Indirect Calorimetry-Based Novel Approach for Evaluating Metabolic Flexibility and Its Association with Circulating Metabolic Markers in Middle-Aged Subjects
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Lactic Acid (LA) Turn-Points
2.3. Maximal Oxygen Consumption
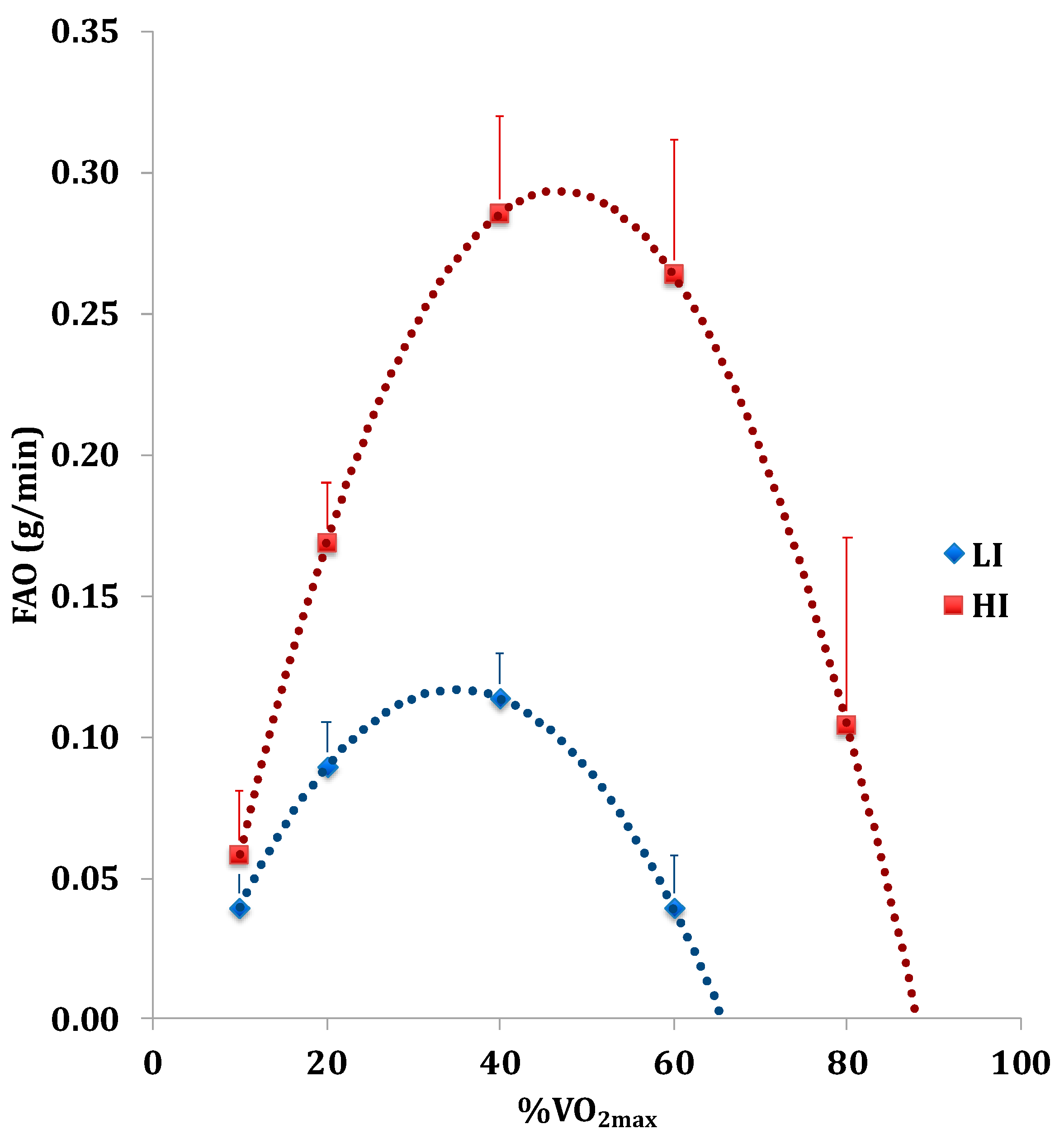
2.4. Fatty Acid (FA) and Carbohydrate (CH) Oxidation Rate Analyses
2.5. Blood Sampling
2.6. Lipid Analyses
2.7. Statistical Analysis
3. Results
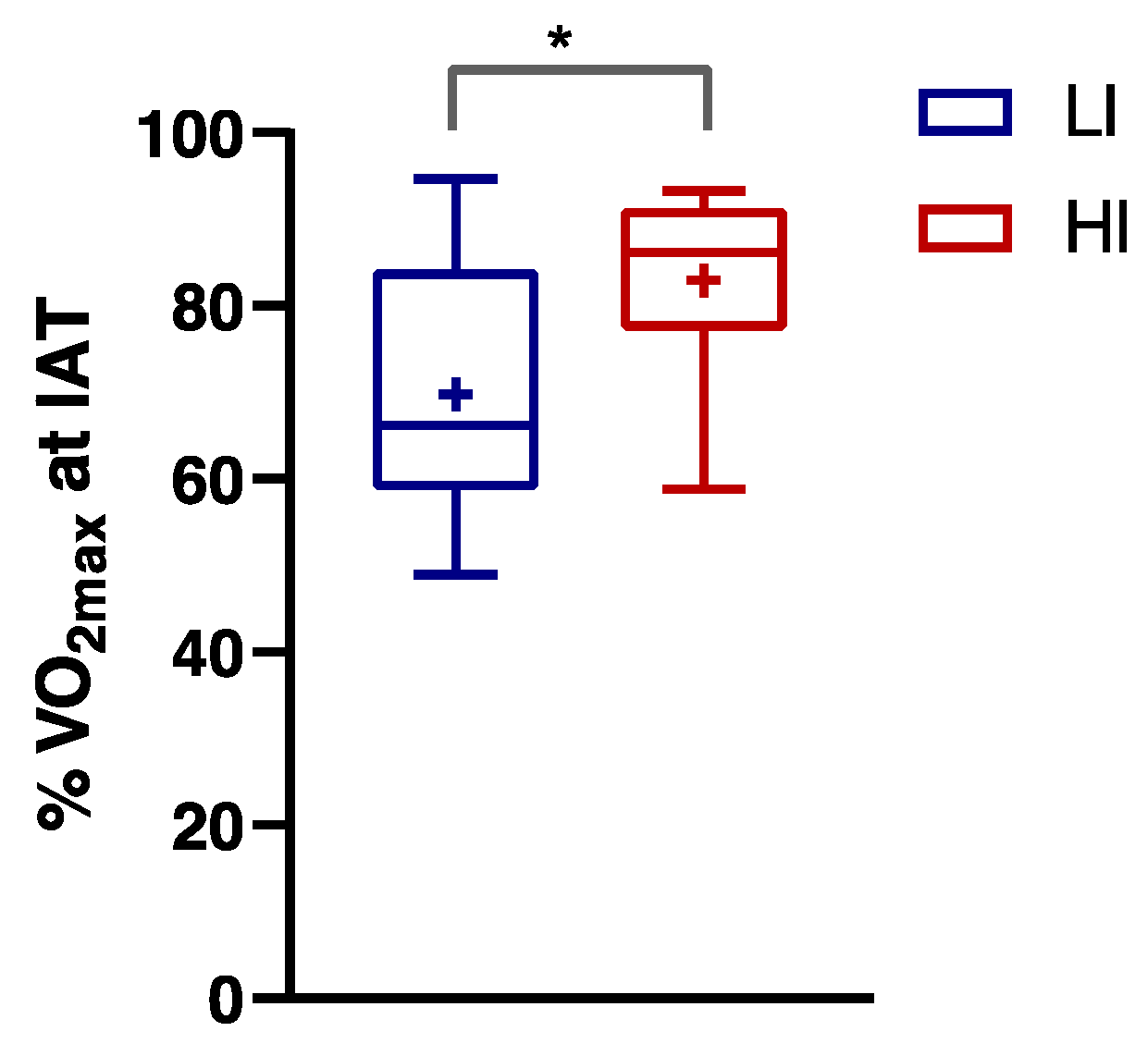
3.1. Anthropometric and Performance Characteristics of Subjects
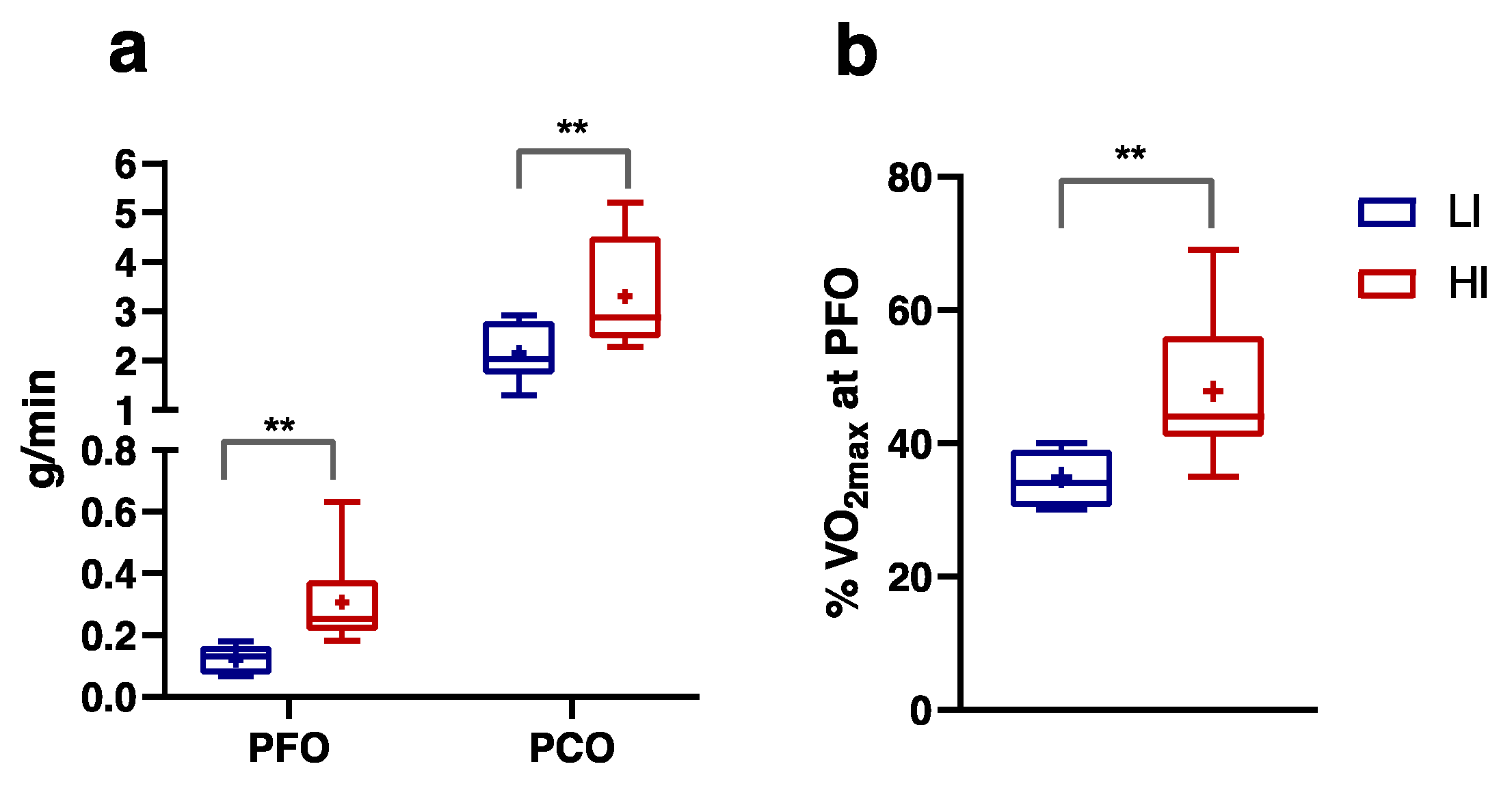
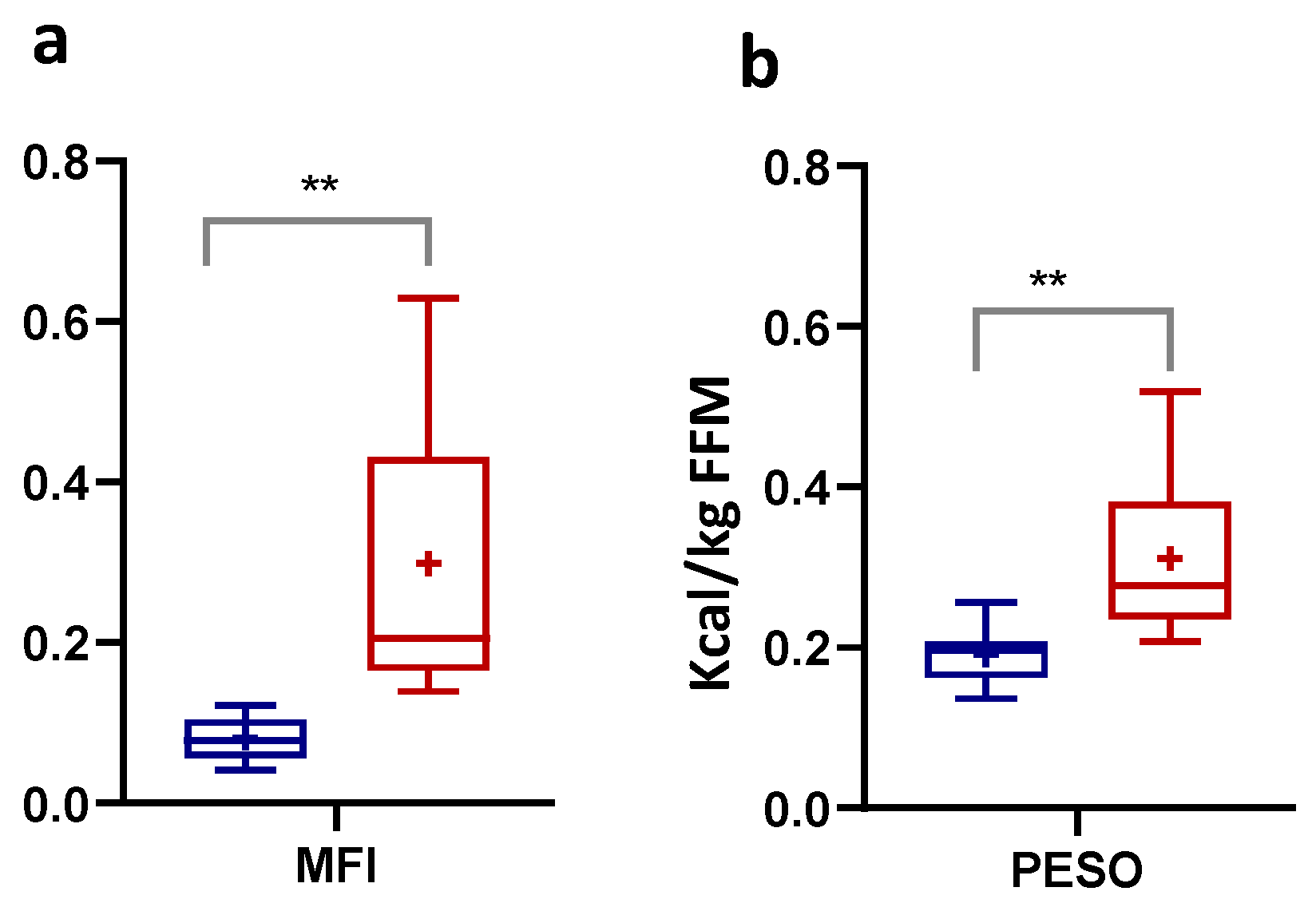
3.2. Numerically Measurable Parameters Indicative of Metabolic Flexibility (MF): MFI and PESO
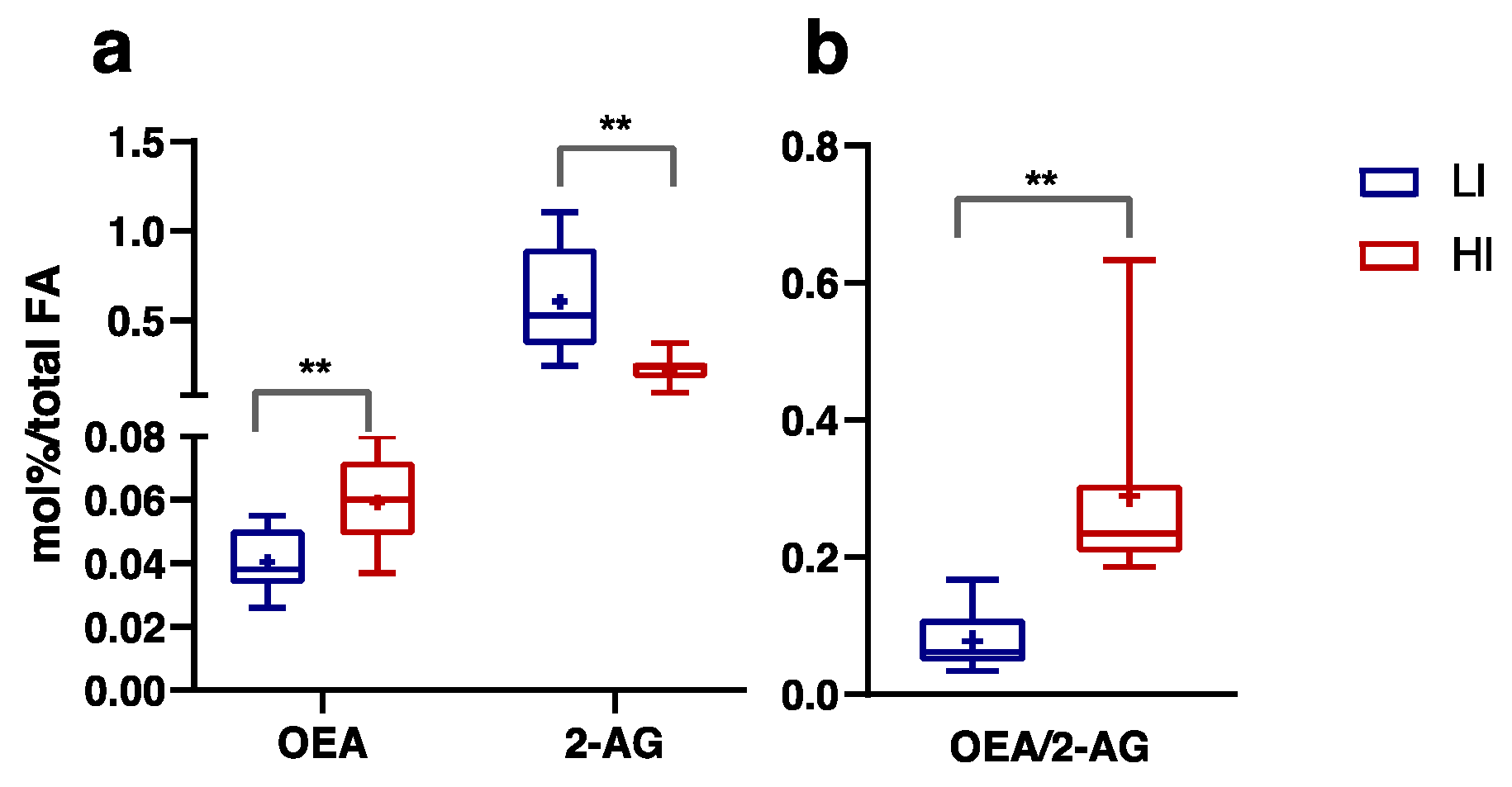
3.3. Plasma FA, Endocannabinoids and Related NAE Profiles
3.4. Correlations among the Key Markers of the Metabolic Flexibility (MF)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Smith, R.L.; Soeters, M.R.; Wüst, R.C.I.; Houtkooper, R.H. Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease. Endocr. Rev. 2018, 39, 489–517. [Google Scholar] [CrossRef]
- Chen, W.W.; Freinkman, E.; Wang, T.; Birsoy, K.; Sabatini, D.M. Absolute Quantification of Matrix Metabolites Reveals the Dynamics of Mitochondrial Metabolism. Cell 2016, 166, 1324–1337.e1311. [Google Scholar] [CrossRef]
- Corpeleijn, E.; Saris, W.H.M.; Blaak, E.E. Metabolic flexibility in the development of insulin resistance and type 2 diabetes: Effects of lifestyle. Obes. Rev. 2009, 10, 178–193. [Google Scholar] [CrossRef]
- Krssak, M.; Falk Petersen, K.; Dresner, A.; DiPietro, L.; Vogel, S.M.; Rothman, D.L.; Shulman, G.I.; Roden, M. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: A 1 H NMR spectroscopy study. Diabetologia 1999, 42, 113–116. [Google Scholar] [CrossRef]
- Muoio, D.M. Metabolic Inflexibility: When Mitochondrial Indecision Leads to Metabolic Gridlock. Cell 2014, 159, 1253–1262. [Google Scholar] [CrossRef]
- Broskey, N.T.; Greggio, C.; Boss, A.; Boutant, M.; Dwyer, A.; Schlueter, L.; Hans, D.; Gremion, G.; Kreis, R.; Boesch, C.; et al. Skeletal Muscle Mitochondria in the Elderly: Effects of Physical Fitness and Exercise Training. J. Clin. Endocrinol. Metab. 2014, 99, 1852–1861. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Choi, C.S.; Birkenfeld, A.L.; Alves, T.C.; Jornayvaz, F.R.; Jurczak, M.J.; Zhang, D.; Woo, D.K.; Shadel, G.S.; Ladiges, W.; et al. Targeted Expression of Catalase to Mitochondria Prevents Age-Associated Reductions in Mitochondrial Function and Insulin Resistance. Cell Metab. 2010, 12, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Short, K.R.; Bigelow, M.L.; Kahl, J.; Singh, R.; Coenen-Schimke, J.; Raghavakaimal, S.; Nair, K.S. Decline in skeletal muscle mitochondrial function with aging in humans. Proc. Natl. Acad. Sci. USA 2005, 102, 5618–5623. [Google Scholar] [CrossRef] [PubMed]
- Stull, A.J.; Galgani, J.E.; Johnson, W.D.; Cefalu, W.T. The contribution of race and diabetes status to metabolic flexibility in humans. Metabolism 2010, 59, 1358–1364. [Google Scholar] [CrossRef]
- Blasco-Lafarga, C.; Monferrer-Marín, J.; Roldán, A.; Monteagudo, P.; Chulvi-Medrano, I. Metabolic Flexibility and Mechanical Efficiency in Women Over-60. Front. Physiol. 2022, 13, 869534. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M.; Aguado, X.; Gonzalez, R.; López, J.L.; Häkkinen, K. Maximal and explosive force production capacity and balance performance in men of different ages. Eur. J. Appl. Physiol. 1999, 79, 260–267. [Google Scholar] [CrossRef]
- Hester, G.M.; VanDusseldorp, T.A.; Ha, P.L.; Kiani, K.; Olmos, A.A.; Jabbari, M.; Kalladanthyil, S.; An, S.; Bailly, A.R.; Dalton, B.E.; et al. Microbiopsy Sampling for Examining Age-Related Differences in Skeletal Muscle Fiber Morphology and Composition. Front. Physiol. 2022, 12, 756626. [Google Scholar] [CrossRef]
- Carneiro, P.; Kraftman, L.; Mason, G.; Moore, L.; Rasul, I.; Scott, M. The Impacts of a Multifaceted Prenatal Intervention on Human Capital Accumulation in Early Life. Am. Econ. Rev. 2021, 111, 2506–2549. [Google Scholar] [CrossRef]
- Brooks, G.A.; Mercier, J. Balance of carbohydrate and lipid utilization during exercise: The “crossover” concept. J. Appl. Physiol. 1994, 76, 2253–2261. [Google Scholar] [CrossRef] [PubMed]
- Purdom, T.; Kravitz, L.; Dokladny, K.; Mermier, C. Understanding the factors that effect maximal fat oxidation. J. Int. Soc. Sports Nutr. 2018, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Venables, M.C.; Achten, J.; Jeukendrup, A.E. Determinants of fat oxidation during exercise in healthy men and women: A cross-sectional study. J. Appl. Physiol. 2005, 98, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Romijn, J.A.; Coyle, E.F.; Sidossis, L.S.; Gastaldelli, A.; Horowitz, J.F.; Endert, E.; Wolfe, R.R. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol.-Endocrinol. Metab. 1993, 265, E380–E391. [Google Scholar] [CrossRef]
- Achten, J.; Jeukendrup, A.E. Maximal Fat Oxidation During Exercise in Trained Men. Int. J. Sports Med. 2003, 24, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Hurley, B.F.; Nemeth, P.M.; Martin, W.H.; Hagberg, J.M.; Dalsky, G.P.; Holloszy, J.O. Muscle triglyceride utilization during exercise: Effect of training. J. Appl. Physiol. 1986, 60, 562–567. [Google Scholar] [CrossRef]
- Bircher, S.; Knechtle, B. Relationship between Fat Oxidation and Lactate Threshold in Athletes and Obese Women and Men. J. Sports Sci. Med. 2004, 3, 174–181. [Google Scholar]
- Goodpaster, B.H.; Sparks, L.M. Metabolic Flexibility in Health and Disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef]
- Zierath, J.R.; Hawley, J.A. Skeletal Muscle Fiber Type: Influence on Contractile and Metabolic Properties. PLoS Biol. 2004, 2, e348. [Google Scholar] [CrossRef] [PubMed]
- San-Millán, I.; Brooks, G.A. Assessment of Metabolic Flexibility by Means of Measuring Blood Lactate, Fat, and Carbohydrate Oxidation Responses to Exercise in Professional Endurance Athletes and Less-Fit Individuals. Sports Med. 2018, 48, 467–479. [Google Scholar] [CrossRef]
- Di Marzo, V.; Matias, I. Endocannabinoid control of food intake and energy balance. Nat. Neurosci. 2005, 8, 585–589. [Google Scholar] [CrossRef]
- Tantimonaco, M.; Ceci, R.; Sabatini, S.; Catani, M.V.; Rossi, A.; Gasperi, V.; Maccarrone, M. Physical activity and the endocannabinoid system: An overview. Cell. Mol. Life Sci. 2014, 71, 2681–2698. [Google Scholar] [CrossRef]
- Banni, S.; Carta, G.; Murru, E.; Cordeddu, L.; Giordano, E.; Sirigu, A.; Berge, K.; Vik, H.; Maki, K.C.; Di Marzo, V.; et al. Krill oil significantly decreases 2-arachidonoylglycerol plasma levels in obese subjects. Nutr. Metab 2011, 8, 7. [Google Scholar] [CrossRef]
- Côté, M.; Matias, I.; Lemieux, I.; Petrosino, S.; Alméras, N.; Després, J.P.; Di Marzo, V. Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int. J. Obes. 2007, 31, 692–699. [Google Scholar] [CrossRef]
- Iannotti, F.A.; Vitale, R.M. The Endocannabinoid System and PPARs: Focus on Their Signalling Crosstalk, Action and Transcriptional Regulation. Cells 2021, 10, 586. [Google Scholar] [CrossRef]
- Bensinger, S.J.; Tontonoz, P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature 2008, 454, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Schönke, M.; Martinez-Tellez, B.; Rensen, P.C.N. Role of the endocannabinoid system in the regulation of the skeletal muscle response to exercise. Curr. Opin. Pharmacol. 2020, 52, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Oveisi, F.; Gaetani, S.; Lin, E.; Piomelli, D. Oleoylethanolamide, an endogenous PPAR-alpha agonist, lowers body weight and hyperlipidemia in obese rats. Neuropharmacology 2005, 48, 1147–1153. [Google Scholar] [CrossRef]
- Tian, F.; Chen, L.; Qian, Z.M.; Xia, H.; Zhang, Z.; Zhang, J.; Wang, C.; Vaughn, M.G.; Tabet, M.; Lin, H. Ranking age-specific modifiable risk factors for cardiovascular disease and mortality: Evidence from a population-based longitudinal study. EClinicalMedicine 2023, 64, 102230. [Google Scholar] [CrossRef] [PubMed]
- Stegmann, H.; Kindermann, W.; Schnabel, A. Lactate Kinetics and Individual Anaerobic Threshold*. Int. J. Sports Med. 1981, 02, 160–165. [Google Scholar] [CrossRef]
- Howley, E.T.; Bassett, D.R.; Welch, H.G. Criteria for maximal oxygen uptake: Review and commentary. Med. Sci. Sports Exerc. 1995, 27, 1292–1301. [Google Scholar] [CrossRef]
- Simonson, D.C.; DeFronzo, R.A. Indirect calorimetry: Methodological and interpretative problems. Am. J. Physiol. -Endocrinol. Metab. 1990, 258, E399–E412. [Google Scholar] [CrossRef]
- Frayn, K.N. Calculation of substrate oxidation rates in vivo from gaseous exchange. J. Appl. Physiol. 1983, 55, 628–634. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Chiang, S.D.; Gessert, C.F.; Lowry, O.H. Colorimetric determination of extracted lipids. An adaptation for microgram amounts of lipids obtained from cerumen. Curr. List Med. Lit. Res. Rep. 1957, 33, 56. [Google Scholar]
- Banni, S.; Carta, G.; Contini, M.S.; Angioni, E.; Deiana, M.; Dessì, M.A.; Melis, M.P.; Corongiu, F.P. Characterization of conjugated diene fatty acids in milk, dairy products, and lamb tissues. J. Nutr. Biochem. 1996, 7, 150–155. [Google Scholar] [CrossRef]
- Murru, E.; Lopes, P.A.; Carta, G.; Manca, C.; Abolghasemi, A.; Guil-Guerrero, J.L.; Prates, J.A.M.; Banni, S. Different Dietary N-3 Polyunsaturated Fatty Acid Formulations Distinctively Modify Tissue Fatty Acid and N-Acylethanolamine Profiles. Nutrients 2021, 13, 625. [Google Scholar] [CrossRef] [PubMed]
- Shang, T.; Liu, L.; Zhou, J.; Zhang, M.; Hu, Q.; Fang, M.; Wu, Y.; Yao, P.; Gong, Z. Protective effects of various ratios of DHA/EPA supplementation on high-fat diet-induced liver damage in mice. Lipids Health Dis. 2017, 16, 65. [Google Scholar] [CrossRef]
- Murru, E.; Banni, S.; Carta, G. Nutritional Properties of Dietary Omega-3-Enriched Phospholipids. BioMed. Res. Int. 2013, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bobbioni-Harsch, E.; Habicht, F.; Lehmann, T.; James, R.W.; Rohner-Jeanrenaud, F.; Golay, A. Energy expenditure and substrates oxidative patterns, after glucose, fat or mixed load in normal weight subjects. Eur. J. Clin. Nutr. 1997, 51, 370–374. [Google Scholar] [CrossRef] [PubMed]
- McDougal, D.H.; Marlatt, K.L.; Beyl, R.A.; Redman, L.M.; Ravussin, E. A Novel Approach to Assess Metabolic Flexibility Overnight in a Whole-Body Room Calorimeter. Obesity 2020, 28, 2073–2077. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Griinari, M.; Carta, G.; Murru, E.; Ligresti, A.; Cordeddu, L.; Giordano, E.; Bisogno, T.; Collu, M.; Batetta, B.; et al. Dietary krill oil increases docosahexaenoic acid and reduces 2-arachidonoylglycerol but not N-acylethanolamine levels in the brain of obese Zucker rats. Int. Dairy J. 2010, 20, 231–235. [Google Scholar] [CrossRef]
- Law, T.D.; Clark, L.A.; Clark, B.C. Resistance Exercise to Prevent and Manage Sarcopenia and Dynapenia. Annu. Rev. Gerontol Geriatr 2016, 36, 205–228. [Google Scholar] [CrossRef] [PubMed]
- Knuiman, P.; Hopman, M.T.E.; Mensink, M. Glycogen availability and skeletal muscle adaptations with endurance and resistance exercise. Nutr. Metab. 2015, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Hatta, R.D.; Putra, I.B.; Jusuf, N.K. The Correlation between Serum Cortisol Levels with Stretch Marks in Gymnastic Male. Open Access Maced. J. Med. Sci. 2022, 10, 150–153. [Google Scholar] [CrossRef]
- Howlett, K.; Galbo, H.; Lorentsen, J.; Bergeron, R.; Zimmerman-Belsing, T.; Bülow, J.; Feldt-Rasmussen, U.; Kjær, M. Effect of adrenaline on glucose kinetics during exercise in adrenalectomised humans. J. Physiol. 1999, 519, 911–921. [Google Scholar] [CrossRef]
- Messonnier, L.A.; Emhoff, C.-A.W.; Fattor, J.A.; Horning, M.A.; Carlson, T.J.; Brooks, G.A. Lactate kinetics at the lactate threshold in trained and untrained men. J. Appl. Physiol. 2013, 114, 1593–1602. [Google Scholar] [CrossRef]
- Juel, C.; Halestrap, A.P. Lactate transport in skeletal muscle—Role and regulation of the monocarboxylate transporter. J. Physiol. 1999, 517, 633–642. [Google Scholar] [CrossRef]
- Gmada, N.; Bouhlel, E.; Mrizak, I.; Debabi, H.; Ben Jabrallah, M.; Tabka, Z.; Feki, Y.; Amri, M. Effect of Combined Active Recovery from Supramaximal Exercise on Blood Lactate Disappearance in Trained and Untrained Man. Int. J. Sports Med. 2005, 26, 874–879. [Google Scholar] [CrossRef]
- Gollnick, P.D. Peripheral factors as limitations to exercise capacity. Can. J. Appl. Sport Sci. 1982, 7, 14–21. [Google Scholar]
- Edvardsen, E.; Hem, E.; Anderssen, S.A. End Criteria for Reaching Maximal Oxygen Uptake Must Be Strict and Adjusted to Sex and Age: A Cross-Sectional Study. PLoS ONE 2014, 9, e85276. [Google Scholar] [CrossRef]
- Annunziata, C.; Lama, A.; Pirozzi, C.; Cavaliere, G.; Trinchese, G.; Di Guida, F.; Nitrato Izzo, A.; Cimmino, F.; Paciello, O.; De Biase, D.; et al. Palmitoylethanolamide counteracts hepatic metabolic inflexibility modulating mitochondrial function and efficiency in diet-induced obese mice. FASEB J. 2020, 34, 350–364. [Google Scholar] [CrossRef]
- Jamshidi, N.; Taylor, D.A. Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats: Special Report. Br. J. Pharmacol. 2001, 134, 1151–1154. [Google Scholar] [CrossRef]
- Batetta, B.; Griinari, M.; Carta, G.; Murru, E.; Ligresti, A.; Cordeddu, L.; Giordano, E.; Sanna, F.; Bisogno, T.; Uda, S.; et al. Endocannabinoids May Mediate the Ability of (n-3) Fatty Acids to Reduce Ectopic Fat and Inflammatory Mediators in Obese Zucker Rats. J. Nutr. 2009, 139, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Murru, E.; Carta, G.; Cordeddu, L.; Melis, M.; Desogus, E.; Ansar, H.; Chilliard, Y.; Ferlay, A.; Stanton, C.; Coakley, M.; et al. Dietary Conjugated Linoleic Acid-Enriched Cheeses Influence the Levels of Circulating n-3 Highly Unsaturated Fatty Acids in Humans. Int J Mol Sci 2018, 19, 1730. [Google Scholar] [CrossRef]
- Pintus, S.; Murru, E.; Carta, G.; Cordeddu, L.; Batetta, B.; Accossu, S.; Pistis, D.; Uda, S.; Elena Ghiani, M.; Mele, M.; et al. Sheep cheese naturally enriched in α-linolenic, conjugated linoleic and vaccenic acids improves the lipid profile and reduces anandamide in the plasma of hypercholesterolaemic subjects. Br. J. Nutr. 2013, 109, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.; Carta, G.; Murru, E.; Lopes, P.A.; Martins, S.V.; Prates, J.A.M.; Banni, S. Effects of dietary CLA on n-3 HUFA score and N-acylethanolamides biosynthesis in the liver of obese Zucker rats. Prostaglandins Leukot. Essent. Fat. Acids 2015, 98, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Trinchese, G.; Cavaliere, G.; Cimmino, F.; Catapano, A.; Carta, G.; Pirozzi, C.; Murru, E.; Lama, A.; Meli, R.; Bergamo, P.; et al. Decreased Metabolic Flexibility in Skeletal Muscle of Rat Fed with a High-Fat Diet Is Recovered by Individual CLA Isomer Supplementation via Converging Protective Mechanisms. Cells 2020, 9, 823. [Google Scholar] [CrossRef] [PubMed]

| Anthropometric Measures | LI (n = 9) | HI (n = 11) | p-Value | Eta2 |
|---|---|---|---|---|
| Sex | ♀ = 5, ♂ = 4 | ♀ F = 5, ♂ M = 6 | ||
| Age (years) | 52.00 ± 8.03 | 48.73 ± 6.57 | 0.329 | 0.053 |
| Height (m) | 1.66 ± 0.09 | 1.69 ± 0.07 | 0.462 | 0.030 |
| Body Weight (kg) | 69.22 ± 13.41 | 64.27 ± 9.00 | 0.337 | 0.051 |
| BMI (kg/m2) | 25.02 ± 3.78 | 22.45 ± 1.64 | 0.056 | 0.188 |
| FM (%) | 25.53 ± 6.41 | 18.05 ± 4.66 | 0.007 | 0.323 |
| FFM (%) | 75.64 ± 4.73 | 81.98 ± 4.16 | 0.005 | 0.323 |
| Resting Parameters | LI (n = 9) | HI (n = 11) | p-Value | Eta2 |
|---|---|---|---|---|
| Gly (mg/dL) | 108.71 ± 8.04 | 102.70 ± 10.35 | 0.218 | 0.099 |
| Total lipids (mg/mL) | 4.99 ± 0.93 | 4.69 ± 0.68 | 0.419 | 0.037 |
| SBP (mmHg) | 118.89 ± 9.28 | 113.18 ± 14.19 | 0.314 | 0.056 |
| DBP (mmHg) | 76.67 ± 7.07 | 75.45 ± 8.20 | 0.731 | 0.007 |
| HR (bpm) | 88.00 ± 15.94 | 71.43 ± 5.47 | 0.005 | 0.367 |
| LA (mmol/L) | 1.59 ± 0.27 | 2.01 ± 0.38 | 0.018 | 0.304 |
| RER (VCO2/VO2) | 0.85 ± 0.11 | 0.78 ± 0.08 | 0.129 | 0.120 |
| Post-Exercise Parameters | LI (n = 9) | HI (n = 11) | p-Value | Eta2 |
|---|---|---|---|---|
| Maximum workload (watt) | 123.89 ± 38.87 | 214.09 ± 67.41 | 0.002 | 0.412 |
| VO2max (ml/min/kg) | 23.31 ± 4.43 | 38.38 ± 10.97 | 0.001 | 0.453 |
| RER at VO2max | 1.15 ± 0.08 | 1.06 ± 0.05 | 0.007 | 0.347 |
| Gly at recovery (% of basal value) | 93.96 ± 10.44 | 118.56 ± 24.15 | 0.002 | 0.297 |
| HR at VO2max (bpm) | 168.11 ± 15.48 | 164.48 ± 15.35 | 0.606 | 0.015 |
| HRR (%) | 95.35 ± 32.40 | 130.99 ± 23.74 | 0.011 | 0.309 |
| HRr (%) | 6.43 ± 1.20 | 9.64 ± 2.59 | 0.006 | 0.408 |
| LA at VO2max (mmol/L) | 6.13 ± 1.62 | 7.94 ± 2.82 | 0.107 | 0.138 |
| Parameters | Age | Weight | %FM | BMI | WLmax | %VO2max | %VO2 max at PFO | PFO | PCO | MFI | PESO | %Gly at Recovery | DHA/ EPA | OEA | 2AG | OEA/ 2-AG |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 1 | |||||||||||||||
| Weight | 0.46 * | 1 | ||||||||||||||
| %FM | 0.19 | 0.03 | 1 | |||||||||||||
| BMI | 0.49 * | 0.81 *** | 0.42 | 1 | ||||||||||||
| WLmax | −0.21 | 0.15 | −0.81 *** | −0.12 | 1 | |||||||||||
| %VO2max | −0.37 | −0.15 | −0.80 *** | −0.33 | 0.92 *** | 1 | ||||||||||
| %VO2 max at PFO | −0.09 | −0.30 | −0.63 ** | −0.39 | 0.60 ** | 0.72 *** | 1 | |||||||||
| PFO | −0.22 | −0.06 | −0.79 *** | −0.27 | 0.85 *** | 0.92 *** | 0.80 *** | 1 | ||||||||
| PCO | −0.21 | 0.10 | −0.65 ** | −0.06 | 0.75 *** | 0.75 *** | 0.40 | 0.73 *** | 1 | |||||||
| MFI | −0.25 | −0.29 | −0.71 *** | −0.42 | 0.71 *** | 0.85 *** | 0.92 *** | 0.94 *** | 0.60 ** | 1 | ||||||
| PESO | −0.41 | −0.36 | −0.60 ** | −0.40 | 0.59 ** | 0.74 *** | 0.57 ** | 0.72 *** | 0.88 *** | 0.73 *** | 1 | |||||
| %Gly at recovery | −0.43 | −0.22 | −0.37 | −0.32 | 0.47 * | 0.55 * | 0.33 | 0.57 * | 0.64 ** | 0.50 * | 0.69 ** | 1 | ||||
| DHA/EPA | −0.46 * | −0.58 ** | −0.44 * | −0.53 * | 0.31 | 0.56 ** | 0.39 | 0.40 | 0.48 * | 0.48 * | 0.70 ** | 0.49 * | 1 | |||
| OEA | −0.29 | −0.39 | −0.42 | −0.53 ** | 0.37 | 0.47 * | 0.37 | 0.42 | 0.35 | 0.43 | 0.50 * | 0.64 ** | 0.58 ** | 1 | ||
| 2AG | 0.09 | 0.45 | 0.27 | 0.53 * | −0.30 | −0.34 | −0.35 | −0.32 | −0.18 | −0.36 | −0.30 | −0.25 | −0.50 * | −0.39 | 1 | |
| OEA/2-AG | −0.21 | −0.50 * | −0.29 | −0.54 * | 0.21 | 0.31 | 0.31 | 0.23 | 0.13 | 0.28 | 0.31 | 0.35 | 0.72 *** | 0.62 ** | −0.69 ** | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murru, E.; Manca, C.; Carta, G.; Ruggiu, M.; Solinas, R.; Montisci, R.; Hodson, L.; Dearlove, D.; Mollica, M.P.; Tocco, F.; et al. Indirect Calorimetry-Based Novel Approach for Evaluating Metabolic Flexibility and Its Association with Circulating Metabolic Markers in Middle-Aged Subjects. Nutrients 2024, 16, 525. https://doi.org/10.3390/nu16040525
Murru E, Manca C, Carta G, Ruggiu M, Solinas R, Montisci R, Hodson L, Dearlove D, Mollica MP, Tocco F, et al. Indirect Calorimetry-Based Novel Approach for Evaluating Metabolic Flexibility and Its Association with Circulating Metabolic Markers in Middle-Aged Subjects. Nutrients. 2024; 16(4):525. https://doi.org/10.3390/nu16040525
Chicago/Turabian StyleMurru, Elisabetta, Claudia Manca, Gianfranca Carta, Michele Ruggiu, Roberto Solinas, Roberta Montisci, Leanne Hodson, David Dearlove, Maria Pina Mollica, Filippo Tocco, and et al. 2024. "Indirect Calorimetry-Based Novel Approach for Evaluating Metabolic Flexibility and Its Association with Circulating Metabolic Markers in Middle-Aged Subjects" Nutrients 16, no. 4: 525. https://doi.org/10.3390/nu16040525
APA StyleMurru, E., Manca, C., Carta, G., Ruggiu, M., Solinas, R., Montisci, R., Hodson, L., Dearlove, D., Mollica, M. P., Tocco, F., & Banni, S. (2024). Indirect Calorimetry-Based Novel Approach for Evaluating Metabolic Flexibility and Its Association with Circulating Metabolic Markers in Middle-Aged Subjects. Nutrients, 16(4), 525. https://doi.org/10.3390/nu16040525









