Association between Low Energy Availability (LEA) and Impaired Sleep Quality in Young Rugby Players
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
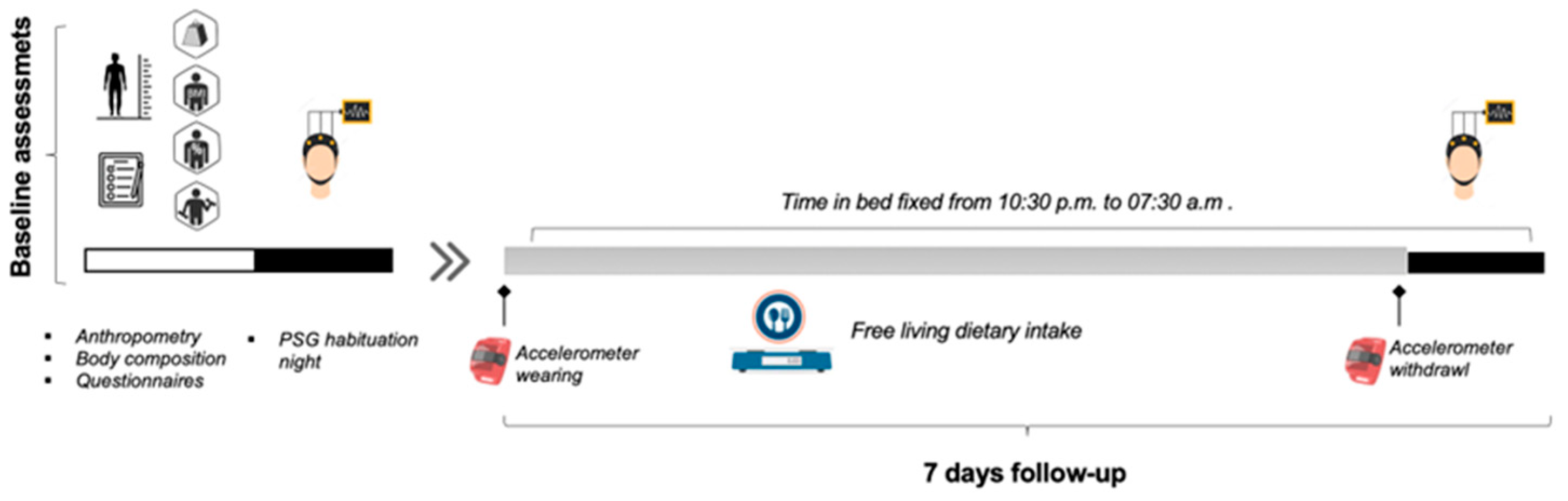
2.2. Study Setting and Procedure
2.3. Measurements
2.3.1. Anthropometric and Body Composition Measurements
2.3.2. Energy Intake (EI)
2.3.3. Exercise Energy Expenditure (EEE)
2.3.4. Energy Availability (EA)
2.3.5. Sleep Assessment
2.4. Statistical Analysis
3. Results
3.1. Participants Characteristics
3.2. Sleep Outcomes among Participants by Energy Availability Status
3.3. Relationship between Energy Availability (EA) and Sleep Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halson, S.L. Sleep in Elite Athletes and Nutritional Interventions to Enhance Sleep. Sport. Med. 2014, 44 (Suppl. S1), S13–S23. [Google Scholar] [CrossRef] [PubMed]
- Fowler, P.M.; Knez, W.; Crowcroft, S.; Mendham, A.E.; Miller, J.; Sargent, C.; Halson, S.; Duffield, R. Greater Effect of East versus West Travel on Jet Lag, Sleep, and Team Sport Performance. Med. Sci. Sports Exerc. 2017, 49, 2548–2561. [Google Scholar] [CrossRef] [PubMed]
- Saidi, O.; Doré, E.; Maso, F.; Mack-Inocentio, D.; Walrand, S.; Pereira, B.; Duché, P. Acute Effect of an Intensified Exercise Program on Subsequent Sleep, Dietary Intake, and Performance in Junior Rugby Players. Eur. J. Appl. Physiol. 2019, 119, 2075–2082. [Google Scholar] [CrossRef]
- Suppiah, H.T.; Low, C.Y.; Choong, G.C.W.; Chia, M. Restricted and Unrestricted Sleep Schedules of Asian Adolescent, High-Level Student Athletes: Effects on Sleep Durations, Marksmanship and Cognitive Performance. Biol. Rhythm Res. 2016, 47, 505–518. [Google Scholar] [CrossRef]
- Suppiah, H.T.; Swinbourne, R.; Wee, J.; Tay, V.; Gastin, P. Sleep Characteristics of Elite Youth Athletes: A Clustering Approach to Optimize Sleep Support Strategies. Int. J. Sports Physiol. Perform. 2021, 16, 1225–1233. [Google Scholar] [CrossRef]
- Milewski, M.D.; Skaggs, D.L.; Bishop, G.A.; Pace, J.L.; Ibrahim, D.A.; Wren, T.A.L.; Barzdukas, A. Chronic Lack of Sleep Is Associated with Increased Sports Injuries in Adolescent Athletes. J. Pediatr. Orthop. 2014, 34, 129–133. [Google Scholar] [CrossRef]
- von Rosen, P.; Frohm, A.; Kottorp, A.; Fridén, C.; Heijne, A. Too Little Sleep and an Unhealthy Diet Could Increase the Risk of Sustaining a New Injury in Adolescent Elite Athletes. Scand. J. Med. Sci. Sport. 2017, 27, 1364–1371. [Google Scholar] [CrossRef]
- Silverberg, N.D.; Berkner, P.D.; Atkins, J.E.; Zafonte, R.; Iverson, G.L. Relationship between Short Sleep Duration and Preseason Concussion Testing. Clin. J. Sport Med. 2016, 26, 226–231. [Google Scholar] [CrossRef]
- Taylor, L.; Chrismas, B.C.R.; Dascombe, B.; Chamari, K.; Fowler, P.M. The Importance of Monitoring Sleep within Adolescent Athletes: Athletic, Academic, and Health Considerations. Front. Physiol. 2016, 7, 101. [Google Scholar] [CrossRef]
- Gupta, L.; Morgan, K.; Gilchrist, S. Does Elite Sport Degrade Sleep Quality? A Systematic Review. Sports Med. 2017, 47, 1317–1333. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.S.H.; Teo, W.P.; Warmington, S.A. Effects of Training and Competition on the Sleep of Elite Athletes: A Systematic Review and Meta-Analysis. Br. J. Sports Med. 2019, 53, 513–522. [Google Scholar] [CrossRef]
- Desbrow, B.; McCormack, J.; Burke, L.M.; Cox, G.R.; Fallon, K.; Hislop, M.; Logan, R.; Marino, N.; Sawyer, S.M.; Shaw, G.; et al. Sports Dietitians Australia Position Statement: Sports Nutrition for the Adolescent Athlete. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 570–584. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Wilborn, C.D.; Roberts, M.D.; Smith-Ryan, A.; Kleiner, S.M.; Jäger, R.; Collins, R.; Cooke, M.; Davis, J.N.; Galvan, E.; et al. ISSN Exercise & Sports Nutrition Review Update: Research & Recommendations. J. Int. Soc. Sports Nutr. 2018, 15, 38. [Google Scholar] [CrossRef]
- Bell, M.; Ghatora, R.; Retsidou, M.I.; Chatzigianni, E.; Klentrou, P. Energy Expenditure, Dietary Energy Intake, and Nutritional Supplements in Adolescent Volleyball Athletes versus Nonathletic Controls. Nutrients 2023, 15, 1788. [Google Scholar] [CrossRef]
- Burrows, T.; Harries, S.K.; Williams, R.L.; Lum, C.; Callister, R. The Diet Quality of Competitive Adolescent Male Rugby Union Players with Energy Balance Estimated Using Different Physical Activity Coefficients. Nutrients 2016, 8, 548. [Google Scholar] [CrossRef]
- Mielgo-Ayuso, J.; Zourdos, M.C.; Calleja-González, J.; Urdampilleta, A.; Ostojic, S.M. Dietary Intake Habits and Controlled Training on Body Composition and Strength in Elite Female Volleyball Players during the Season. Appl. Physiol. Nutr. Metab. 2015, 40, 827–834. [Google Scholar] [CrossRef]
- Holway, F.E.; Spriet, L.L. Sport-Specific Nutrition: Practical Strategies for Team Sports. J. Sports Sci. 2011, 29, S115–S125. [Google Scholar] [CrossRef]
- Reid, M.M.; Duffield, R.; Minett, G.M.; Sibte, N.; Murphy, A.P.; Baker, J. Physiological, Perceptual, and Technical Responses to on-Court Tennis Training on Hard and Clay Courts. J. Strength Cond. Res. 2013, 27, 1487–1495. [Google Scholar] [CrossRef]
- Lastella, M.; Roach, G.D.; Vincent, G.E.; Scanlan, A.T.; Halson, S.L.; Sargent, C. The Impact of Training Load on Sleep during a 14-Day Training Camp in Elite, Adolescent, Female Basketball Players. Int. J. Sports Physiol. Perform. 2020, 15, 724–730. [Google Scholar] [CrossRef]
- Dave, S.C.; Fisher, M. Relative Energy Deficiency in Sport (RED-S). Curr. Probl. Pediatr. Adolesc. Health Care 2022, 52, 101242. [Google Scholar] [CrossRef]
- Tarnowski, C.A.; Wardle, S.L.; O’Leary, T.J.; Gifford, R.M.; Greeves, J.P.; Wallis, G.A. Measurement of Energy Intake Using the Principle of Energy Balance Overcomes a Critical Limitation in the Assessment of Energy Availability. Sport. Med.—Open 2023, 9, 16. [Google Scholar] [CrossRef]
- Jurov, I.; Keay, N.; Spudić, D.; Rauter, S. Inducing Low Energy Availability in Trained Endurance Male Athletes Results in Poorer Explosive Power. Eur. J. Appl. Physiol. 2022, 122, 503–513. [Google Scholar] [CrossRef]
- Tornberg, Å.B.; Melin, A.; Koivula, F.M.; Johansson, A.; Skouby, S.; Faber, J.; Sjödin, A. Reduced Neuromuscular Performance in Amenorrheic Elite Endurance Athletes. Med. Sci. Sports Exerc. 2017, 49, 2478–2485. [Google Scholar] [CrossRef]
- Mountjoy, M.; Ackerman, K.E.; Bailey, D.M.; Burke, L.M.; Constantini, N.; Hackney, A.C.; Heikura, I.A.; Melin, A.; Pensgaard, A.M.; Stellingwerff, T.; et al. 2023 International Olympic Committee’s (IOC) Consensus Statement on Relative Energy Deficiency in Sport (REDs). Br. J. Sports Med. 2023, 57, 1073–1097. [Google Scholar] [CrossRef] [PubMed]
- Pardue, A.; Trexler, E.T.; Sprod, L.K. Case Study: Unfavorable but Transient Physiological Changes during Contest Preparation in a Drug-Free Male Bodybuilder. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Gillbanks, L.; Mountjoy, M.; Filbay, S.R. Lightweight Rowers’ Perspectives of Living with Relative Energy Deficiency in Sport (RED-S). PLoS ONE 2022, 17, e0265268. [Google Scholar] [CrossRef] [PubMed]
- Garron, T.; Klein, D.J. Male Army ROTC Cadets Fail to Meet Military Dietary Reference Intakes and Exhibit a High Prevalence of Low Energy Availability and Poor Sleep Quality. J. Funct. Morphol. Kinesiol. 2023, 8, 95. [Google Scholar] [CrossRef]
- Thomas, C.; Langan-Evans, C.; Germaine, M.; Artukovic, M.; Jones, H.; Whitworth-Turner, C.; Close, G.L.; Louis, J. Case Report: Effect of Low Energy Availability and Training Load on Sleep in a Male Combat Sport Athlete. Front. Sport. Act. Living 2023, 4, 981755. [Google Scholar] [CrossRef] [PubMed]
- Saidi, O.; Pereira, B.; Peyrel, P.; Maso, F.; Doré, E.; Rochette, E.; Ratel, S.; Walrand, S.; Duché, P. Sleep Pattern and Staging in Elite Adolescent Rugby Players during the In-Season Competitive Phase Compared to an Age Matched Non-Athlete Population. Eur. J. Sport Sci. 2022, 22, 499–510. [Google Scholar] [CrossRef]
- Shahid, A.; Wilkinson, K.; Marcu, S.; Shapiro, C.M. STOP, THAT and One Hundred Other Sleep Scales; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Evenson, K.R.; Catellier, D.J.; Gill, K.; Ondrak, K.S.; McMurray, R.G. Calibration of Two Objective Measures of Physical Activity for Children. J. Sports Sci. 2008, 26, 1557–1565. [Google Scholar] [CrossRef]
- Trost, S.G.; Loprinzi, P.D.; Moore, R.; Pfeiffer, K.A. Comparison of Accelerometer Cut Points for Predicting Activity Intensity in Youth. Med. Sci. Sports Exerc. 2011, 43, 1360–1368. [Google Scholar] [CrossRef]
- Loucks, A.B.; Kiens, B.; Wright, H.H. Energy Availability in Athletes. J. Sports Sci. 2011, 29, S7–S15. [Google Scholar] [CrossRef]
- Levendowski, D.J.; Popovic, D.; Berka, C.; Westbrook, P.R. Retrospective Cross-Validation of Automated Sleep Staging Using Electroocular Recording in Patients with and without Sleep Disordered Breathing. Int. Arch. Med. 2012, 5, 21. [Google Scholar] [CrossRef]
- Popovic, D.; Khoo, M.; Westbrook, P. Automatic Scoring of Sleep Stages and Cortical Arousals Using Two Electrodes on the Forehead: Validation in Healthy Adults. J. Sleep Res. 2014, 23, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Levendowski, D.J.; Ferini-Strambi, L.; Gamaldo, C.; Cetel, M.; Rosenberg, R.; Westbrook, P.R. The Accuracy, Night-To-Night Variability, and Stability of Frontopolar Sleep Electroencephalography Biomarkers. J. Clin. Sleep Med. 2017, 13, 791–803. [Google Scholar] [CrossRef]
- Berry, R.B.; Brooks, R.; Gamaldo, C.; Harding, S.M.; Lloyd, R.M.; Quan, S.F.; Troester, M.T.; Vaughn, B.V. AASM Scoring Manual Updates for 2017 (Version 2.4). J. Clin. Sleep Med. 2017, 13, 665–666. [Google Scholar] [CrossRef]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. American College of Sports Medicine Joint Position Statement. Nutrition and Athletic Performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar] [CrossRef]
- Koehler, K.; Achtzehn, S.; Braun, H.; Mester, J.; Schaenzer, W. Comparison of Self-Reported Energy Availability and Metabolic Hormones to Assess Adequacy of Dietary Energy Intake in Young Elite Athletes. Appl. Physiol. Nutr. Metab. 2013, 38, 725–733. [Google Scholar] [CrossRef]
- McCormack, W.P.; Shoepe, T.C.; LaBrie, J.; Almstedt, H.C. Bone Mineral Density, Energy Availability, and Dietary Restraint in Collegiate Cross-Country Runners and Non-Running Controls. Eur. J. Appl. Physiol. 2019, 119, 1747–1756. [Google Scholar] [CrossRef]
- Areta, J.L.; Taylor, H.L.; Koehler, K. Low Energy Availability: History, Definition and Evidence of Its Endocrine, Metabolic and Physiological Effects in Prospective Studies in Females and Males. Eur. J. Appl. Physiol. 2021, 121, 1–21. [Google Scholar] [CrossRef]
- Elliott-Sale, K.J.; Tenforde, A.S.; Parziale, A.L.; Holtzman, B.; Ackerman, K.E. Endocrine Effects of Relative Energy Deficiency in Sport. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 335–349. [Google Scholar] [CrossRef]
- Nattiv, A.; De Souza, M.J.; Koltun, K.J.; Misra, M.; Kussman, A.; Williams, N.I.; Barrack, M.T.; Kraus, E.; Joy, E.; Fredericson, M. The Male Athlete Triad—A Consensus Statement From the Female and Male Athlete Triad Coalition Part 1: Definition and Scientific Basis. Clin. J. Sport Med. 2021, 31, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Fredericson, M.; Kussman, A.; Misra, M.; Barrack, M.T.; De Souza, M.J.; Kraus, E.; Koltun, K.J.; Williams, N.I.; Joy, E.; Nattiv, A. The Male Athlete Triad—A Consensus Statement From the Female and Male Athlete Triad Coalition Part II: Diagnosis, Treatment, and Return-To-Play. Clin. J. Sport Med. 2021, 31, 349–366. [Google Scholar] [CrossRef] [PubMed]
- Koehler, K.; Hoerner, N.R.; Gibbs, J.C.; Zinner, C.; Braun, H.; De Souza, M.J.; Schaenzer, W. Low Energy Availability in Exercising Men Is Associated with Reduced Leptin and Insulin but Not with Changes in Other Metabolic Hormones. J. Sports Sci. 2016, 34, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Mäestu, J.; Jürimäe, J.; Valter, I.; Jürimäe, T. Increases in Ghrelin and Decreases in Leptin without Altering Adiponectin during Extreme Weight Loss in Male Competitive Bodybuilders. Metabolism. 2008, 57, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Ruddick-Collins, L.C.; Morgan, P.J.; Johnstone, A.M. Mealtime: A Circadian Disruptor and Determinant of Energy Balance? J. Neuroendocrinol. 2020, 32, e12886. [Google Scholar] [CrossRef] [PubMed]
- Toshinai, K.; Date, Y.; Murakami, N.; Shimada, M.; Mondal, M.S.; Shimbara, T.; Guan, J.L.; Wang, Q.P.; Funahashi, H.; Sakurai, T.; et al. Ghrelin-Induced Food Intake Is Mediated via the Orexin Pathway. Endocrinology 2003, 144, 1506–1512. [Google Scholar] [CrossRef]
- Mogavero, M.P.; Godos, J.; Grosso, G.; Caraci, F.; Ferri, R. Rethinking the Role of Orexin in the Regulation of REM Sleep and Appetite. Nutrients 2023, 15, 3679. [Google Scholar] [CrossRef] [PubMed]
- Falkenberg, E.; Aisbett, B.; Lastella, M.; Roberts, S.; Condo, D. Nutrient Intake, Meal Timing and Sleep in Elite Male Australian Football Players. J. Sci. Med. Sport 2021, 24, 7–12. [Google Scholar] [CrossRef]
- Sakurai, T. Roles of Orexin/Hypocretin in Regulation of Sleep/Wakefulness and Energy Homeostasis. Sleep Med. Rev. 2005, 9, 231–241. [Google Scholar] [CrossRef]
- Sakurai, T.; Mieda, M.; Tsujino, N. The orexin system: Roles in sleep/wake regulation. Ann. N. Y. Acad. Sci. 2010, 1200, 149–161. [Google Scholar] [CrossRef]
- St-Onge, M.P. The Role of Sleep Duration in the Regulation of Energy Balance: Effects on Energy Intakes and Expenditure. J. Clin. Sleep Med. 2013, 9, 73–80. [Google Scholar] [CrossRef]
- Kojima, C.; Ishibashi, A.Y.A.; Tanabe, Y.; Iwayama, K.; Kamei, A.; Takahashi, H.; Goto, K. Muscle Glycogen Content during Endurance Training under Low Energy Availability. Med. Sci. Sports Exerc. 2020, 52, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, M.; Elliott-Sale, K.J.; Parsons, A.; Tang, J.C.Y.; Greeves, J.P.; Fraser, W.D.; Sale, C. Effects of Reduced Energy Availability on Bone Metabolism in Women and Men. Bone 2017, 105, 191–199. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.P.; Cherta-Murillo, A.; Darimont, C.; Mantantzis, K.; Martin, F.P.; Owen, L. The Interrelationship between Sleep, Diet, and Glucose Metabolism. Sleep Med. Rev. 2023, 69, 101788. [Google Scholar] [CrossRef] [PubMed]
- Jauch-Chara, K.; Schultes, B. Sleep and the Response to Hypoglycaemia. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 801–815. [Google Scholar] [CrossRef] [PubMed]
- Jauch-Chara, K.; Hallschmid, M.; Gais, S.; Oltmanns, K.M.; Peters, A.; Born, J.; Schultes, B. Awakening and Counterregulatory Response to Hypoglycemia during Early and Late Sleep. Diabetes 2007, 56, 1938–1942. [Google Scholar] [CrossRef][Green Version]
- Souabni, M.; Hammouda, O.; Souabni, M.; Romdhani, M.; Souissi, W.; Ammar, A.; Driss, T. Nap Improved Game-Related Technical Performance and Physiological Response during Small-Sided Basketball Game in Professional Players. Biol. Sport 2023, 40, 389–397. [Google Scholar] [CrossRef]
- Souabni, M.; Hammouda, O.; Romdhani, M.; Trabelsi, K.; Ammar, A.; Driss, T. Benefits of Daytime Napping Opportunity on Physical and Cognitive Performances in Physically Active Participants: A Systematic Review. Sport. Med. 2021, 51, 2115–2146. [Google Scholar] [CrossRef]


| Total | LEA (n = 20) EA < 30 kcal·kg FFM−1·day−1 | REA (n = 15) 30 ≤ EA < 45 kcal·kg FFM−1·day−1 | OEA (n = 7) EA ≥ 45 kcal·kg FFM−1·day−1 | p | η2 | |
|---|---|---|---|---|---|---|
| Age (year) | 16.2 (0.88) | 16 (0.74) | 16.4 (0.97) | 16.2 (1.12) | 0.389 | 0.04 |
| Height (cm) | 181 (6.76) | 180 (6.72) | 182 (6.77) | 183 (7.48) | 0.643 | 0.02 |
| BM (kg) | 82.2 (11.3) | 86.6 (11.9) a | 79.3 (9.19) | 73 (4.84) a | 0.007 | 0.20 |
| BMI (kg·m−2) | 25.1 (3.40) | 26.7 (3.36) ab | 24.1 (2.76) b | 21.9 (0.76) a | 0.001 | 0.28 |
| BF (%) | 15.8 (5.36) | 18.8 (5.13) ab | 13.1 (3.46) b | 10.9 (1.56) a | <0.001 | 0.38 |
| FFM (kg) | 68.8 (7.54) | 69.7 (8.50) | 68.8 (6.70) | 65.8 (5.29) | 0.493 | 0.03 |
| EI (kcal·day−1) | 3551 (567) | 3164 (314) ab | 3811 (448) bc | 4373 (149) ac | <0.001 | 0.64 |
| EI (kcal·kg−1·day−1) | 44.3 (10.5) | 37.3 (6.55) ab | 58.5 (6.71) bc | 60.1 (4.14) ac | <0.001 | 0.65 |
| EEE (kcal·day−1) | 1552 (196) | 1583 (185) a | 1589 (150) b | 1360 (233) ab | 0.016 | 0.17 |
| EA (kcal·kg FFM−1·day−1) | 29.3 (9.14) | 22.8 (4.16) ab | 32.3 (4.88) bc | 45.8 (0.61) ac | <0.001 | 0.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saidi, O.; Souabni, M.; Del Sordo, G.C.; Maviel, C.; Peyrel, P.; Maso, F.; Vercruyssen, F.; Duché, P. Association between Low Energy Availability (LEA) and Impaired Sleep Quality in Young Rugby Players. Nutrients 2024, 16, 609. https://doi.org/10.3390/nu16050609
Saidi O, Souabni M, Del Sordo GC, Maviel C, Peyrel P, Maso F, Vercruyssen F, Duché P. Association between Low Energy Availability (LEA) and Impaired Sleep Quality in Young Rugby Players. Nutrients. 2024; 16(5):609. https://doi.org/10.3390/nu16050609
Chicago/Turabian StyleSaidi, Oussama, Maher Souabni, Giovanna C. Del Sordo, Clément Maviel, Paul Peyrel, Freddy Maso, Fabrice Vercruyssen, and Pascale Duché. 2024. "Association between Low Energy Availability (LEA) and Impaired Sleep Quality in Young Rugby Players" Nutrients 16, no. 5: 609. https://doi.org/10.3390/nu16050609
APA StyleSaidi, O., Souabni, M., Del Sordo, G. C., Maviel, C., Peyrel, P., Maso, F., Vercruyssen, F., & Duché, P. (2024). Association between Low Energy Availability (LEA) and Impaired Sleep Quality in Young Rugby Players. Nutrients, 16(5), 609. https://doi.org/10.3390/nu16050609






