Bulbils of Aerial Yam Attenuate Ethanol-Induced Hepatotoxicity in HepG2 Cells through Inhibition of Oxidative Stress by Activation of the Nuclear Factor Erythroid-2-Related Factor 2 Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Bulbil of Yam (BY) Extract
2.2. Cell Culture and the Treatment of Aerial Bulbil of Yam and EtOH
2.3. Cell Viability Assay
2.4. Hoechst 33342 Staining
2.5. Measurement of Mitochondrial Membrane Potential (MMP)
2.6. Western Blot Analysis
2.7. Nuclear Fractionation of HepG2 Cells
2.8. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.9. DPPH Assay for Antioxidant Activity
2.10. Total Amount of Phenolic Compounds in the BY Extract
2.11. Statistical Analysis
3. Results
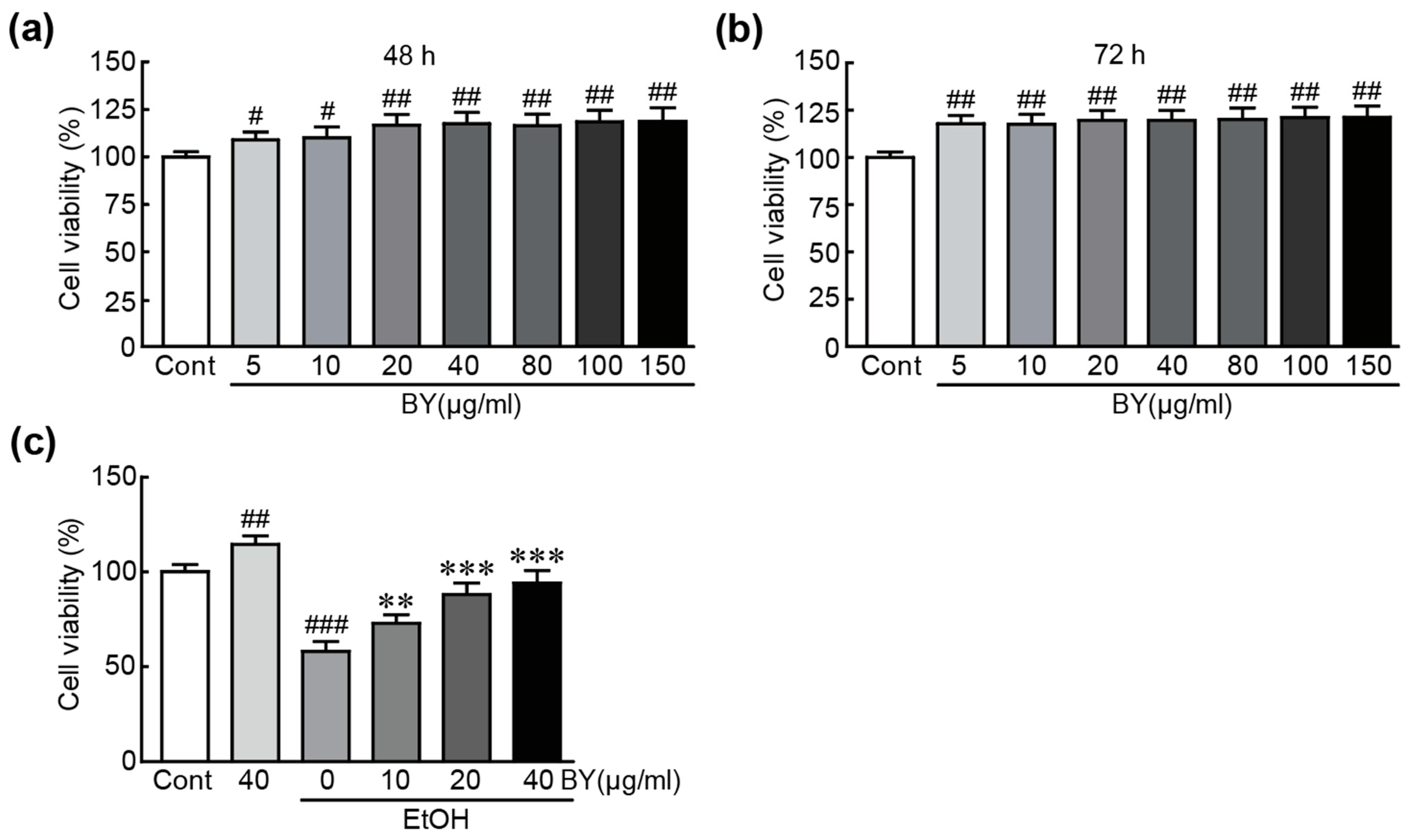
3.1. BY Extract Rescues HepG2 Liver Cells from EtOH-Induced Cytotoxicity
3.2. BY Extract Suppresses EtOH-Induced Apoptotic Responses in HepG2 Cells
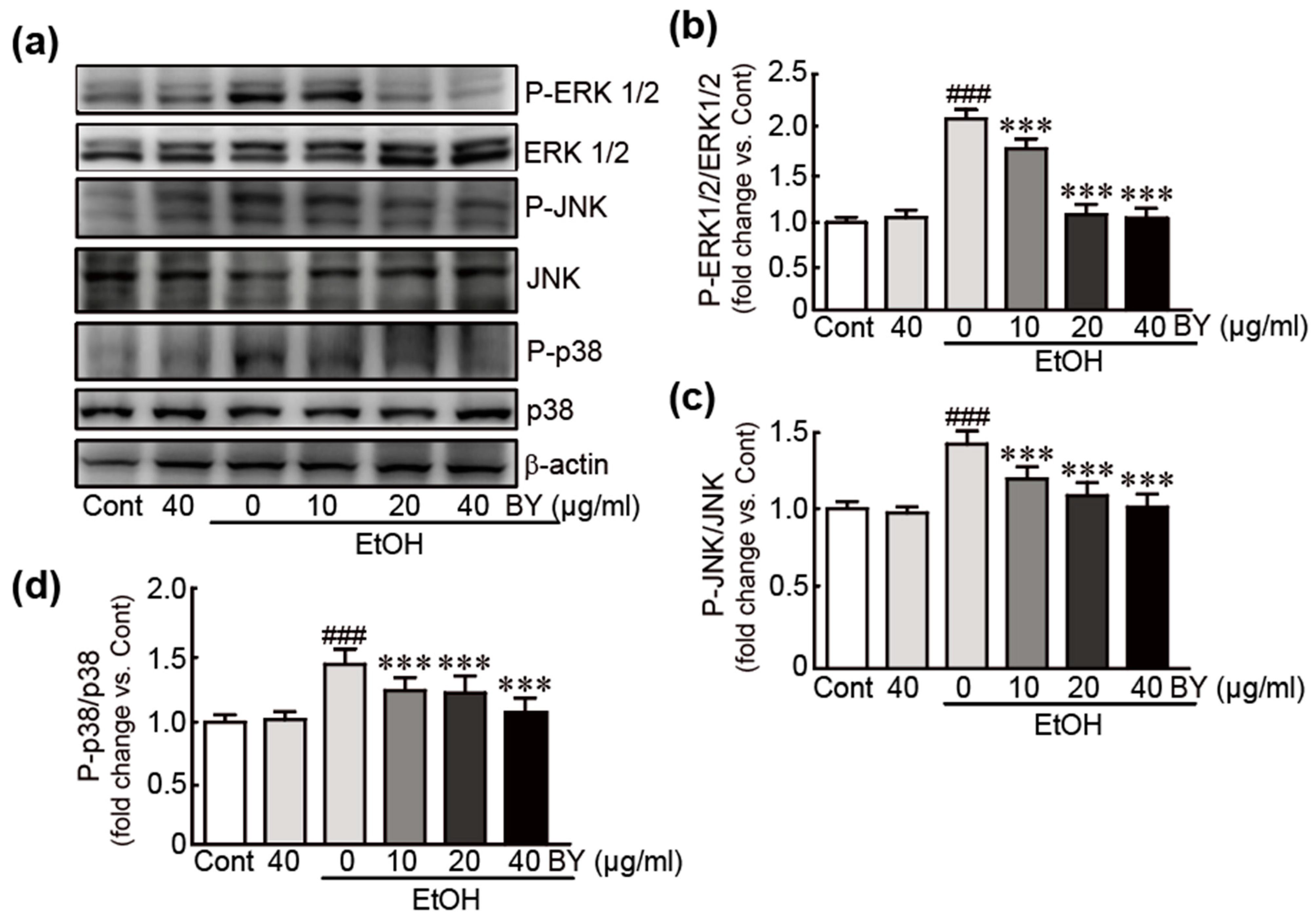
3.3. BY Extract Inhibits the Activation of MAPK Signaling Induced by EtOH in HepG2 Cells
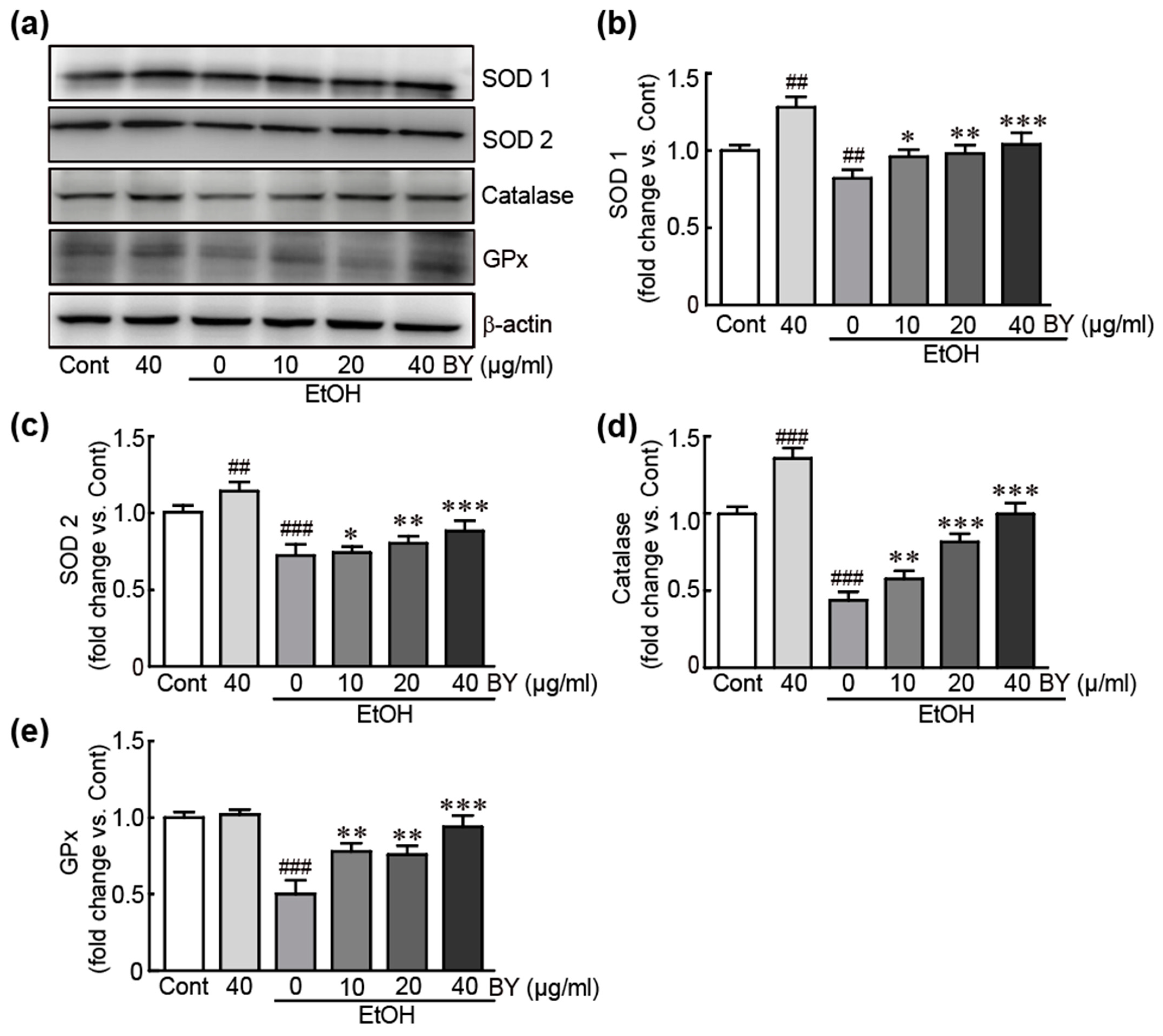
3.4. BY Extract Inhibits EtOH-Induced Oxidative Stress in HepG2 Cells
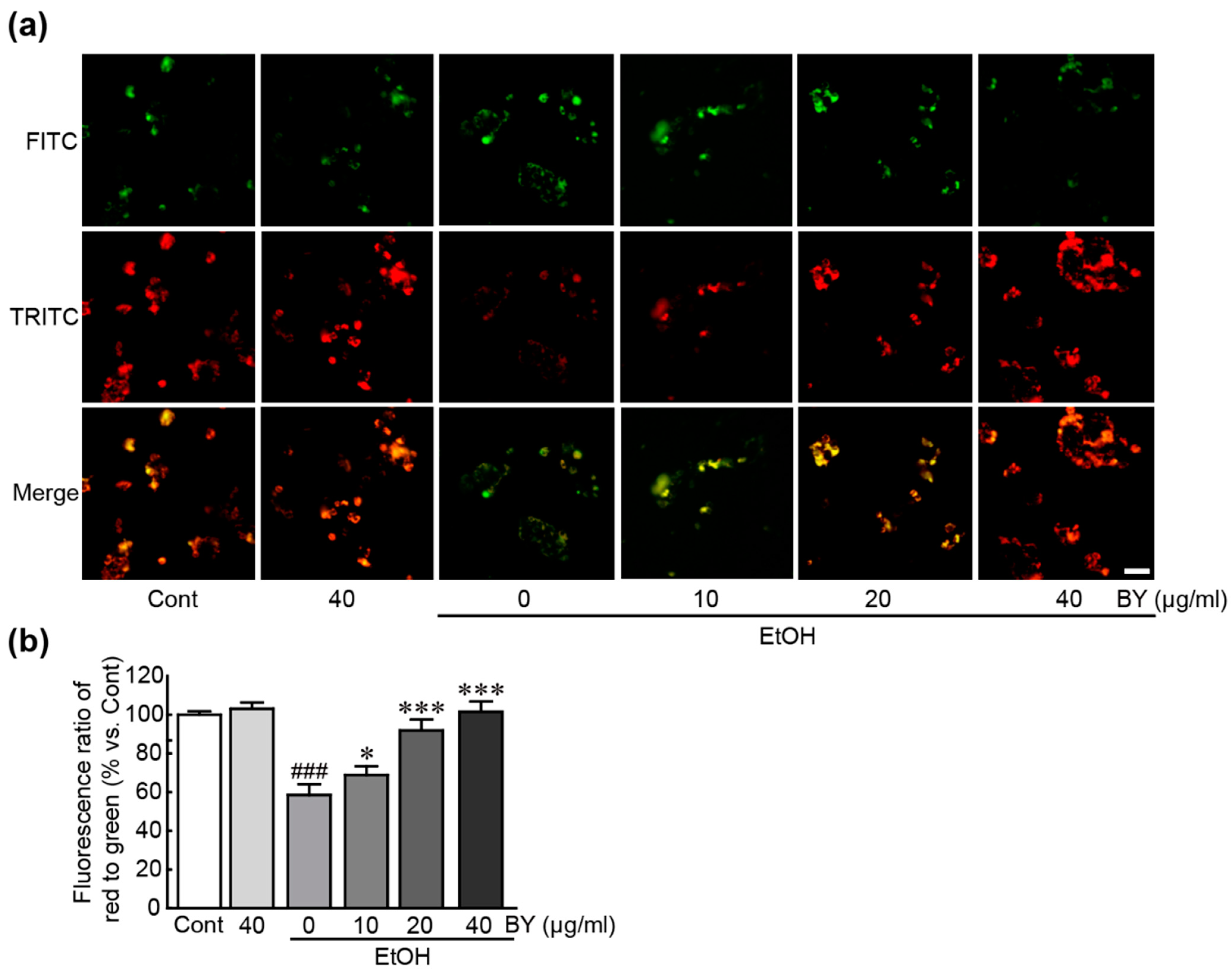
3.5. BY Extract Attenuates Mitochondrial Dysfunction Induced by EtOH in HepG2 Cells
3.6. BY Extract Inhibits the Activation of Endoplasmic Reticulum (ER) Stress Induced by EtOH in HepG2 Cells
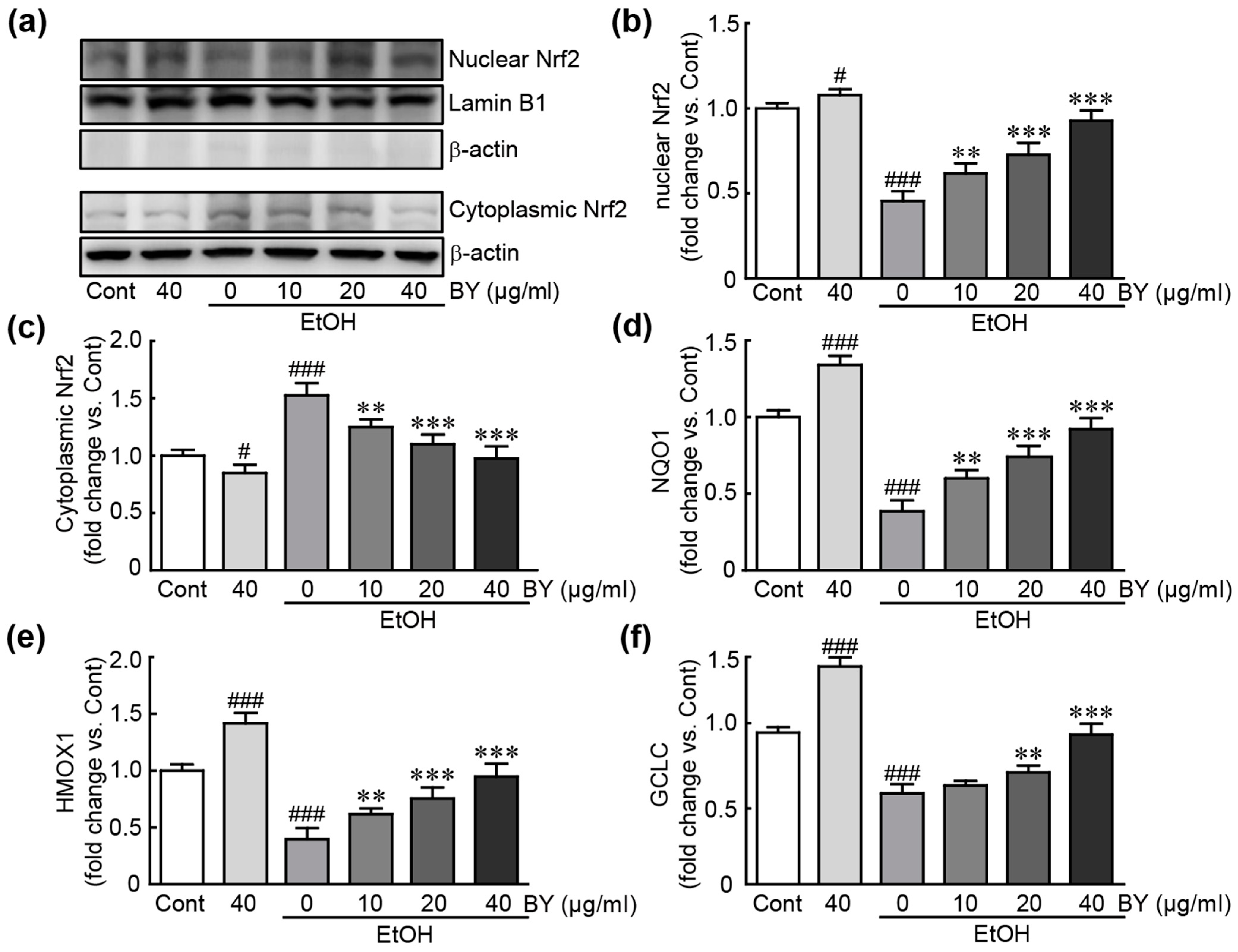
3.7. BY Extract Activates Nrf2-Related Signaling Pathway in EtOH-Treated HepG2 Cells
3.8. DPPH Radical Scavenging Activity and Total Phenolic Compounds in BY Extract
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeng, X.; Yuan, X.; Cai, Q.; Tang, C.; Gao, J. Circular RNA as An Epigenetic Regulator in Chronic Liver Diseases. Cells 2021, 10, 1945. [Google Scholar] [CrossRef]
- Thomes, P.G.; Rasineni, K.; Saraswathi, V.; Kharbanda, K.K.; Clemens, D.L.; Sweeney, S.A.; Kubik, J.L.; Donohue, T.M., Jr.; Casey, C.A. Natural Recovery by the Liver and Other Organs after Chronic Alcohol Use. Alcohol. Res. 2021, 41, 05. [Google Scholar] [CrossRef]
- WHO. Global Status Report on Alcohol and Health 2018; World Health Organization: Rome, Italy, 2018. [Google Scholar]
- Pohl, K.; Moodley, P.; Dhanda, A.D. Alcohol’s Impact on the Gut and Liver. Nutrients 2021, 13, 3170. [Google Scholar] [CrossRef]
- Roth, N.C.; Qin, J. Histopathology of Alcohol-Related Liver Diseases. Clin. Liver. Dis. 2019, 23, 11–23. [Google Scholar] [CrossRef]
- Chu, H.; Duan, Y.; Lang, S.; Jiang, L.; Wang, Y.; Llorente, C.; Liu, J.; Mogavero, S.; Bosques-Padilla, F.; Abraldes, J.G.; et al. The Candida albicans exotoxin candidalysin promotes alcohol-associated liver disease. J. Hepatol. 2020, 72, 391–400. [Google Scholar] [CrossRef]
- Sukketsiri, W.; Sawangjaroen, K.; Tanasawet, S. Anti-apoptotic effects of phyllanthin against alcohol-induced liver cell death. Trop. J. Pharm. Res. 2016, 15, 981–988. [Google Scholar] [CrossRef]
- Schuppan, D.; Afdhal, N.H. Liver cirrhosis. Lancet 2008, 371, 838–851. [Google Scholar] [CrossRef]
- Mathurin, P.; O’Grady, J.; Carithers, R.L.; Phillips, M.; Louvet, A.; Mendenhall, C.L.; Ramond, M.J.; Naveau, S.; Maddrey, W.C.; Morgan, T.R. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: Meta-analysis of individual patient data. Gut 2011, 60, 255–260. [Google Scholar] [CrossRef]
- Louvet, A.; Wartel, F.; Castel, H.; Dharancy, S.; Hollebecque, A.; Canva-Delcambre, V.; Deltenre, P.; Mathurin, P. Infection in patients with severe alcoholic hepatitis treated with steroids: Early response to therapy is the key factor. Gastroenterology 2009, 137, 541–548. [Google Scholar] [CrossRef]
- Naveau, S.; Chollet-Martin, S.; Dharancy, S.; Mathurin, P.; Jouet, P.; Piquet, M.A.; Davion, T.; Oberti, F.; Broet, P.; Emilie, D.; et al. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology 2004, 39, 1390–1397. [Google Scholar] [CrossRef]
- Mathurin, P. Early liver transplantation for acute alcoholic hepatitis: We can’t say no. J. Hepatol. 2021, 75, 718–722. [Google Scholar] [CrossRef]
- Luo, G.F.; Podolyan, A.; Kidanemariam, D.B.; Pilotti, C.; Houliston, G.; Sukal, A.C. A Review of Viruses Infecting Yam (Dioscorea spp.). Viruses 2022, 14, 662. [Google Scholar] [CrossRef]
- Chiang, C.T.; Way, T.D.; Tsai, S.J.; Lin, J.K. Diosgenin, a naturally occurring steroid, suppresses fatty acid synthase expression in HER2-overexpressing breast cancer cells through modulating Akt, mTOR and JNK phosphorylation. FEBS Lett. 2007, 581, 5735–5742. [Google Scholar] [CrossRef]
- Chen, H.; Wang, C.; Chang, C.; Wang, T. Effects of Taiwanese yam (Dioscorea japonica Thunb var. pseudojaponica Yamamoto) on upper gut function and lipid metabolism in Balb/c mice. Nutrition 2003, 19, 646–651. [Google Scholar] [CrossRef]
- Guo, X.X.; Sha, X.H.; Liu, J.; Cai, S.B.; Wang, Y.; Ji, B.P. Chinese Purple Yam (Dioscorea alata L.) Extracts Inhibit Diabetes-Related Enzymes and Protect HepG2 Cells Against Oxidative Stress and Insulin Resistance Induced by FFA. Food Sci. Technol. Res. 2015, 21, 677–683. [Google Scholar] [CrossRef]
- Amat, N.; Amat, R.; Abdureyim, S.; Hoxur, P.; Osman, Z.; Mamut, D.; Kijjoa, A. Aqueous extract of dioscorea opposita thunb. normalizes the hypertension in 2K1C hypertensive rats. BMC Complement. Altern. Med. 2014, 14, 36. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Fan, Y.; Man, S.; Liu, Z.; Gao, W.; Wang, T. Antioxidant and Antitumor Activities of the Extracts from Chinese Yam (Dioscorea opposite Thunb.) Flesh and Peel and the Effective Compounds. J. Food Sci. 2016, 81, H1553–H1564. [Google Scholar] [CrossRef]
- Perera, P.K.; Li, Y. Functional herbal food ingredients used in type 2 diabetes mellitus. Pharmacogn. Rev. 2012, 6, 37–45. [Google Scholar] [CrossRef]
- Wang, C.C.L.; Hess, C.N.; Hiatt, W.R.; Goldfine, A.B. Clinical Update: Cardiovascular Disease in Diabetes Mellitus Atherosclerotic Cardiovascular Disease and Heart Failure in Type 2 Diabetes Mellitus—Mechanisms, Management, and Clinical Considerations. Circulation 2016, 133, 2459–2502. [Google Scholar] [CrossRef]
- Huong, P.T.; Lee, C.H.; Li, M.H.; Lee, M.Y.; Kim, J.K.; Lee, S.M.; Seon, J.H.; Lee, D.C.; Jeon, Y.J. Characterization and immunopotentiating effects of the glycoprotein isolated from dioscorea batatas. Korean J. Physiol. Pharmacol. 2011, 15, 101–106. [Google Scholar] [CrossRef]
- Epping, J.; Laibach, N. An underutilized orphan tuber crop-Chinese yam: A review. Planta 2020, 252, 58. [Google Scholar] [CrossRef]
- Edwards, A.L.; Jenkins, R.L.; Davenport, L.J.; Duke, J.A. Presence of diosgenin in Dioscorea batatas (Dioscoreaceae). Econ. Bot. 2002, 56, 204–206. [Google Scholar] [CrossRef]
- Fu, Y.C.; Ferng, L.H.A.; Huang, P.Y. Quantitative analysis of allantoin and allantoic acid in yam tuber, mucilage, skin and bulbil of the Dioscorea species. Food Chem. 2006, 94, 541–549. [Google Scholar] [CrossRef]
- Kim, M.J.; Son, S.Y.; Jeon, S.G.; Kim, J.G.; Lee, C.H. Metabolite Profiling of Dioscorea (Yam) Leaves to Identify Bioactive Compounds Reveals Their Potential as Renewable Resources. Plant 2021, 10, 1751. [Google Scholar] [CrossRef]
- Zhou, L.; Shi, X.; Ren, X.; Qin, Z. Chemical composition and antioxidant activity of phenolic compounds from Dioscorea (Yam) leaves. Pak. J. Pharm. Sci. 2018, 31, 1031–1038. [Google Scholar]
- Ikiriza, H.; Ogwang, P.E.; Peter, E.L.; Hedmon, O.; Tolo, C.U.; Abubaker, M.; Abdalla, A.A.M. Dioscorea bulbifera, a highly threatened African medicinal plant, a review. Cogent Biol. 2019, 5, 1631561. [Google Scholar] [CrossRef]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Marzioni, D.; Mazzucchelli, R.; Roberta, M. Cellular Modulators of the NRF2/KEAP1 Signaling Pathway in Prostate Cancer. Front. Biosci. 2023, 28, 143. [Google Scholar] [CrossRef]
- Xia, L.; Ma, W.; Afrashteh, A.; Sajadi, M.A.; Fakheri, H.; Valilo, M. The nuclear factor erythroid 2-related factor 2/p53 axis in breast cancer. Biochem. Med. 2023, 33, 030504. [Google Scholar] [CrossRef]
- De Plano, L.M.; Calabrese, G.; Rizzo, M.G.; Oddo, S.; Caccamo, A. The Role of the Transcription Factor Nrf2 in Alzheimer’s Disease: Therapeutic Opportunities. Biomolecules 2023, 13, 549. [Google Scholar] [CrossRef]
- Aroor, A.R.; Shukla, S.D. MAP kinase signaling in diverse effects of ethanol. Life Sci. 2004, 74, 2339–2364. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta. Mol. Cell. Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free-Radical Method to Evaluate Antioxidant Activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2005, 579, 200–213. [Google Scholar] [CrossRef]
- Mbiantcha, M.; Kamanyi, A.; Teponno, R.B.; Tapondjou, A.L.; Watcho, P.; Nguelefack, T.B. Analgesic and Anti-Inflammatory Properties of Extracts from the Bulbils of Dioscorea bulbifera L. var sativa (Dioscoreaceae) in Mice and Rats. Evid.-Based Complement. Altern. Med. 2011, 2011, 912935. [Google Scholar] [CrossRef]
- Lawan, A.; Bennett, A.M. Mitogen-Activated Protein Kinase Regulation in Hepatic Metabolism. Trends. Endocrinol. Metab. 2017, 28, 868–878. [Google Scholar] [CrossRef]
- Mandrekar, P.; Szabo, G. Signalling pathways in alcohol-induced liver inflammation. J. Hepatol. 2009, 50, 1258–1266. [Google Scholar] [CrossRef]
- Ambade, A.; Mandrekar, P. Oxidative stress and inflammation: Essential partners in alcoholic liver disease. Int. J. Hepatol. 2012, 2012, 853175. [Google Scholar] [CrossRef]
- Lieber, C.S. ALCOHOL: Its metabolism and interaction with nutrients. Annu. Rev. Nutr. 2000, 20, 395–430. [Google Scholar] [CrossRef]
- Leung, T.M.; Lu, Y. Alcoholic Liver Disease: From CYP2E1 to CYP2A5. Curr. Mol. Pharmacol. 2017, 10, 172–178. [Google Scholar] [CrossRef]
- Cederbaum, A.I.; Wu, D.; Mari, M.; Bai, J. CYP2E1-dependent toxicity and oxidative stress in HepG2 cells. Free Radic. Biol. Med. 2001, 31, 1539–1543. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, D.; Wang, X.; Ward, S.C.; Cederbaum, A.I. Chronic alcohol-induced liver injury and oxidant stress are decreased in cytochrome P4502E1 knockout mice and restored in humanized cytochrome P4502E1 knock-in mice. Free Radic. Biol. Med. 2010, 49, 1406–1416. [Google Scholar] [CrossRef]
- Cahill, A.; Cunningham, C.C.; Adachi, M.; Ishii, H.; Bailey, S.M.; Fromenty, B.; Davies, A. Effects of alcohol and oxidative stress on liver pathology: The role of the mitochondrion. Alcohol Clin. Exp. Res. 2002, 26, 907–915. [Google Scholar] [CrossRef]
- San-Millan, I. The Key Role of Mitochondrial Function in Health and Disease. Antioxidants 2023, 12, 782. [Google Scholar] [CrossRef]
- Wilson, D.F. Oxidative phosphorylation: Regulation and role in cellular and tissue metabolism. J. Physiol. 2017, 595, 7023–7038. [Google Scholar] [CrossRef]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef]
- Liu, X.; Green, R.M. Endoplasmic reticulum stress and liver diseases. Liver Res. 2019, 3, 55–64. [Google Scholar] [CrossRef]
- Malhi, H.; Kaufman, R.J. Endoplasmic reticulum stress in liver disease. J. Hepatol. 2011, 54, 795–809. [Google Scholar] [CrossRef]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell. Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef]
- Xu, D.; Xu, M.; Jeong, S.; Qian, Y.; Wu, H.; Xia, Q.; Kong, X. The Role of Nrf2 in Liver Disease: Novel Molecular Mechanisms and Therapeutic Approaches. Front. Pharmacol. 2018, 9, 1428. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Zhou, J.; Zheng, Q.; Chen, Z. The Nrf2 Pathway in Liver Diseases. Front. Cell. Dev. Biol. 2022, 10, 826204. [Google Scholar] [CrossRef]
- Dong, J.; Sulik, K.K.; Chen, S.Y. Nrf2-mediated transcriptional induction of antioxidant response in mouse embryos exposed to ethanol in vivo: Implications for the prevention of fetal alcohol spectrum disorders. Antioxid. Redox Signal. 2008, 10, 2023–2033. [Google Scholar] [CrossRef]
- Zhou, R.; Lin, J.; Wu, D. Sulforaphane induces Nrf2 and protects against CYP2E1-dependent binge alcohol-induced liver steatosis. Biochim. Biophys. Acta 2014, 1840, 209–218. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, S.; Tao, S.; Hou, G.; Zhao, F.; Tan, S.; Meng, Q. Dioscorea spp.: Bioactive compounds and potential for the treatment of inflammatory and metabolic Diseases. Molecules 2023, 28, 2878. [Google Scholar] [CrossRef]
- Chaniad, P.; Tewtrakul, S.; Sudsai, T.; Langyanai, S.; Kaewdana, K. Anti-inflammatory, wound healing and antioxidant potential of compounds from Dioscorea bulbifera L. bulbils. PLoS ONE 2020, 15, e0243632. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.K.; Tungalag, T.; Kang, H.-S. Bulbils of Aerial Yam Attenuate Ethanol-Induced Hepatotoxicity in HepG2 Cells through Inhibition of Oxidative Stress by Activation of the Nuclear Factor Erythroid-2-Related Factor 2 Signaling Pathway. Nutrients 2024, 16, 542. https://doi.org/10.3390/nu16040542
Yang DK, Tungalag T, Kang H-S. Bulbils of Aerial Yam Attenuate Ethanol-Induced Hepatotoxicity in HepG2 Cells through Inhibition of Oxidative Stress by Activation of the Nuclear Factor Erythroid-2-Related Factor 2 Signaling Pathway. Nutrients. 2024; 16(4):542. https://doi.org/10.3390/nu16040542
Chicago/Turabian StyleYang, Dong Kwon, Tsendsuren Tungalag, and Hyung-Sub Kang. 2024. "Bulbils of Aerial Yam Attenuate Ethanol-Induced Hepatotoxicity in HepG2 Cells through Inhibition of Oxidative Stress by Activation of the Nuclear Factor Erythroid-2-Related Factor 2 Signaling Pathway" Nutrients 16, no. 4: 542. https://doi.org/10.3390/nu16040542
APA StyleYang, D. K., Tungalag, T., & Kang, H.-S. (2024). Bulbils of Aerial Yam Attenuate Ethanol-Induced Hepatotoxicity in HepG2 Cells through Inhibition of Oxidative Stress by Activation of the Nuclear Factor Erythroid-2-Related Factor 2 Signaling Pathway. Nutrients, 16(4), 542. https://doi.org/10.3390/nu16040542






