [CrossRef]
- Louie, J.C.Y. Objective Biomarkers for Total Added Sugar Intake—Are We on a Wild Goose Chase? Adv. Nutr. 2020, 11, 1429–1436. [Google Scholar] [CrossRef]
- DiFrancesco, L.; Fulgoni, V.L., III; Gaine, P.C.; Scott, M.O.; Ricciuto, L. Trends in added sugars intake and sources among U.S. adults using the National Health and Nutrition Examination Survey (NHANES) 2001–2018. Front. Nutr. 2022, 9, 897952. [Google Scholar] [CrossRef] [PubMed]
- Dietary Guidelines for Americans 2020–2025. Available online: https://www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf (accessed on 1 June 2023).
- World Health Organization. Guideline: Sugars Intake for Adults and Children [Internet]; World Health Organization: Geneva, Switzerland, 2015; 49p, Available online: https://apps.who.int/iris/handle/10665/149782 (accessed on 10 July 2023).
- Dhurandhar, N.V.; Schoeller, D.; Brown, A.W.; Heymsfield, S.B.; Thomas, D.; Sørensen, T.I.; Speakman, J.R.; Jeansonne, M.; Allison, D.B. Energy balance measurement: When something is not better than nothing. Int. J. Obes. 2015, 39, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, S.I.; Troiano, R.P.; Barrett, B.; Cunningham, C.; Subar, A.F.; Park, Y.; Bowles, H.R.; Freedman, L.S.; Kipnis, V.; Rimm, E.B.; et al. Measurement Error Affecting Web- and Paper-Based Dietary Assessment Instruments: Insights from the Multi-Cohort Eating and Activity Study for Understanding Reporting Error. Am. J. Epidemiol. 2022, 191, 1125–1139. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, M.N.; Schoeller, D.A. Traditional Self-Reported Dietary Instruments Are Prone to Inaccuracies and New Approaches Are Needed. Front. Nutr. 2020, 7, 538983. [Google Scholar] [CrossRef] [PubMed]
- Krebs-Smith, S.M.; Graubard, B.I.; Kahle, L.L.; Subar, A.F.; Cleveland, L.E.; Ballard-Barbash, R. Low energy reporters vs. others: A comparison of reported food intakes. Eur. J. Clin. Nutr. 2000, 54, 281–287. [Google Scholar] [CrossRef]
- Whitton, C.; Ramos-García, C.; I Kirkpatrick, S.; Healy, J.D.; Dhaliwal, S.S.; Boushey, C.J.; E Collins, C.; E Rollo, M.; A Kerr, D. A Systematic Review Examining Contributors to Misestimation of Food and Beverage Intake Based on Short-Term Self-Report Dietary Assessment Instruments Administered to Adults. Adv. Nutr. Int. Rev. J. 2022, 13, 2620–2665. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.A.; Tayyiba, M.; Agarwal, A.; Mejia, S.B.; de Souza, R.J.; Wolever, T.M.; Leiter, L.A.; Kendall, C.W.; Jenkins, D.J.; Sievenpiper, J.L. Relation of Total Sugars, Sucrose, Fructose, and Added Sugars with the Risk of Cardiovascular Disease: A Systematic Review and Dose-Response Meta-analysis of Prospective Cohort Studies. Mayo Clin. Proc. 2019, 94, 2399–2414. [Google Scholar] [CrossRef] [PubMed]
- Tsilas, C.S.; de Souza, R.J.; Mejia, S.B.; Mirrahimi, A.; Cozma, A.I.; Jayalath, V.H.; Ha, V.; Tawfik, R.; Di Buono, M.; Jenkins, A.L.; et al. Relation of total sugars, fructose and sucrose with incident type 2 diabetes: A systematic review and meta-analysis of prospective cohort studies. Can. Med. Assoc. J. 2017, 189, E711–E720. [Google Scholar] [CrossRef]
- Yamamoto, N.; Ejima, K.; Zoh, R.S.; Brown, A.W. Bias in nutrition-health associations is not eliminated by excluding extreme reporters in empirical or simulation studies. eLife 2023, 12, e83616. [Google Scholar] [CrossRef]
- Park, Y.; Dodd, K.W.; Kipnis, V.; E Thompson, F.; Potischman, N.; A Schoeller, D.; Baer, D.J.; Midthune, D.; Troiano, R.P.; Bowles, H.; et al. Comparison of self-reported dietary intakes from the Automated Self-Administered 24-h recall, 4-d food records, and food-frequency questionnaires against recovery biomarkers. Am. J. Clin. Nutr. 2018, 107, 80–93. [Google Scholar] [CrossRef]
- Tasevska, N.; Pettinger, M.; Kipnis, V.; Midthune, D.; Tinker, L.F.; Potischman, N.; Neuhouser, M.L.; Beasley, J.M.; Van Horn, L.; Howard, B.V.; et al. Associations of Biomarker-Calibrated Intake of Total Sugars with the Risk of Type 2 Diabetes and Cardiovascular Disease in the Women’s Health Initiative Observational Study. Am. J. Epidemiol. 2018, 187, 2126–2135. [Google Scholar] [CrossRef]
- Zheng, C.; Pettinger, M.; Gowda, G.N.; Lampe, J.W.; Raftery, D.; Tinker, L.F.; Huang, Y.; Navarro, S.L.; O’Brien, D.M.; Snetselaar, L.; et al. Biomarker-Calibrated Red and Combined Red and Processed Meat Intakes with Chronic Disease Risk in a Cohort of Postmenopausal Women. J. Nutr. 2022, 152, 1711–1720. [Google Scholar] [CrossRef]
- Tasevska, N. Urinary Sugars—A Biomarker of Total Sugars Intake. Nutrients 2015, 7, 5816–5833. [Google Scholar] [CrossRef] [PubMed]
- Luceri, C.; Caderni, G.; Lodovici, M.; Spagnesi, M.T.; Monserrat, C.; Lancioni, L.; Dolara, P. Urinary excretion of sucrose and fructose as a predictor of sucrose intake in dietary intervention studies. Cancer Epidemiol. Biomark. Prev. 1996, 5, 167–171. [Google Scholar]
- Tasevska, N.; Runswick, S.A.; McTaggart, A.; Bingham, S.A. Urinary Sucrose and Fructose as Biomarkers for Sugar Consumption. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1287–1294. [Google Scholar] [CrossRef]
- Tasevska, N.; Sagi-Kiss, V.; A Palma-Duran, S.; Barrett, B.; Chaloux, M.; Commins, J.; O’brien, D.M.; Johnston, C.S.; Midthune, D.; Kipnis, V.; et al. Investigating the performance of 24-h urinary sucrose and fructose as a biomarker of total sugars intake in US participants—A controlled feeding study. Am. J. Clin. Nutr. 2021, 114, 721–730. [Google Scholar] [CrossRef]
- Intemann, T.; Pigeot, I.; De Henauw, S.; Eiben, G.; Lissner, L.; Krogh, V.; Dereń, K.; Molnár, D.; Moreno, L.A.; Russo, P.; et al. Urinary sucrose and fructose to validate self-reported sugar intake in children and adolescents: Results from the I. Family study. Eur. J. Nutr. 2019, 58, 1247–1258. [Google Scholar] [CrossRef] [PubMed]
- Perrar, I.; Gray, N.; Kuhnle, G.G.; Remer, T.; Buyken, A.E.; Alexy, U. Sugar intake among German adolescents: Trends from 1990 to 2016 based on biomarker excretion in 24-h urine samples. Br. J. Nutr. 2020, 124, 164–172. [Google Scholar] [CrossRef]
- Ramne, S.; Gray, N.; Hellstrand, S.; Brunkwall, L.; Enhörning, S.; Nilsson, P.M.; Engström, G.; Orho-Melander, M.; Ericson, U.; Kuhnle, G.G.C.; et al. Comparing Self-Reported Sugar Intake with the Sucrose and Fructose Biomarker from Overnight Urine Samples in Relation to Cardiometabolic Risk Factors. Front. Nutr. 2020, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Te Morenga, L.; Kruimer, D.; McLean, R.; Sabadel, A.J.M.; van Hale, R.; Tatin, X.; Hindmarsh, J.H.; Mann, J.; Merriman, T. Associations between Sugars Intakes and Urinary Sugars Excretion and Carbon Stable Isotope Ratios in Red Blood Cells as Biomarkers of Sugars Intake in a Predominantly Māori Population. Front. Nutr. 2021, 8, 637267. [Google Scholar] [CrossRef]
- Venti, C.A.; Votruba, S.B.; Franks, P.W.; Krakoff, J.; Salbe, A.D. Reproducibility of ad libitum energy intake with the use of a computerized vending machine system. Am. J. Clin. Nutr. 2010, 91, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Ravussin, E.; Lillioja, S.; Anderson, T.E.; Christin, L.; Bogardus, C. Determinants of 24-hour Energy Expenditure in Man: Methods and Results Using a Respiratory Chamber. J. Clin. Investig. 1986, 78, 1568–1578. [Google Scholar] [CrossRef]
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- Abreu, T.C.; Hulshof, P.J.M.; Boshuizen, H.C.; Trijsburg, L.; Gray, N.; de Vries, J.H.M. Validity Coefficient of Repeated Measurements of Urinary Marker of Sugar Intake Is Comparable to Urinary Nitrogen as Marker of Protein Intake in Free-living Subjects. Cancer Epidemiol. Biomark. Prev. 2021, 30, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Joosen, A.M.C.P.; Kuhnle, G.G.C.; A Runswick, S.; A Bingham, S. Urinary sucrose and fructose as biomarkers of sugar consumption: Comparison of normal weight and obese volunteers. Int. J. Obes. 2008, 32, 1736–1740. [Google Scholar] [CrossRef]
- Freedman, L.S.; Schatzkin, A.; Midthune, D.; Kipnis, V. Dealing with Dietary Measurement Error in Nutritional Cohort Studies. JNCI J. Natl. Cancer Inst. 2011, 103, 1086–1092. [Google Scholar] [CrossRef]
- White, E.; Kushiz, L.H.; Pepe, M.S. The effect of exposure variance and exposure measurement error on study sample size: Implications for the design of epidemiologic studies. J. Clin. Epidemiol. 1994, 47, 873–880. [Google Scholar] [CrossRef]
- FoodData Central [Internet]. Available online: https://fdc.nal.usda.gov/index.html (accessed on 30 January 2024).
- Larrick, B.; Kretser, A.; McKillop, K. Update on “A Partnership for Public Health: USDA Global Branded Food Products Database”. J. Food Compos. Anal. 2022, 105, 104250. [Google Scholar] [CrossRef]
- Tasevska, N.; Palma-Duran, S.A.; Sagi-Kiss, V.; Commins, J.; Barrett, B.; Kipnis, V.; Midthune, D.; O’brien, D.M.; Freedman, L.S. Urinary Sucrose and Fructose from Spot Urine May Be Used as a Predictive Biomarker of Total Sugar Intake–Findings from a Controlled Feeding Study. J. Nutr. 2023, 153, 1816–1824. [Google Scholar] [CrossRef] [PubMed]
| Variable | Total | Female | Male | p-Value |
|---|---|---|---|---|
| Demographics, Body Composition, and other Covariates | ||||
| n (%) | 62 | 25 (40.3) | 37 (59.7) | |
| Age (years) | 39.1 (12.5) | 35.9 (12.1) | 41.2 (12.5) | 0.099 |
| Age Category, n (%) | 0.85 | |||
| 18–30 | 20 (32.3) | 9 (36) | 11 (29.7) | |
| 31–45 | 20 (32.3) | 8 (32) | 12 (32.4) | |
| 45+ | 22 (35.5) | 8 (32) | 14 (37.8) | |
| BMI (kg/m2) | 30.6 (7.6) | 32.2 (7.6) | 29.5 (7.5) | 0.18 |
| BMI Category, n (%) | 0.067 | |||
| Normal weight | 12 (20.6) | 4 (16) | 8 (21.6) | |
| Overweight | 26 (41.3) | 7 (28) | 19 (51.4) | |
| Obese | 24 (38.1) | 14 (56) | 10 (27) | |
| Race/ethnicity, n (%) | 0.017 * | |||
| AI/AN | 36 (58.1%) | 19 (76%) | 17 (45.9%) | |
| AA/Other | 9 (14.5%) | 4 (16%) | 5 (13.5%) | |
| White | 17 (27.4%) | 2 (8%) | 15 (40.5%) | |
| Body Fat (%) | 32.5 (9.1) | 39.8 (6.1) | 27.5 (7.2) | <0.0001 ** |
| Fat-free Mass (kg) | 57.2 (11.6) | 48.2 (7.9) | 63.4 (9.5) | <0.0001 ** |
| Fat Mass (kg) | 28.9 (14.1) | 33.3 (12.4) | 25.9 (14.5) | 0.0423 * |
| Height (cm) | 168.0 (10.1) | 159.2 (4.5) | 174.1 (8.2) | <0.0001 ** |
| Weight (kg) | 86.1 (21.5) | 81.5 (19.5) | 89.3 (22.6) | 0.16 |
| Physical Activity (SPA) | 7.9 (3.4) | 7.5 (3.1) | 8.2 (3.6) | 0.51 |
| Creatinine | 0.8 (0.2) | 0.7 (0.2) | 0.9 (0.1) | <0.0001 ** |
| Dietary Intake | ||||
| Total Sugars (g/d) | 197.7 (78.9) | 160.7 (53.9) | 219.8 (83.8) | 0.0028 ** |
| Non-sugar CHO (g/d) | 214.0 (65.9) | 186.1 (59.4) | 232.9 (64.1) | 0.0052 ** |
| Soda Intake (kcal/d) | 198.5 (193.6) | 169.2 (169.7) | 218.3 (208.1) | 0.33 |
| Total CHO (g/d) | 413.6 (126.7) | 348.9 (91.0) | 457.3 (129.6) | 0.0006 ** |
| Total Energy (kcal/d) | 3141 (916) | 2621 (693) | 3492 (888) | <0.0001 ** |
| Protein Intake (g/d) | 99.3 (32.5) | 77.1 (24.2) | 114.2 (28.7) | <0.0001 ** |
| Fat Intake (g/d) | 123.8 (41.2) | 104.4 (34.2) | 136.8 (40.7) | 0.0018 ** |
| Urine Sugars | ||||
| Urinary Fructose (mg/d) | 72.3 (80.6) | 58.8 (31.0) | 81.9 (101.8) | 0.2774 |
| Urinary Sucrose (mg/d) | 29.6 (23.9) | 22.3 (12.4) | 34.5 (28.5) | 0.0531 |
| Biomarker | ||||
| 24hruSF (mg/d) | 101.7 (91.9) | 79.2 (32.6) | 117.5 (115.0) | 0.1190 |
| N | r log 24hruSF vs. Total Sugars | p-Value | |
|---|---|---|---|
| Sex | |||
| Males | 34 | 0.23 | 0.19 |
| Females | 24 | 0.45 | 0.028 |
| Age category | |||
| 18–30 | 17 | 0.44 | 0.079 |
| 31–45 | 20 | 0.37 | 0.11 |
| >45 | 21 | 0.31 | 0.17 |
| Race/ethnicity | |||
| White | 16 | 0.21 | 0.43 |
| AI/AN | 33 | 0.52 | 0.0023 |
| AA/other | 9 | 0.21 | 0.59 |
| BMI | |||
| Normal | 11 | 0.66 | 0.027 |
| Overweight | 23 | 0.073 | 0.74 |
| Obese | 24 | 0.53 | 0.0076 |
| Variable | Beta Estimate | p-Value | 95% CI Lower | 95% CI Upper |
|---|---|---|---|---|
| Total Sugars Intake (g/d) | 0.0027 | <0.0001 | 0.0016 | 0.0038 |
| Race/ethnicity | ||||
| AI/AN | 0.57 | 0.0017 | 0.22 | 0.91 |
| AA | 0.73 | 0.0097 | 0.18 | 1.28 |
| Other | 0.17 | 0.47 | −0.30 | 0.65 |
| Sex (Male) | 0.0057 | 0.97 | −0.35 | 0.36 |
| Age | −0.0044 | 0.43 | −0.015 | 0.0067 |
| Body fat (%) | −0.026 | 0.015 | −0.047 | −0.0052 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahern, M.M.; Stinson, E.J.; Votruba, S.B.; Krakoff, J.; Tasevska, N. Twenty-Four-Hour Urinary Sugars Biomarker in a Vending Machine Intake Paradigm in a Diverse Population. Nutrients 2024, 16, 610. https://doi.org/10.3390/nu16050610
Ahern MM, Stinson EJ, Votruba SB, Krakoff J, Tasevska N. Twenty-Four-Hour Urinary Sugars Biomarker in a Vending Machine Intake Paradigm in a Diverse Population. Nutrients. 2024; 16(5):610. https://doi.org/10.3390/nu16050610
Chicago/Turabian StyleAhern, Mary M., Emma J. Stinson, Susanne B. Votruba, Jonathan Krakoff, and Natasha Tasevska. 2024. "Twenty-Four-Hour Urinary Sugars Biomarker in a Vending Machine Intake Paradigm in a Diverse Population" Nutrients 16, no. 5: 610. https://doi.org/10.3390/nu16050610
APA StyleAhern, M. M., Stinson, E. J., Votruba, S. B., Krakoff, J., & Tasevska, N. (2024). Twenty-Four-Hour Urinary Sugars Biomarker in a Vending Machine Intake Paradigm in a Diverse Population. Nutrients, 16(5), 610. https://doi.org/10.3390/nu16050610






