Association between Dietary Inflammatory Index and Bone Mineral Density Changes among Pregnant Women: A Prospective Study in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. BMD Measurement
2.3. Assessment of Inflammatory Factors of Blood Samples
2.4. Measurement of Bone Turnover Markers
2.5. Dietary Assessment
2.6. DII Calculation
2.7. Covariates
2.8. Statistical Analysis
3. Results
3.1. Demographic Characteristics of Participants across Tertiles of DII
3.2. Distribution of Food Groups across Tertiles of DII
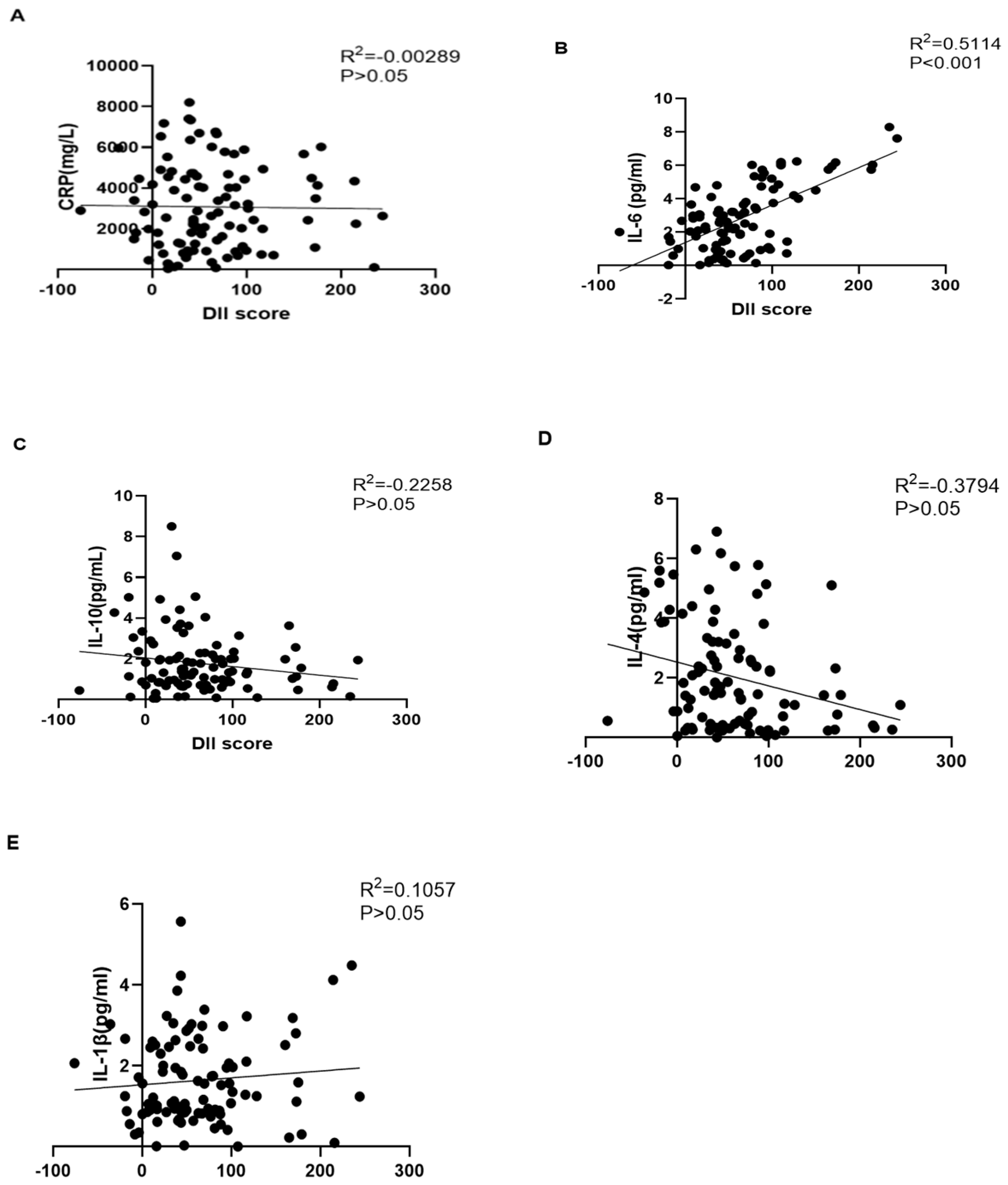
3.3. The Serum Inflammation Factor Level across the Tertiles of DII
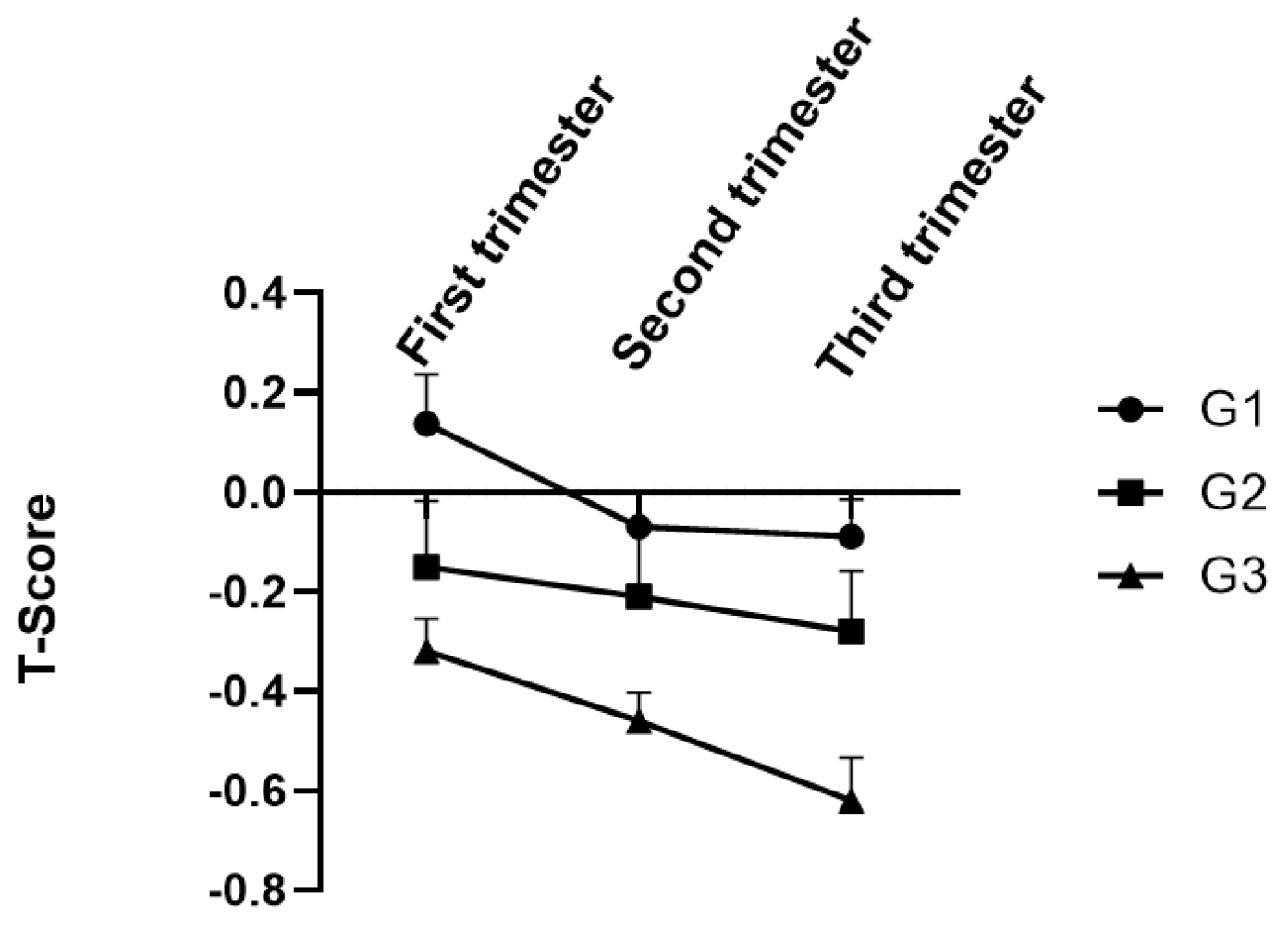
3.4. Maternal BMD Levels of DII Score Groups
3.5. The Linear Association between DII and BMD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fowler, M.E.; Akinyemiju, T.F. Meta-analysis of the association between dietary inflammatory index (DII) and cancer outcomes. Int. J. Cancer 2017, 141, 2215–2227. [Google Scholar] [CrossRef]
- Tabung, F.K.; E Steck, S.; Liese, A.D.; Zhang, J.; Ma, Y.; Caan, B.; Chlebowski, R.T.; Freudenheim, J.L.; Hou, L.; Mossavar-Rahmani, Y.; et al. Association between dietary inflammatory potential and breast cancer incidence and death: Results from the Women’s Health Initiative. Br. J. Cancer 2016, 114, 1277–1285. [Google Scholar] [CrossRef]
- López-Alarcón, M.; Perichart-Perera, O.; Flores-Huerta, S.; Inda-Icaza, P.; Rodríguez-Cruz, M.; Armenta-Álvarez, A.; Bram-Falcón, M.T.; Mayorga-Ochoa, M. Excessive refined carbohydrates and scarce micronutrients intakes increase inflammatory mediators and insulin resistance in prepubertal and pubertal obese children independently of obesity. Mediat. Inflamm. 2014, 2014, 849031. [Google Scholar] [CrossRef]
- Bendsen, N.T.; Stender, S.; Szecsi, P.B.; Pedersen, S.B.; Basu, S.; Hellgren, L.I.; Newman, J.W.; Larsen, T.M.; Haugaard, S.B.; Astrup, A. Effect of industrially produced trans fat on markers of systemic inflammation: Evidence from a randomized trial in women. J. Lipid Res. 2011, 52, 1821–1828. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef]
- Darbandi, M.; Hamzeh, B.; Ayenepour, A.; Rezaeian, S.; Najafi, F.; Shakiba, E.; Pasdar, Y. Anti-inflammatory diet consumption reduced fatty liver indices. Sci. Rep. 2021, 11, 22601. [Google Scholar] [CrossRef]
- Bahr, L.S.; Franz, K.; Mähler, A. Assessing the (anti)-inflammatory potential of diets. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Hebert, J.R.; Marcos, A.; Diaz, L.-E.; Gomez, S.; Nova, E.; Michels, N.; Arouca, A.; González-Gil, E.; Frederic, G.; et al. Association between dietary inflammatory index and inflammatory markers in the HELENA study. Mol. Nutr. Food Res. 2017, 61, 1600707. [Google Scholar] [CrossRef] [PubMed]
- Dutra, M.T.; Avelar, B.P.; Souza, V.C.; Bottaro, M.; Oliveira, R.J.; Nóbrega, O.T.; Moreno Lima, R. Relationship between sarcopenic obesity-related phenotypes and inflammatory markers in postmenopausal women. Clin. Physiol. Funct. Imaging 2017, 37, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, Y.A.M.; Kravchychyn, A.C.P.; de Castro Ferreira Vicente, S.; Campos, R.; Tock, L.; Oyama, L.M.; Boldarine, V.T.; Masquio, D.C.L.; Thivel, D.; Shivappa, N.; et al. An interdisciplinary weight loss program improves body composition and metabolic profile in adolescents with obesity: Associations with the dietary inflammatory index. Front. Nutr. 2019, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Kenđel Jovanović, G.; Pavičić Žeželj, S.; Klobučar Majanović, S.; Mrakovcic-Sutic, I.; Šutić, I. Metabolic syndrome and its association with the Dietary Inflammatory Index (DII)® in a Croatian working population. J. Hum. Nutr. Diet. 2020, 33, 128–137. [Google Scholar] [CrossRef]
- Pocovi-Gerardino, G.; Correa-Rodríguez, M.; Callejas-Rubio, J.-L.; Ríos-Fernández, R.; Martín-Amada, M.; Cruz-Caparros, M.-G.; Rueda-Medina, B.; Ortego-Centeno, N. Dietary inflammatory index score and cardiovascular disease risk markers in women with systemic lupus erythematosus. J. Acad. Nutr. Diet. 2020, 120, 280–287. [Google Scholar] [CrossRef]
- Amakye, W.K.; Zhang, Z.; Wei, Y.; Shivappa, N.; Hebert, J.R.; Wang, J.; Su, Y.; Mao, L. The relationship between dietary inflammatory index (DII) and muscle mass and strength in Chinese children aged 6–9 years. Asia Pac. J. Clin. Nutr. 2018, 27, 1315–1324. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Lee, J.; Kim, J. Association between dietary inflammatory index and metabolic syndrome in the general korean population. Nutrients 2018, 10, 648. [Google Scholar] [CrossRef]
- Shivappa, N.; Hébert, J.R.; Karamati, M.; Shariati-Bafghi, S.-E.; Rashidkhani, B. Increased inflammatory potential of diet is associated with bone mineral density among postmenopausal women in Iran. Eur. J. Nutr. 2016, 55, 561–568. [Google Scholar] [CrossRef]
- Kyozuka, H.; Murata, T.; Fukuda, T.; Yamaguchi, A.; Kanno, A.; Yasuda, S.; Sato, A.; Ogata, Y.; Kuse, M.; Hosoya, M.; et al. Dietary inflammatory index during pregnancy and the risk of intrapartum fetal asphyxia: The Japan Environment and Children’s Study. Nutrients 2020, 12, 3482. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Rifas-Shiman, S.L.; Shivappa, N.; Wirth, M.D.; Hébert, J.R.; Gold, D.R.; Gillman, M.W.; Oken, E. Dietary Inflammatory Potential during Pregnancy Is Associated with Lower Fetal Growth and Breastfeeding Failure: Results from Project Viva. J. Nutr. 2015, 146, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Amor, S.; Puentes, F.; Baker, D.; Van Der Valk, P. Inflammation in neurodegenerative diseases. Immunology 2010, 129, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Rane, M. Interleukin-6 signaling and anti-interleukin-6 therapeutics in cardiovascular disease. Circ. Res. 2021, 128, 1728–1746. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Yin, Y.; Gao, Y.; Lau, S.; Shen, F.; Zhao, M.; Chen, Q. The increased maternal serum levels of IL-6 are associated with the severity and onset of preeclampsia. Cytokine 2012, 60, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Fuggle, N.R.; Westbury, L.D.; Syddall, H.E.; Duggal, N.A.; Shaw, S.C.; Maslin, K.; Dennison, E.M.; Lord, J.; Cooper, C. Relationships between markers of inflammation and bone density: Findings from the Hertfordshire Cohort Study. Osteoporos. Int. 2018, 29, 1581–1589. [Google Scholar] [CrossRef]
- Cauley, J.A.; E Barbour, K.; Harrison, S.L.; Cloonan, Y.K.; Danielson, M.E.; E Ensrud, K.; Fink, H.A.; Orwoll, E.S.; Boudreau, R. Inflammatory markers and the risk of hip and vertebral fractures in men: The osteoporotic fractures in men (MrOS). J. Bone Miner. Res. 2016, 31, 2129–2138. [Google Scholar] [CrossRef]
- Lin, C.C.; Li, T.C.; Liu, C.S.; Yang, C.W.; Lin, C.H.; Hsiao, J.H.; Meng, N.H.; Lin, W.Y.; Liao, L.N.; Li, C.I.; et al. Associations of TNF-α and IL-6 polymorphisms with osteoporosis through joint effects and interactions with LEPR gene in Taiwan, Taichung Community Health Study for Elders (TCHS-E). Mol. Biol. Rep. 2016, 43, 1179–1191. [Google Scholar] [CrossRef] [PubMed]
- Scheidt-Nave, C.; Bismar, H.; Leidig-Bruckner, G.; Woitge, H.; Seibel, M.J.; Ziegler, R.; Pfeilschifter, J. Serum interleukin 6 is a major predictor of bone loss in women specific to the first decade past menopause. J. Clin. Endocrinol. Metab. 2001, 86, 2032–2042. [Google Scholar] [CrossRef] [PubMed]
- Hong, N.; Kim, J.E.; Lee, S.J.; Kim, S.H.; Rhee, Y. Changes in bone mineral density and bone turnover markers during treatment with teriparatide in pregnancy-and lactation-associated osteoporosis. Clin. Endocrinol. 2018, 88, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.S.; Wang, L.; de Bakker, C.M.J.; Lai, X. Mechanical regulation of the maternal skeleton during reproduction and lactation. Curr. Osteoporos. Rep. 2019, 17, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, C.S. Fetal calcium metabolism. In Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism, 8th ed.; Clifford, J., Rosen, M.D., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 180–187. [Google Scholar]
- Scholl, T.O.; Chen, X.; Stein, T.P. Maternal calcium metabolic stress and fetal growth. Am. J. Clin. Nutr. 2014, 99, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Møller, U.K.; Við Streym, S.; Mosekilde, L.; Rejnmark, L. Changes in bone mineral density and body composition during pregnancy and post partum. A controlled cohort study. Osteoporos. Int. 2012, 23, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Woolford, S.J.; D’angelo, S.; Mancano, G.; Curtis, E.M.; Ashai, S.; Shivappa, N.; Hébert, J.R.; Crozier, S.R.; Phillips, C.M.; Suderman, M.; et al. Associations Between Late Pregnancy Dietary Inflammatory Index (DII) and Offspring Bone Mass, A Meta-Analysis of the Southampton Women’s Survey (SWS) and the Avon Longitudinal Study of Parents and Children (ALSPAC). J. Bone Miner. Res. 2022, 37, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Dibley, M.J.; D’Este, C. Reliability and validity of a food-frequency questionnaire for Chinese postmenopausal women. Public Health Nutr. 2004, 7, 91–98. [Google Scholar] [CrossRef][Green Version]
- Zhang, Z.-Q.; Cao, W.-T.; Shivappa, N.; Hebert, J.R.; Li, B.-L.; He, J.; Tang, X.-Y.; Liang, Y.-Y.; Chen, Y.-M. Association between diet inflammatory index and osteoporotic hip fracture in elderly chinese population. J. Am. Med. Dir. Assoc. 2017, 18, 671–677. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef]
- Chen, Y.; Xiang, J.; Wang, Z.; Xiao, Y.; Zhang, D.; Chen, X.; Li, H.; Liu, M.; Zhang, Q. Associations of bone mineral density with lean mass, fat mass, and dietary patterns in postmenopausal chinese women: A 2-year prospective study. PLoS ONE 2015, 10, e0137097. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.-F.; Wu, B.-H.; Fan, F.; Xie, H.-L.; Xue, W.-Q.; Zhu, H.-L.; Chen, Y.-M. Dietary patterns and the risk of hip fractures in elderly chinese: A matched case-control study. J. Clin. Endocrinol. Metab. 2013, 98, 2347–2355. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.; Harris, S.S.; Must, A.; Phillips, S.M.; Rand, W.M.; Dawson-Hughes, B. Household tobacco smoke exposure is negatively associated with premenopausal bone mass. Osteoporos. Int. 2002, 13, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Okubo, H.; Sasaki, S.; Horiguchi, H.; Oguma, E.; Miyamoto, K.; Hosoi, Y.; Kim, M.-K.; Kayama, F. Dietary patterns associated with bone mineral density in premenopausal Japanese farmwomen. Am. J. Clin. Nutr. 2006, 83, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Ocké, M.C. Evaluation of methodologies for assessing the overall diet: Dietary quality scores and dietary pattern analysis. Proc. Nutr. Soc. 2013, 72, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Sponholtz, T.R.; Zhang, X.; Fontes, J.D.T.; Meigs, J.B.; Cupples, L.A.; Kiel, D.P.; Hannan, M.T.; McLean, R.R. Association between inflammatory biomarkers and bone mineral density in a community-based cohort of men and women. Arthritis Care Res. 2014, 66, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Ehnert, S.; Baur, J.; Schmitt, A.; Neumaier, M.; Lucke, M.; Dooley, S.; Vester, H.; Wildemann, B.; Stöckle, U.; Nussler, A.K. TGF-β1 as possible link between loss of bone mineral density and chronic inflammation. PLoS ONE 2010, 5, e14073. [Google Scholar] [CrossRef]
- Esmaillzadeh, A.; Kimiagar, M.; Mehrabi, Y.; Azadbakht, L.; Hu, F.B.; Willett, W.C. Dietary patterns and markers of systemic inflammation among iranian women. J. Nutr. 2007, 137, 992–998. [Google Scholar] [CrossRef]
- Tartibian, B.; Maleki, B.H.; Kanaley, J.; Sadeghi, K. Long-term aerobic exercise and omega-3 supplementation modulate osteoporosis through inflammatory mechanisms in post-menopausal women: A randomized, repeated measures study. Nutr. Metab. 2011, 8, 71. [Google Scholar] [CrossRef]
- Karlsson, M.K.; Ahlborg, H.G.; Karlsson, C. Maternity and bone mineral density. Acta Orthop. 2005, 76, 2–13. [Google Scholar] [CrossRef]
- Degennaro, V.A.; Brandi, M.L.; Cagninelli, G.; Casciaro, S.; Ciardo, D.; Conversano, F.; Di Pasquo, E.; Gonnelli, S.; Lombardi, F.A.; Pisani, P.; et al. First assessment of bone mineral density in healthy pregnant women by means of Radiofrequency Echographic Multi Spectrometry (REMS) technology. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 263, 44–49. [Google Scholar] [CrossRef]
- Hu, Y.; Li, X.; Zhi, X.; Cong, W.; Huang, B.; Chen, H.; Wang, Y.; Li, Y.; Wang, L.; Fang, C.; et al. RANKL from bone marrow adipose lineage cells promotes osteoclast formation and bone loss. EMBO Rep. 2021, 22, e52481. [Google Scholar] [CrossRef]
- Yao, Z.; Getting, S.J.; Locke, I.C. Regulation of TNF-induced osteoclast differentiation. Cells 2021, 11, 132. [Google Scholar] [CrossRef]
- Epsley, S.; Tadros, S.; Farid, A.; Kargilis, D.; Mehta, S.; Rajapakse, C.S. The effect of inflammation on bone. Front. Physiol. 2021, 11, 1695. [Google Scholar] [CrossRef] [PubMed]
- Ishimi, Y. Soybean isoflavones in bone health. Food Factors Health Promot. 2009, 61, 104–116. [Google Scholar]
- Zheng, X.; Lee, S.K.; Chun, O.K. Soy isoflavones and osteoporotic bone loss: A review with an emphasis on modulation of bone remodeling. J. Med. Food 2016, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Corbi, G.; Nobile, V.; Conti, V.; Cannavo, A.; Sorrenti, V.; Medoro, A.; Scapagnini, G.; Davinelli, S. Equol and Resveratrol Improve Bone Turnover Biomarkers in Postmenopausal Women: A Clinical Trial. Int. J. Mol. Sci. 2023, 24, 12063. [Google Scholar] [CrossRef]
- Orchard, T.; Yildiz, V.; Steck, S.E.; Hébert, J.R.; Ma, Y.; Cauley, J.A.; Li, W.; Mossavar-Rahmani, Y.; Johnson, K.C.; Sattari, M.; et al. Dietary inflammatory index, bone mineral density, and risk of fracture in postmenopausal women: Results from the women’s health initiative. J. Bone Miner. Res. 2017, 32, 1136–1146. [Google Scholar] [CrossRef]
- Lujano-Negrete, A.Y.; Rodríguez-Ruiz, M.C.; Skinner-Taylor, C.M.; Perez-Barbosa, L.; de la Garza, J.A.C.; García-Hernández, P.A.; Espinosa-Banuelos, L.G.; Gutierrez-Leal, L.F.; Jezzini-Martínez, S.; Galarza-Delgado, D. Bone metabolism and osteoporosis during pregnancy and lactation. Arch. Osteoporos. 2022, 17, 36. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Tertiles of DII | p Value | ||
|---|---|---|---|---|
| G1 (n = 96) | G2 (n = 96) | G3 (n = 97) | ||
| Age, years | 28.98 | 29.95 | 28.73 | 0.087 |
| BMI, kg/m2 | 22.06 | 21.66 | 21.84 | 0.696 |
| Educational level, n | ||||
| High school or below | 56 | 45 | 55 | 0.071 |
| College | 32 | 38 | 28 | |
| Postgraduate or above | 6 | 11 | 10 | |
| Time spent for physical activity, n | ||||
| <2 h/week | 24 | 23 | 21 | 0.556 |
| 2–4 h/week | 24 | 35 | 33 | |
| 4–6 h/week | 24 | 18 | 26 | |
| >6 h/week | 22 | 18 | 13 | |
| Nutrient intake | ||||
| Calcium, mg/day | 1140 | 1143 | 1161 | 0.805 |
| Vitamin D, IU/day | 218.16 | 239.36 | 217.36 | 0.524 |
| Isoflavone, mg/day | 37.3 | 42.7 | 49.9 | 0.039 |
| Time for sunshine, h/day | 1.65 | 1.72 | 1.68 | 0.878 |
| Food Groups | Tertiles of DII | p Value | ||
|---|---|---|---|---|
| G1 (n = 96) | G2 (n = 96) | G3 (n = 97) | ||
| Dietary energy, kcal/day | 1957.74 | 2106.18 | 2310.87 | |
| Refined Cereals, g/day | 250.04 | 263.99 | 344.62 | <0.001 |
| Whole Cereals, g/day | 11.97 | 23.95 | 39.99 | <0.001 |
| Soy and soy product, g/day | 62.94 | 113.47 | 170.27 | <0.001 |
| Dark leafy vegetables, g/day | 110.62 | 158.59 | 292.33 | <0.001 |
| Light vegetables, g/day | 134.57 | 204.52 | 312.69 | <0.001 |
| Dark fruits, g/day | 88.51 | 155.88 | 221.80 | <0.001 |
| Light fruits, g/day | 116.08 | 152.61 | 189.10 | <0.001 |
| Poultry, g/day | 9.18 | 18.02 | 24.77 | <0.001 |
| Meat and Processed meats, g/day | 33.69 | 47.75 | 52.72 | 0.041 |
| Fish and aquatic products, g/day | 28.89 | 34.40 | 33.06 | 0.518 |
| Eggs, g/day | 44.68 | 61.69 | 52.44 | 0.013 |
| Milk and Dairy products, g/day | 208.31 | 221.71 | 270.17 | 0.046 |
| Fungus and Alga, g/day | 16.43 | 28.23 | 53.54 | <0.001 |
| Nuts, g/day | 9.03 | 12.19 | 19.84 | <0.001 |
| Backed Bread, g/day | 15.18 | 17.60 | 21.36 | 0.245 |
| Candies, g/day | 1.47 | 1.54 | 2.14 | 0.612 |
| Junk food, g/day | 1.70 | 1.31 | 1.19 | 0.724 |
| Fruit and vegetable juice, mL/day | 4.20 | 4.16 | 5.43 | 0.871 |
| Soft drinks, mL/day | 30.33 | 35.58 | 29.14 | 0.854 |
| Item | Tertiles of DII | p Value | ||
|---|---|---|---|---|
| G1 (n = 96) | G2 (n = 96) | G3 (n = 97) | ||
| CRP (mg/L) | 3045.902 | 3104.019 | 3095.260 | 0.653 |
| IL-6 (pg/mL) | 2.019 | 2.474 | 2.862 | 0.034 |
| IL-10 (pg/mL) | 1.879 | 1.716 | 1.685 | 0.795 |
| IL-4 (pg/mL) | 1.956 | 1.925 | 1.806 | 0.879 |
| IL-1β (pg/mL) | 1.478 | 1.518 | 1.761 | 0.082 |
| Item | Tertiles of DII | p Value | ||
|---|---|---|---|---|
| G1 (n = 96) | G2 (n = 96) | G3 (n = 97) | ||
| OC | 12.87 | 11.29 | 9.55 | 0.415 |
| OPG | 624.65 | 605.62 | 593.41 | 0.209 |
| PINP | 56.49 | 48.72 | 45.58 | 0.063 |
| PTH | 22.86 | 17.84 | 15.93 | 0.112 |
| RANKL | 5.34 | 7.89 | 10.43 | 0.002 |
| Item | Tertiles of DII | p Value | ||
|---|---|---|---|---|
| G1 (n = 96) | G2 (n = 96) | G3 (n = 97) | ||
| SOS | 1399.65 | 1404.09 | 1403.26 | 0.978 |
| Z-score | −0.11 | −0.25 | −0.44 | 0.694 |
| T-score | −0.09 | −0.28 | −0.62 | 0.043 |
| Model Name | Tertiles of DII | p for Trend * | ||
|---|---|---|---|---|
| G1 (n = 96) | G2 (n = 96) | G3 (n = 97) | ||
| Model 1 | 0.03 | 0.01 | −0.09 | 0.153 |
| Model 2 | −0.02 | −0.07 | −0.16 | 0.047 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Zhou, Y.; Wen, Z.; Ye, W.; Gao, L.; Xu, Y. Association between Dietary Inflammatory Index and Bone Mineral Density Changes among Pregnant Women: A Prospective Study in China. Nutrients 2024, 16, 455. https://doi.org/10.3390/nu16030455
Zhu X, Zhou Y, Wen Z, Ye W, Gao L, Xu Y. Association between Dietary Inflammatory Index and Bone Mineral Density Changes among Pregnant Women: A Prospective Study in China. Nutrients. 2024; 16(3):455. https://doi.org/10.3390/nu16030455
Chicago/Turabian StyleZhu, Xiaoyu, Yalin Zhou, Zhang Wen, Wanyun Ye, Lan Gao, and Yajun Xu. 2024. "Association between Dietary Inflammatory Index and Bone Mineral Density Changes among Pregnant Women: A Prospective Study in China" Nutrients 16, no. 3: 455. https://doi.org/10.3390/nu16030455
APA StyleZhu, X., Zhou, Y., Wen, Z., Ye, W., Gao, L., & Xu, Y. (2024). Association between Dietary Inflammatory Index and Bone Mineral Density Changes among Pregnant Women: A Prospective Study in China. Nutrients, 16(3), 455. https://doi.org/10.3390/nu16030455








