A Cohort Study of the Influence of the 12-Component Modified Japanese Diet Index on Oral and Gut Microbiota in the Japanese General Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Clinical Parameters
2.3. Next-Generation Sequence Analysis of Gut Microbiota
2.4. Statistical Analysis
2.5. Ethics Statement
3. Results
3.1. Participant Characteristics
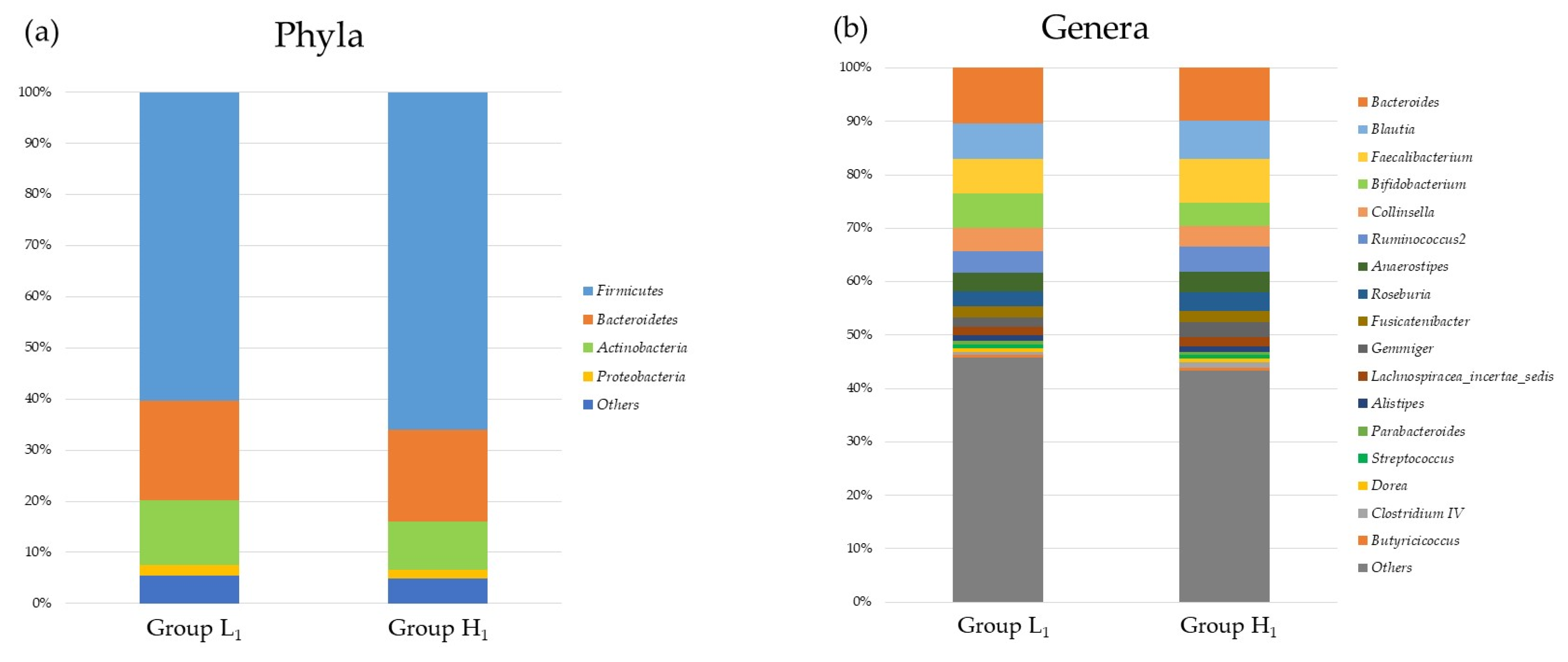
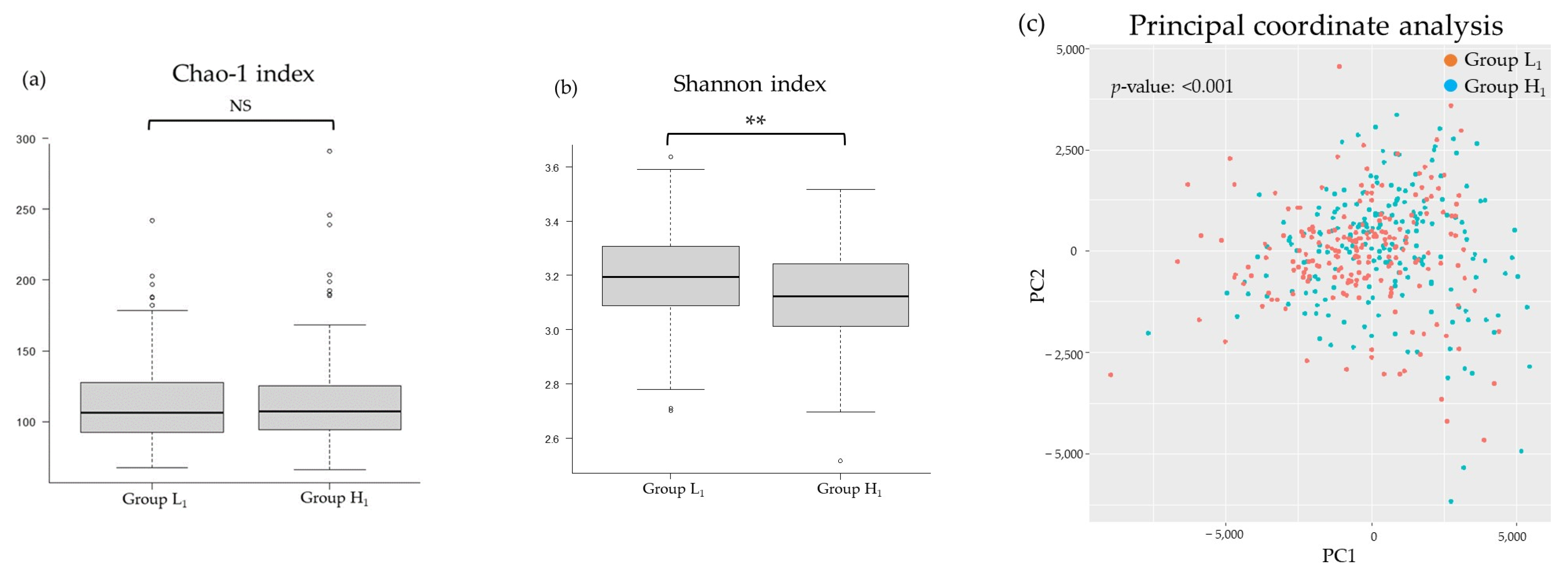
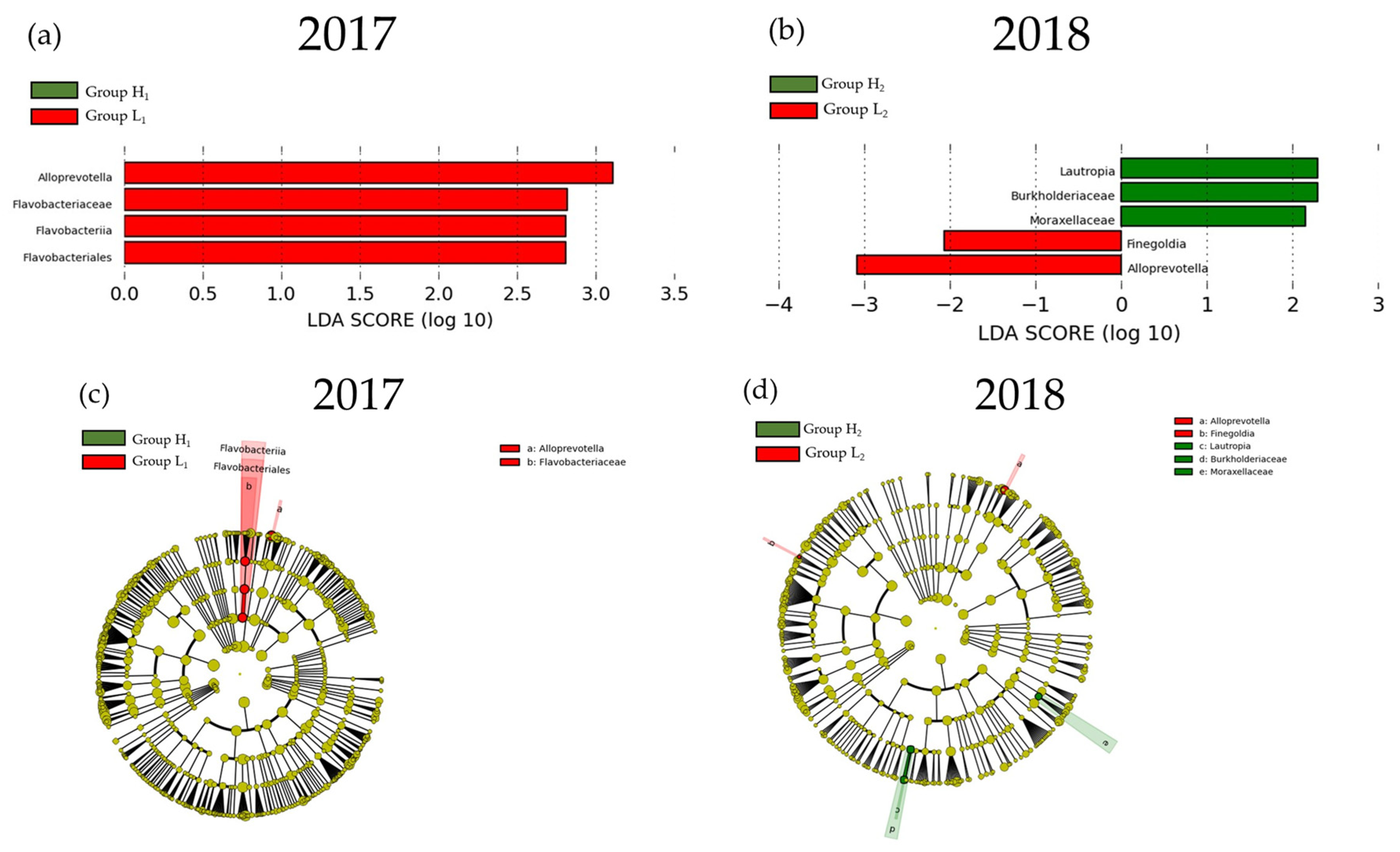
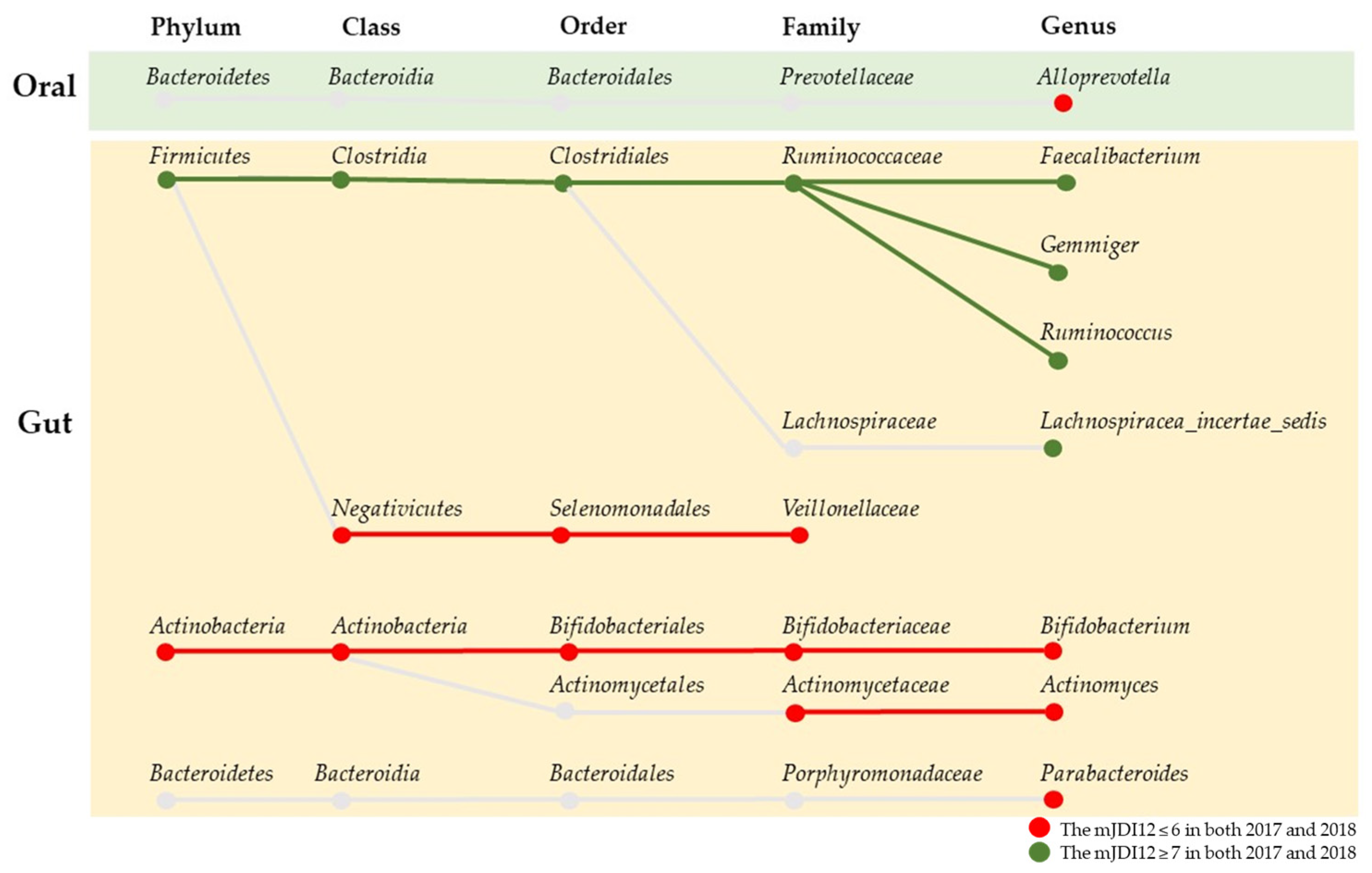
3.2. Comparison of the mJDI12 and the Oral or Gut Bacterial Species in 2017 and 2018
3.3. Participant Characteristics after Grouping Classified by the mJDI12 from 2017 to 2018
3.4. Correlation between Differences in the Intake of Foods and Food Groups and the Relative Abundance of Microbiota Associated with the mJDI12
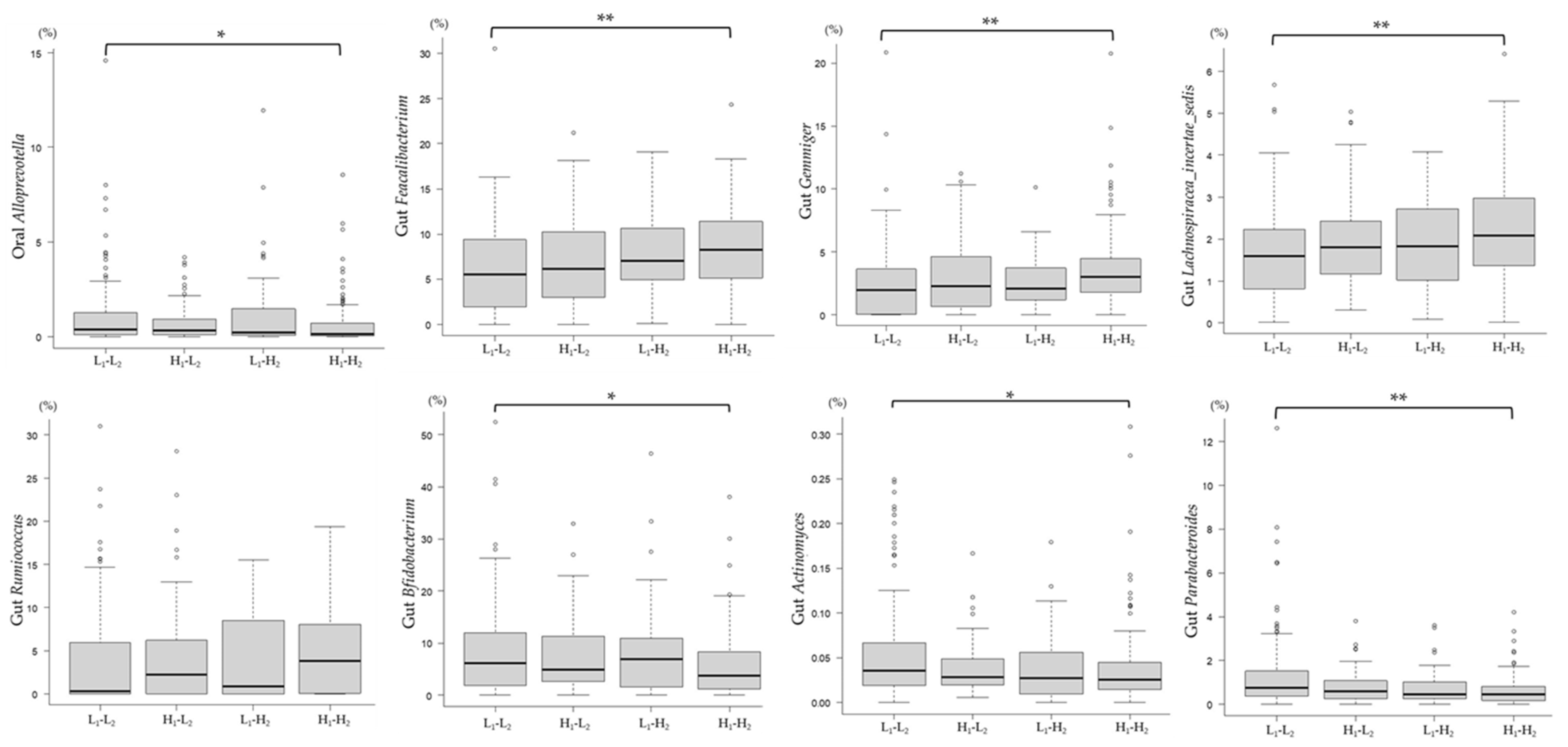
3.5. Association between Changes in the mJDI12 and the Relative Abundance of mJDI12-Related Bacteria
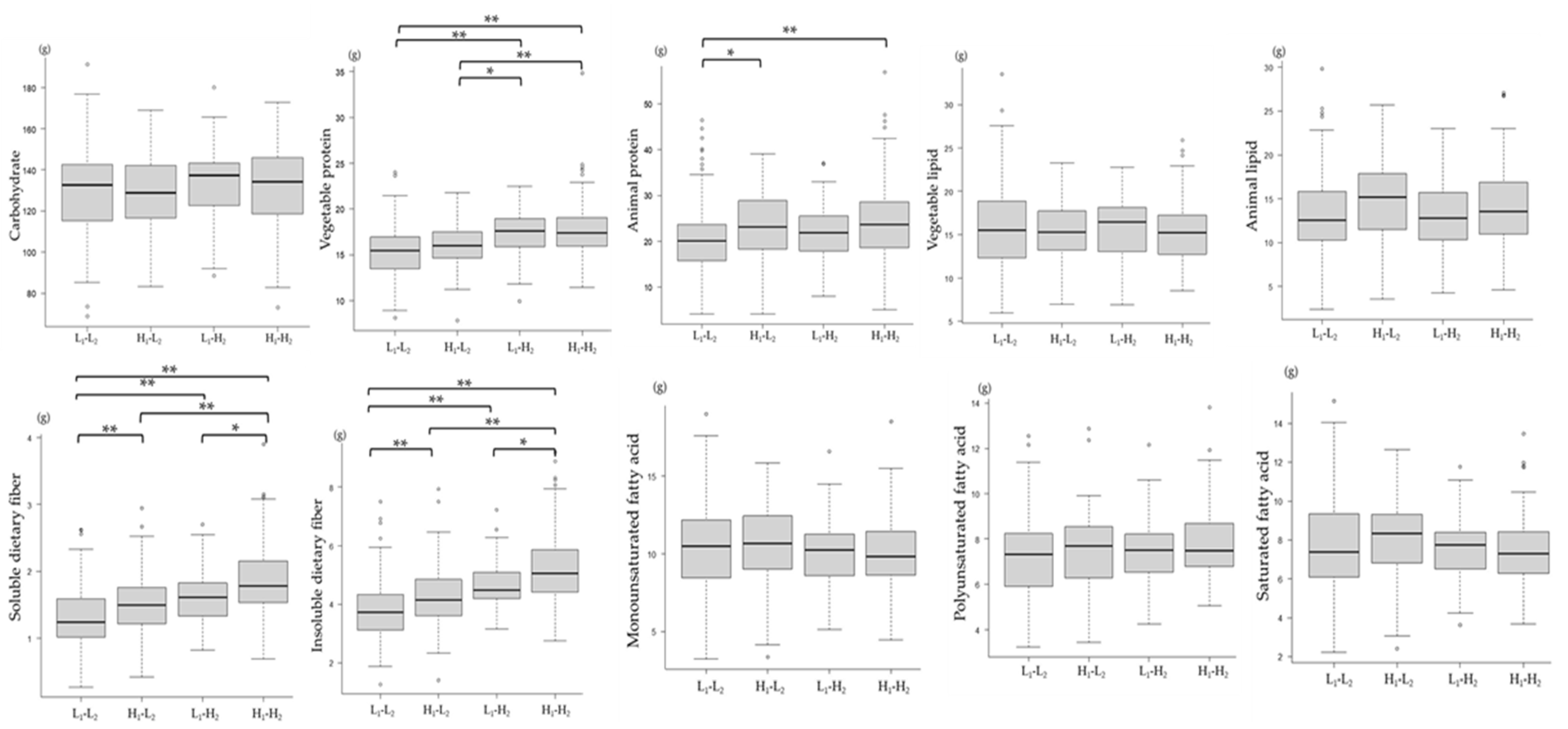
3.6. Association between Changes in the mJDI12 and Nutrient Intake Levels
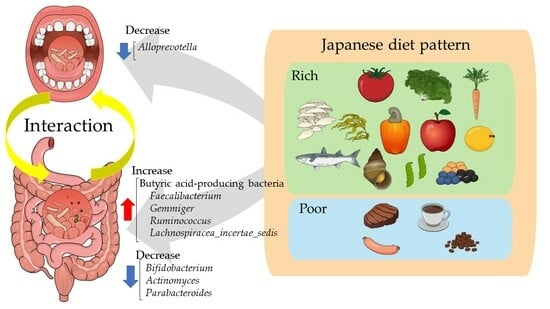
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perler, B.K.; Friedman, E.S.; Wu, G.D. The Role of the Gut Microbiota in the Relationship Between Diet and Human Health. Annu. Rev. Physiol. 2023, 85, 449–468. [Google Scholar] [CrossRef]
- Purdel, C.; Margină, D.; Adam-Dima, I.; Ungurianu, A. The Beneficial Effects of Dietary Interventions on Gut Microbiota-An Up-to-Date Critical Review and Future Perspectives. Nutrients 2023, 15, 5005. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Passarelli, P.C.; Azzolino, D.; Bottalico, L.; Charitos, I.A.; Cazzolla, A.P.; Colella, M.; Topi, S.; Godoy, F.G.; D’Addona, A. Oral microbiota in human health and disease: A perspective. Exp. Biol. Med. 2023, 248, 1288–1301. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Hwang, B.O.; Lim, M.; Ok, S.H.; Lee, S.K.; Chun, K.S.; Park, K.K.; Hu, Y.; Chung, W.Y.; Song, N.Y. Oral-Gut Microbiome Axis in Gastrointestinal Disease and Cancer. Cancers 2021, 13, 2124. [Google Scholar] [CrossRef]
- Suzuki, N.; Goto, Y.; Ota, H.; Kito, K.; Mano, F.; Joo, E.; Ikeda, K.; Inagaki, N.; Nakayama, T. Characteristics of the Japanese Diet Described in Epidemiologic Publications: A Qualitative Systematic Review. J. Nutr. Sci. Vitaminol. 2018, 64, 129–137. [Google Scholar] [CrossRef]
- Psaltopoulou, T.; Sergentanis, T.N.; Panagiotakos, D.B.; Sergentanis, I.N.; Kosti, R.; Scarmeas, N. Mediterranean diet, stroke, cognitive impairment, and depression: A meta-analysis. Ann. Neurol. 2013, 74, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Saji, N.; Tsuduki, T.; Murotani, K.; Hisada, T.; Sugimoto, T.; Kimura, A.; Niida, S.; Toba, K.; Sakurai, T. Relationship between the Japanese-style diet, gut microbiota, and dementia: A cross-sectional study. Nutrition 2022, 94, 111524. [Google Scholar] [CrossRef]
- Tomata, Y.; Watanabe, T.; Sugawara, Y.; Chou, W.T.; Kakizaki, M.; Tsuji, I. Dietary patterns and incident functional disability in elderly Japanese: The Ohsaki Cohort 2006 study. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 843–851. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Fujii, H.; Harima, M.; Okamura, H.; Yukawa-Muto, Y.; Odagiri, N.; Motoyama, H.; Kotani, K.; Kozuka, R.; Kawamura, E.; et al. Severity of Liver Fibrosis Is Associated with the Japanese Diet Pattern and Skeletal Muscle Mass in Patients with Nonalcoholic Fatty Liver Disease. Nutrients 2023, 15, 1175. [Google Scholar] [CrossRef]
- Nagpal, R.; Neth, B.J.; Wang, S.; Craft, S.; Yadav, H. Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer’s disease markers in subjects with mild cognitive impairment. EBioMedicine 2019, 47, 529–542. [Google Scholar] [CrossRef]
- Kushida, M.; Sugawara, S.; Asano, M.; Yamamoto, K.; Fukuda, S.; Tsuduki, T. Effects of the 1975 Japanese diet on the gut microbiota in younger adults. J. Nutr. Biochem. 2019, 64, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Gupta, U.; Dey, P. The oral microbial odyssey influencing chronic metabolic disease. Arch. Physiol. Biochem. 2023, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Hong, Y.; Maishi, N.; Matsuda, A.Y.; Hida, Y.; Hasebe, A.; Kitagawa, Y.; Hida, K. Oral bacterium Streptococcus mutans promotes tumor metastasis through thrombosis formation. Cancer Sci. 2024, 115, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Slouha, E.; Ibrahim, F.; Rezazadah, A.; Esposito, S.; Clunes, L.A.; Kollias, T.F. Anti-diabetics and the Prevention of Dementia: A Systematic Review. Cureus 2023, 15, e49515. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Li, S.; He, M.; Ao, Z.; Wang, J.; Wu, Q.; Wang, Q. Oral Pathogenic Bacteria and the Oral-Gut-Liver Axis: A New Understanding of Chronic Liver Diseases. Diagnostics 2023, 13, 3324. [Google Scholar] [CrossRef] [PubMed]
- Cato, L.E.; McKay, A.K.A.; L’Heureux, J.E.; Vanhatalo, A.; Jones, A.M.; Askew, C.D.; Slater, G.J.; Burke, L.M. Low Carbohydrate, High Fat Diet Alters the Oral Microbiome without Negating the Nitrite Response to Beetroot Juice Supplementation. Nutrients. 2023, 15, 5123. [Google Scholar] [CrossRef]
- Ogce, F.; Ceber, E.; Ekti, R.; Oran, N.T. Comparison of mediterranean, Western and Japanese diets and some recommendations. Asian Pac. J. Cancer Prev. 2008, 9, 351–356. [Google Scholar]
- Nakamoto, M.; Otsuka, R.; Nishita, Y.; Tange, C.; Tomida, M.; Kato, Y.; Imai, T.; Sakai, T.; Ando, F.; Shimokata, H. Soy food and isoflavone intake reduces the risk of cognitive impairment in elderly Japanese women. Eur. J. Clin. Nutr. 2018, 72, 1458–1462. [Google Scholar] [CrossRef]
- Depeint, F.; Tzortzis, G.; Vulevic, J.; I’Anson, K.; Gibson, G.R. Prebiotic evaluation of a novel galactooligosaccharide mixture produced by the enzymatic activity of Bifidobacterium bifidum NCIMB 41171, in healthy humans: A randomized, double-blind, crossover, placebo-controlled intervention study. Am. J. Clin. Nutr. 2008, 87, 785–791. [Google Scholar] [CrossRef]
- Ramnani, P.; Gaudier, E.; Bingham, M.; van Bruggen, P.; Tuohy, K.M.; Gibson, G.R. Prebiotic effect of fruit and vegetable shots containing Jerusalem artichoke inulin: A human intervention study. Br. J. Nutr. 2010, 104, 233–240. [Google Scholar] [CrossRef]
- So, D.; Whelan, K.; Rossi, M.; Morrison, M.; Holtmann, G.; Kelly, J.T.; Shanahan, E.R.; Staudacher, H.M.; Campbell, K.L. Dietary fiber intervention on gut microbiota composition in healthy adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2018, 107, 965–983. [Google Scholar] [CrossRef] [PubMed]
- Caesar, R.; Tremaroli, V.; Kovatcheva-Datchary, P.; Cani, P.D.; Bäckhed, F. Crosstalk between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation through TLR Signaling. Cell Metab. 2015, 22, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, J.; Igarashi, M.; Watanabe, K.; Karaki, S.I.; Mukouyama, H.; Kishino, S.; Li, X.; Ichimura, A.; Irie, J.; Sugimoto, Y.; et al. Gut microbiota confers host resistance to obesity by metabolizing dietary polyunsaturated fatty acids. Nat. Commun. 2019, 10, 4007. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Borriello, S.P.; Hulme, P.; Kirk, D.N.; Axelson, M. Nonsteroidal estrogens of dietary origin: Possible roles in hormone-dependent disease. Am. J. Clin. Nutr. 1984, 40, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.H.; Kern, T.; Bak, E.G.; Kashani, A.; Allin, K.H.; Nielsen, T.; Hansen, T.; Pedersen, O. Impact of a vegan diet on the human salivary microbiota. Sci. Rep. 2018, 8, 5847. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, T.; Matsuo, K.; Furuta, M.; Shibata, Y.; Fukami, K.; Shimazaki, Y.; Akifusa, S.; Han, D.H.; Kim, H.D.; Yokoyama, T.; et al. Distinct composition of the oral indigenous microbiota in South Korean and Japanese adults. Sci. Rep. 2014, 4, 6990. [Google Scholar] [CrossRef]
- Yoshihara, T.; Kioi, M.; Baba, J.; Usuda, H.; Kessoku, T.; Iwaki, M.; Takatsu, T.; Misawa, N.; Ashikari, K.; Matsuura, T.; et al. A prospective interventional trial on the effect of periodontal treatment on Fusobacterium nucleatum abundance in patients with colorectal tumours. Sci. Rep. 2021, 11, 23719. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, F.; Lu, H.; Wang, B.; Chen, Y.; Lei, D.; Wang, Y.; Zhu, B.; Li, L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology 2011, 54, 562–572. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Ridlon, J.M.; Hylemon, P.B.; Thacker, L.R.; Heuman, D.M.; Smith, S.; Sikaroodi, M.; Gillevet, P.M. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G168–G175. [Google Scholar] [CrossRef]
- Zhang, S.; Otsuka, R.; Tomata, Y.; Shimokata, H.; Tange, C.; Tomida, M.; Nishita, Y.; Matsuyama, S.; Tsuji, I. A cross-sectional study of the associations between the traditional Japanese diet and nutrient intakes: The NILS-LSA project. Nutr. J. 2019, 18, 43. [Google Scholar] [CrossRef]
- Gui, X.; Yang, Z.; Li, M.D. Effect of cigarette smoke on gut microbiota: State of knowledge. Front. Physiol. 2021, 12, 673341. [Google Scholar] [CrossRef] [PubMed]
- Nakaji, S.; Ihara, K.; Sawada, K.; Parodi, S.; Umeda, T.; Takahashi, I.; Murashita, K.; Kurauchi, S.; Tokuda, I. Social innovation for life expectancy extension utilizing a platform-centered system used in the Iwaki health promotion project: A protocol paper. SAGE Open Med. 2021, 9, 20503121211002606. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Yanagibori, R.; Amano, K. Self-administered diet history questionnaire developed for health education: A relative validation of the test-version by comparison with 3-day diet record in women. J. Epidemiol. 1998, 8, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Chinda, D.; Shimoyama, T.; Iino, C.; Kudo, S.; Sawada, K.; Mikami, T.; Nakaji, S.; Sakuraba, H.; Fukuda, S. A Cohort Study of the Effects of Daily-Diet Water-Soluble Dietary Fiber on Butyric Acid-Producing Gut Microbiota in Middle-Aged and Older Adults in a Rural Region. Microorganisms 2022, 10, 1813. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.; de Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Fukui, H. Role of gut dysbiosis in liver diseases: What have we learned so far? Diseases 2019, 7, 58. [Google Scholar] [CrossRef]
- Ferolla, S.M.; Armiliato, G.N.; Couto, C.A.; Ferrari, T.C. The role of intestinal bacteria overgrowth in obesity-related nonalcoholic fatty liver disease. Nutrients 2014, 6, 5583–5599. [Google Scholar] [CrossRef]
- Fernando, W.; Martins, I.J.; Morici, M.; Bharadwaj, P.; Rainey-Smith, S.R.; Lim, W.L.F.; Martins, R.N. Sodium Butyrate Reduces Brain Amyloid-β Levels and Improves Cognitive Memory Performance in an Alzheimer’s Disease Transgenic Mouse Model at an Early Disease Stage. J. Alzheimer’s Dis. 2020, 74, 91–99. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, D.; Weng, F.; Jin, Y.; He, L. Sodium butyrate ameliorates the cognitive impairment of Alzheimer’s disease by regulating the metabolism of astrocytes. Psychopharmacology 2022, 239, 215–227. [Google Scholar] [CrossRef]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Daily, J.W.; Park, S. Sarcopenia Is a Cause and Consequence of Metabolic Dysregulation in Aging Humans: Effects of Gut Dysbiosis, Glucose Dysregulation, Diet and Lifestyle. Cells 2022, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Kircher, B.; Woltemate, S.; Gutzki, F.; Schlüter, D.; Geffers, R.; Bähre, H.; Vital, M. Predicting butyrate- and propionate-forming bacteria of gut microbiota from sequencing data. Gut Microbes 2022, 14, 2149019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Song, L.; Wang, Y.; Liu, C.; Zhang, L.; Zhu, S.; Liu, S.; Duan, L. Beneficial effect of butyrate-producing Lachnospiraceae on stress-induced visceral hypersensitivity in rats. J. Gastroenterol. Hepatol. 2019, 34, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Holtrop, G.; Lobley, G.E.; Calder, A.G.; Stewart, C.S.; Flint, H.J. Contribution of acetate to butyrate formation by human faecal bacteria. Br. J. Nutr. 2004, 91, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, K.; Mizuno, S.; Mikami, Y.; Sujino, T.; Saigusa, K.; Matsuoka, K.; Naganuma, M.; Sato, T.; Takada, T.; Tsuji, H. A single species of Clostridium subcluster XIVa decreased in ulcerative colitis patients. Inflamm. Bowel Dis. 2016, 22, 2802–2810. [Google Scholar] [CrossRef] [PubMed]
- Iino, C.; Endo, T.; Mikami, K.; Hasegawa, T.; Kimura, M.; Sawada, N.; Nakaji, S.; Fukuda, S. Significant decrease in Faecalibacterium among gut microbiota in nonalcoholic fatty liver disease: A large BMI-and sex-matched population study. Hepatol. Int. 2019, 13, 748–756. [Google Scholar] [CrossRef]
- Oka, A.; Sartor, R.B. Microbial-based and microbial-targeted therapies for inflammatory bowel diseases. Dig. Dis. Sci. 2020, 65, 757–788. [Google Scholar] [CrossRef]
- Otsuka, T.; Sugimoto, M.; Inoue, R.; Ohno, M.; Ban, H.; Nishida, A.; Inatomi, O.; Takahashi, S.; Naito, Y.; Andoh, A. Influence of potassium-competitive acid blocker on the gut microbiome of Helicobacter pylori-negative healthy individuals. Gut 2017, 66, 1723–1725. [Google Scholar] [CrossRef]
- Noble, E.E.; Olson, C.A.; Davis, E.; Tsan, L.; Chen, Y.W.; Schade, R.; Liu, C.; Suarez, A.; Jones, R.B.; de La Serre, C.; et al. Gut microbial taxa elevated by dietary sugar disrupt memory function. Transl. Psychiatry 2021, 11, 194. [Google Scholar] [CrossRef]
- Pu, S.; Khazanehei, H.; Jones, P.J.; Khafipour, E. Interactions between Obesity Status and Dietary Intake of Monounsaturated and Polyunsaturated Oils on Human Gut Microbiome Profiles in the Canola Oil Multicenter Intervention Trial (COMIT). Front. Microbiol. 2016, 7, 1612. [Google Scholar] [CrossRef] [PubMed]
- Jaquet, M.; Rochat, I.; Moulin, J.; Cavin, C.; Bibiloni, R. Impact of coffee consumption on the gut microbiota: A human volunteer study. Int. J. Food Microbiol. 2009, 130, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Leloup, V. Evaluation of the nutritive value of soluble coffee. In Proceedings of the 21st International Conference on Coffee Science, Montpellier, France, 11–15 September 2006. [Google Scholar]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.Z.; Abe, F.; Osawa, R. Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol. 2016, 16, 90. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.P.; Acosta-Pagán, K.T.; Oramas-Sepúlveda, C.; Castañeda-Avila, M.A.; Vilanova-Cuevas, B.; Ramos-Cartagena, J.M.; Vivaldi, J.A.; Pérez-Santiago, J.; Pérez, C.M.; Godoy-Vitorino, F. Oral microbiota and periodontitis severity among Hispanic adults. Front. Cell. Infect. Microbiol. 2022, 12, 965159. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Liu, C.; Jia, W.; Zhao, D.; Chen, X.; Zhu, X.; Yang, M.; Wang, L. Alcohol drinking alters oral microbiota to modulate the progression of alcohol-related liver disease. iScience 2023, 26, 107977. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Ma, Y.; Guan, K.; Zhao, L.; Ma, Y.; Wang, R. Whey Protein Peptide Pro-Glu-Trp Ameliorates Hyperuricemia by Enhancing Intestinal Uric Acid Excretion, Modulating the Gut Microbiota, and Protecting the Intestinal Barrier in Rats. J. Agric. Food Chem. 2024, 72, 2573–2584. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Jiang, H.; Sun, Z.; Sun, A. Alloprevotella Can be Considered as a Potential Oral Biomarker in Intestinal Metaphase of Gastric Patients. Stud. Health Technol. Inform. 2023, 308, 155–167. [Google Scholar] [CrossRef]




| Characteristics | mJDI12 ≤ 6 (n = 337) | mJDL12 ≥ 7 (n = 237) | p-Value |
|---|---|---|---|
| Sex (male/female) | 139:198 | 103:134 | 0.607 |
| Age (years) | 49.0 (38.0–59.0) | 60.0 (46.0–67.0) | <0.001 |
| BMI (kg/m2) | 22.5 (20.1–24.9) | 22.5 (20.5–24.6) | 0.672 |
| mJDI12 | 5.0 (3.0–6.0) | 8.0 (7.0–9.0) | <0.001 |
| Soybeans and soybean foods | 25.5 (15.1–39.3) | 41.5 (28.6–56.2) | <0.001 |
| Green and yellow vegetables | 28.1 (17.8–44.1) | 46.5 (34.3–65.8) | <0.001 |
| Fruit | 11.5 (4.6–23.7) | 32.0 (16.3–55.5) | <0.001 |
| Fish and shellfish | 33.2 (22.1–47.9) | 52.2 (37.8–68.3) | <0.001 |
| Pickles | 1.1 (0.0–3.9) | 4.0 (0.9–8.4) | <0.001 |
| Mushrooms | 2.9 (1.7–6.0) | 6.2 (4.0–10.3) | <0.001 |
| Seaweeds | 2.9 (1.6–6.5) | 7.3 (5.6–12.0) | <0.001 |
| Green tea | 16.9 (1.7–65.1) | 64.8 (23.3–175.0) | <0.001 |
| Rice | 167.1 (124.3–220.5) | 178.5 (128.4–215.2) | 0.666 |
| Miso soup | 62.9 (38.5–105.2) | 102.5 (66.3–135.5) | <0.001 |
| Beef and pork | 19.1 (13.4–25.4) | 16.2 (8.5–20.3) | <0.001 |
| Coffee | 110.5 (59.6–221.8) | 83.1 (27.0–172.8) | <0.001 |
| Characteristics | Group L1 (n = 198) | Group H1 (n = 198) | p-Value |
|---|---|---|---|
| Sex (male/female) | 85:113 | 79:119 | 0.610 |
| Age (years) | 56.0 (43.0–64.0) | 57.0 (42.8–64.0) | 0.771 |
| BMI (kg/m2) | 23.0 (20.5–24.8) | 22.5 (20.5–24.6) | 0.680 |
| mJDI12 | 5.0 (3.8–6.0) | 8.0 (7.0–9.0) | <0.001 |
| Soybeans and soybean foods | 24.7 (14.6–39.9) | 41.6 (29.2–57.8) | <0.001 |
| Green and yellow vegetables | 30.2 (16.4–48.1) | 46.4 (34.4–66.9) | <0.001 |
| Fruit | 13.3 (5.9–26.2) | 30.6 (15.6–53.9) | <0.001 |
| Fish and shellfish | 33.8 (24.0–48.5) | 50.9 (35.9–67.3) | <0.001 |
| Pickles | 1.6 (0.7–5.2) | 3.5 (0.9–6.8) | 0.004 |
| Mushrooms | 2.9 (1.6–6.9) | 6.2 (4.4–10.1) | <0.001 |
| Seaweeds | 3.1 (1.6–6.9) | 7.3 (5.6–11.6) | <0.001 |
| Green tea | 17.1 (5.1–56.6) | 64.2 (15.4–170.7) | <0.001 |
| Rice | 152.9 (122.7–216.2) | 178.1 (125.2–215.5) | 0.310 |
| Miso soup | 64.8 (37.0–109.6) | 104.2 (66.0–136.2) | <0.001 |
| Beef and pork | 19.0 (11.8–24.7) | 16.3 (8.5–20.5) | 0.002 |
| Coffee | 103.8 (56.2–214.8) | 85.6 (40.5–176.9) | 0.006 |
| Characteristics | L1-L2 (n = 156) | H1-L2 (n = 65) | L1-H2 (n = 42) | H1-H2 (n = 133) | p-Value |
|---|---|---|---|---|---|
| Sex (male/female) | 71:85 | 27:38 | 14:28 | 52:81 | 0.474 |
| Age (years) | 54.5 (42.0–63.0) | 49.0 (37.0–62.0) | 62.0 (53.3–67.0) | 59.0 (50.0–65.0) | <0.001 |
| BMI (kg/m2) | 22.6 (20.4–24.7) | 22.9 (20.4–24.8) | 23.2 (21.6–25.2) | 22.4 (20.5–24.4) | 0.560 |
| mJDI12 | 5.0 (4.0–6.0) | 6.0 (5.0–6.0) | 7.0 (7.0–8.0) | 8.0 (7.0–9.0) | <0.001 |
| Soybeans and soybean foods | 27.0 (15.1–37.5) | 34.5 (20.3–51.6) | 42.0 (32.0–51.9) | 51.5 (35.8–62.5) | <0.001 |
| Green and yellow vegetables | 26.6 (15.4–44.3) | 32.2 (19.6–49.3) | 42.0 (29.1–59.4) | 49.3 (32.8–74.9) | <0.001 |
| Fruit | 11.7 (4.0–26.0) | 16.7 (7.9–45.7) | 28.1 (16.9–48.0) | 31.4 (19.2–56.6) | <0.001 |
| Fish and shellfish | 34.1 (22.9–51.5) | 40.5 (24.9–63.4) | 46.8 (32.1–58.5) | 53.7 (38.3–68.9) | <0.001 |
| Pickles | 1.2 (0.0–4.9) | 1.0 (0.0–4.4) | 2.4 (0.8–4.9) | 3.2 (0.9–7.5) | 0.001 |
| Mushrooms | 3.4 (1.7–6.1) | 4.6 (2.4–7.0) | 5.4 (2.7–7.3) | 6.8 (4.0–11.9) | <0.001 |
| Seaweeds | 2.9 (1.7–5.5) | 4.6 (2.3–7.1) | 7.4 (4.3–12.8) | 7.6 (5.5–12.6) | <0.001 |
| Green tea | 15.5 (5.3–78.8) | 24.8 (5.9–86.5) | 41.8 (6.0–83.5) | 70.7 (26.6–201.1) | <0.001 |
| Rice | 159.6 (104.5–208.8) | 153.2 (126.1–182.5) | 170.6 (129.5–207.0) | 166.9 (126.8–213.3) | 0.247 |
| Miso soup | 63.1 (36.8–96.5) | 70.7 (43.4–118.1) | 91.0 (67.5–125.1) | 102.7 (61.8–144.4) | <0.001 |
| Beef and pork | 19.2 (11.7–26.4) | 19.0 (12.8–28.9) | 16.7 (7.4–20.3) | 15.7 (8.8–20.9) | 0.004 |
| Coffee | 129.5 (60.9–224.1) | 139.4 (53.2–209.6) | 96.7 (63.9–161.6) | 82.4 (40.5–186.3) | 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, S.; Chinda, D.; Iino, C.; Sawada, K.; Mikami, T.; Nakaji, S.; Sakuraba, H.; Fukuda, S. A Cohort Study of the Influence of the 12-Component Modified Japanese Diet Index on Oral and Gut Microbiota in the Japanese General Population. Nutrients 2024, 16, 524. https://doi.org/10.3390/nu16040524
Sato S, Chinda D, Iino C, Sawada K, Mikami T, Nakaji S, Sakuraba H, Fukuda S. A Cohort Study of the Influence of the 12-Component Modified Japanese Diet Index on Oral and Gut Microbiota in the Japanese General Population. Nutrients. 2024; 16(4):524. https://doi.org/10.3390/nu16040524
Chicago/Turabian StyleSato, Satoshi, Daisuke Chinda, Chikara Iino, Kaori Sawada, Tatsuya Mikami, Shigeyuki Nakaji, Hirotake Sakuraba, and Shinsaku Fukuda. 2024. "A Cohort Study of the Influence of the 12-Component Modified Japanese Diet Index on Oral and Gut Microbiota in the Japanese General Population" Nutrients 16, no. 4: 524. https://doi.org/10.3390/nu16040524
APA StyleSato, S., Chinda, D., Iino, C., Sawada, K., Mikami, T., Nakaji, S., Sakuraba, H., & Fukuda, S. (2024). A Cohort Study of the Influence of the 12-Component Modified Japanese Diet Index on Oral and Gut Microbiota in the Japanese General Population. Nutrients, 16(4), 524. https://doi.org/10.3390/nu16040524