Assessment of Epicardial Fat in Children: Its Role as a Cardiovascular Risk Factor and How It Is Influenced by Lifestyle Habits
Abstract
1. Introduction
2. Methods
3. Assessment of Epicardial Adipose Tissue
3.1. Transthoracic Echocardiography
3.2. Magnetic Resonance Imaging (MRI)
3.3. Computed Tomography
4. Epicardial Fat Tissue as a Marker for Cardiovascular Risk in Pediatric Age
4.1. Children with Obesity
4.2. Children with Undernutrition
5. The Impact of Lifestyle Habits and Lifestyle Intervention on Epicardial Adipose Tissue
5.1. Unhealthy Eating Habits
5.2. Sedentary Life and Exercise
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Iacobellis, G. Aging Effects on Epicardial Adipose Tissue. Front. Aging 2021, 2, 666260. [Google Scholar] [CrossRef]
- Iacobellis, G. Epicardial adipose tissue in contemporary cardiology. Nat. Rev. Cardiol. 2022, 19, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Konwerski, M.; Gąsecka, A.; Opolski, G.; Grabowski, M.; Mazurek, T. Role of Epicardial Adipose Tissue in Cardiovascular Diseases: A Review. Biology 2022, 11, 355. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Ribaudo, M.C.; Assael, F.; Vecci, E.; Tiberti, C.; Zappaterreno, A.; Di Mario, U.; Leonetti, F. Echocardiographic Epicardial Adipose Tissue Is Related to Anthropometric and Clinical Parameters of Metabolic Syndrome: A New Indicator of Cardiovascular Risk. J. Clin. Endocrinol. Metab. 2003, 88, 5163–5168. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Assael, F.; Ribaudo, M.C.; Zappaterreno, A.; Alessi, G.; Di Mario, U.; Leonetti, F. Epicardial Fat from Echocardiography: A New Method for Visceral Adipose Tissue Prediction. Obes. Res. 2003, 11, 304–310. [Google Scholar] [CrossRef]
- Hirata, Y.; Kurobe, H.; Akaike, M.; Chikugo, F.; Hori, T.; Bando, Y.; Nishio, C.; Higashida, M.; Nakaya, Y.; Kitagawa, T.; et al. Enhanced Inflammation in Epicardial Fat in Patients with Coronary Artery Disease. Int. Heart J. 2011, 52, 139–142. [Google Scholar] [CrossRef]
- Rajsheker, S.; Manka, D.; Blomkalns, A.L.; Chatterjee, T.K.; Stoll, L.L.; Weintraub, N.L. Crosstalk between perivascular adipose tissue and blood vessels. Curr. Opin. Pharmacol. 2010, 10, 191–196. [Google Scholar] [CrossRef]
- Mazurek, T.; Zhang, L.; Zalewski, A.; Mannion, J.D.; Diehl, J.T.; Arafat, H.; Sarov-Blat, L.; O’Brien, S.; Keiper, E.A.; Johnson, A.G.; et al. Human Epicardial Adipose Tissue Is a Source of Inflammatory Mediators. Circulation 2003, 108, 2460–2466. [Google Scholar] [CrossRef]
- Ozdemir, O.; Hizli, S.; Abaci, A.; Agladioglu, K.; Aksoy, S. Echocardiographic Measurement of Epicardial Adipose Tissue in Obese Children. Pediatr. Cardiol. 2010, 31, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Natale, F.; Tedesco, M.A.; Mocerino, R.; De Simone, V.; Di Marco, G.M.; Aronne, L.; Credendino, M.; Siniscalchi, C.; Calabro, P.; Cotrufo, M.; et al. Visceral adiposity and arterial stiffness: Echocardiographic epicardial fat thickness reflects, better than waist circumference, carotid arterial stiffness in a large population of hypertensives. Eur. J. Echocardiogr. 2009, 10, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.J.; Worthley, M.I.; Psaltis, P.J.; Carbone, A.; Dundon, B.K.; Duncan, R.F.; Piantadosi, C.; Lau, D.H.; Sanders, P.; Wittert, G.A.; et al. Validation of cardiovascular magnetic resonance assessment of pericardial adipose tissue volume. J. Cardiovasc. Magn. Reson. 2009, 11, 15. [Google Scholar] [CrossRef]
- Goel, R.; Alharthi, M.; Jiamsripong, P.; Cha, S.; Mookadam, F. Epicardial fat and its association with cardiovascular risk: A cross-sectional observational study. Heart Views 2010, 11, 103. [Google Scholar] [CrossRef]
- Ross, R.; Shaw, K.; Martel, Y.; De Guise, J.; Avruch, L. Adipose tissue distribution measured by magnetic resonance imaging in obese women. Am. J. Clin. Nutr. 1993, 57, 470–475. [Google Scholar] [CrossRef]
- Society for Cardiovascular Magnetic Resonance; Board of Trustees Task Force on Standardized Protocols; Kramer, C.M.; Barkhausen, J.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. J. Cardiovasc. Magn. Reson. 2013, 15, 91. [Google Scholar] [CrossRef] [PubMed]
- Doesch, C.; Haghi, D.; Flüchter, S.; Suselbeck, T.; Schoenberg, S.O.; Michaely, H.; Borggrefe, M.; Papavassiliu, T. Epicardial adipose tissue in patients with heart failure. J. Cardiovasc. Magn. Reson. 2010, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Flüchter, S.; Haghi, D.; Dinter, D.; Heberlein, W.; Kühl, H.P.; Neff, W.; Sueselbeck, T.; Borggrefe, M.; Papavassiliu, T. Volumetric Assessment of Epicardial Adipose Tissue with Cardiovascular Magnetic Resonance Imaging. Obesity 2007, 15, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Hsu, F.-C.; Harris, T.B.; Liu, Y.; Kritchevsky, S.B.; Szklo, M.; Ouyang, P.; Espeland, M.A.; Lohman, K.K.; Criqui, M.H.; et al. The association of pericardial fat with incident coronary heart disease: The Multi-Ethnic Study of Atherosclerosis (MESA). Am. J. Clin. Nutr. 2009, 90, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hsu, H.; Hung, W.; Yu, T.; Chen, Y.; Chiu, C.; Lu, L.; Chung, F.; Shin, S.; Lee, Y. Increased epicardial adipose tissue (EAT) volume in type 2 diabetes mellitus and association with metabolic syndrome and severity of coronary atherosclerosis. Clin. Endocrinol. 2009, 70, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Gorter, P.M.; De Vos, A.M.; Van Der Graaf, Y.; Stella, P.R.; Doevendans, P.A.; Meijs, M.F.L.; Prokop, M.; Visseren, F.L.J. Relation of Epicardial and Pericoronary Fat to Coronary Atherosclerosis and Coronary Artery Calcium in Patients Undergoing Coronary Angiography. Am. J. Cardiol. 2008, 102, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.S.; Gona, P.; Hoffmann, U.; Porter, S.A.; Salton, C.J.; Massaro, J.M.; Levy, D.; Larson, M.G.; D’Agostino, R.B.; O’Donnell, C.J.; et al. Pericardial Fat, Intrathoracic Fat, and Measures of Left Ventricular Structure and Function: The Framingham Heart Study. Circulation 2009, 119, 1586–1591. [Google Scholar] [CrossRef]
- Abbara, S.; Desai, J.C.; Cury, R.C.; Butler, J.; Nieman, K.; Reddy, V. Mapping epicardial fat with multi-detector computed tomography to facilitate percutaneous transepicardial arrhythmia ablation. Eur. J. Radiol. 2006, 57, 417–422. [Google Scholar] [CrossRef]
- Schusterova, I.; Leenen, F.H.H.; Jurko, A.; Sabol, F.; Takacova, J. Epicardial adipose tissue and cardiometabolic risk factors in overweight and obese children and adolescents. Pediatr. Obes. 2014, 9, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Morricone, L.; Malavazos, A.E.; Coman, C.; Donati, C.; Hassan, T.; Caviezel, F. Echocardiographic Abnormalities in Normotensive Obese Patients: Relationship with Visceral Fat. Obes. Res. 2002, 10, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Alp, H.; Eklioğlu, B.S.; Atabek, M.E.; Karaarslan, S.; Baysal, T.; Altın, H.; Karataş, Z.; Şap, F. Evaluation of epicardial adipose tissue, carotid intima-media thickness and ventricular functions in obese children and adolescents. J. Pediatr. Endocrinol. Metab. 2014, 27, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Villasante Fricke, A.C.; Iacobellis, G. Epicardial Adipose Tissue: Clinical Biomarker of Cardio-Metabolic Risk. Int. J. Mol. Sci. 2019, 20, 5989. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Arya, M. Sharma Epicardial Adipose Tissue as New Cardio-Metabolic Risk Marker and Potential Therapeutic Target in the Metabolic Syndrome. Curr. Pharm. Des. 2007, 13, 2180–2184. [Google Scholar] [CrossRef] [PubMed]
- Mazur, A.; Ostański, M.; Telega, G.; Malecka-Tendera, E. Is epicardial fat tissue a marker of metabolic syndrome in obese children? Atherosclerosis 2010, 211, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, G.; Piedimonte, A.; Podagrosi, M.; Mercurio, R.; Mosca, A.; D’Avanzo, M.; Vania, A. Epicardial adipose tissue and signs of metabolic syndrome in children. Eat. Weight Disord.-Stud. Anorex. Bulim. Obes. 2016, 21, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Trandafir, L.M.; Cojocaru, E.; Moscalu, M.; Leon Constantin, M.M.; Miron, I.; Mastaleru, A.; Teslariu, O.; Datcu, M.E.; Fotea, S.; Frăsinariu, O. Predictive Markers of Early Cardiovascular Impairment and Insulin Resistance in Obese Pediatric Patients. Diagnostics 2021, 11, 735. [Google Scholar] [CrossRef]
- Güneş, H.; Güneş, H.; Temiz, F. Relação entre o Tecido Adiposo Epicárdico e Resistência à Insulina em Crianças Obesa: Tecido adiposo epicárdico e resistência à insulina. Arq. Bras. Cardiol. 2020, 11, 675–682. [Google Scholar] [CrossRef]
- Abacı, A.; Tascılar, M.E.; Sarıtas, T.; Yozgat, Y.; Yesilkaya, E.; Kılıc, A.; Okutan, V.; Lenk, M.K. Threshold value of subepicardial adipose tissue to detect insulin resistance in obese children. Int. J. Obes. 2009, 33, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Güneş, H.; Güneş, H.; Özmen, Ş.; Çelik, E.; Temiz, F. Effects of metformin on epicardial adipose tissue and atrial electromechanical delay of obese children with insulin resistance. Cardiol. Young 2020, 30, 1429–1432. [Google Scholar] [CrossRef] [PubMed]
- Elshorbagy, H.H.; Fouda, E.R.; Kamal, N.M.; Bassiouny, M.M.; Fathi, W.M. Evaluation oF Epicardial Fat and Carotid Intima-Media Thickness in Obese Children. Iran. J. Pediatr. 2016, 26, e2968. [Google Scholar] [CrossRef] [PubMed]
- Van Hoek, E.; Koopman, L.P.; Feskens, E.J.M.; Janse, A.J. Assessment of epicardial adipose tissue in young obese children. Child Adolesc. Obes. 2019, 2, 96–107. [Google Scholar] [CrossRef]
- Schweighofer, N.; Rupreht, M.; Marčun Varda, N.; Caf, P.; Povalej Bržan, P.; Kanič, V. Epicardial Adipose Tissue: A Piece of The Puzzle in Pediatric Hypertension. J. Clin. Med. 2023, 12, 2192. [Google Scholar] [CrossRef] [PubMed]
- Bedir, A.; Mehmet, B.; Cevriye, A. Relationship of Epicardial Adipose Tissue Thickness with Early Indicators of Atherosclerosis and Cardiac Functional Changes in Obese Adolescents with Metabolic Syndrome. J. Clin. Res. Pediatr. Endocrinol. 2013, 5, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Aldiss, P.; Davies, G.; Woods, R.; Budge, H.; Sacks, H.S.; Symonds, M.E. ‘Browning’ the cardiac and peri-vascular adipose tissues to modulate cardiovascular risk. Int. J. Cardiol. 2017, 228, 265–274. [Google Scholar] [CrossRef]
- Calcaterra, V.; Cena, H.; Casali, P.M.; Iacobellis, G.; Albertini, R.; De Amici, M.; de Silvestri, A.; Comparato, C.; Pelizzo, G. Epicardial Fat Thickness in Non-Obese Neurologically Impaired Children: Association with Unfavorable Cardiometabolic Risk Profile. Ann. Nutr. Metab. 2018, 72, 96–103. [Google Scholar] [CrossRef]
- Turkmen, K.; Ozer, H.; Kusztal, M. The Relationship of Epicardial Adipose Tissue and Cardiovascular Disease in Chronic Kidney Disease and Hemodialysis Patients. J. Clin. Med. 2022, 11, 1308. [Google Scholar] [CrossRef]
- Aydin, E.; Altin, C.; Sakallioglu, O.; Yilmaz, M.; Gezmis, E.; Sade, L.E.; Müderrisoglu, H. Epicardial Adipose Tissue Thickness and Carotid Intima-Media Thickness in Hemodialysis Patients. Acta Cardiol. Sin. 2017, 33, 266–272. [Google Scholar] [CrossRef]
- Cano Megías, M.; Guisado Vasco, P.; Bouarich, H.; Aguilera, I.L.; De Arriba-de La Fuente, G.; Rodríguez-Puyol, D. Tejido graso epicárdico, calcificación arterial coronaria y mortalidad en pacientes con enfermedad renal crónica avanzada y hemodiálisis. Nefrología 2021, 41, 174–181. [Google Scholar] [CrossRef]
- Pecoits-Filho, R.; Lindholm, B.; Stenvinkel, P. The malnutrition, inflammation, and atherosclerosis (MIA) syndrome—The heart of the matter. Nephrol. Dial. Transplant. 2002, 17, 28–31. [Google Scholar] [CrossRef]
- Pelizzo, G.; Calcaterra, V.; Acierno, C.; Cena, H. Malnutrition and Associated Risk Factors Among Disabled Children. Special Considerations in the Pediatric Surgical “Fragile” Patients. Front. Pediatr. 2019, 7, 86. [Google Scholar] [CrossRef]
- Ozcicek, A.; Ozcicek, F.; Yildiz, G.; Timuroglu, A.; Demirtas, L.; Buyuklu, M.; Kuyrukluyildiz, U.; Akbas, E.M.; Topal, E.; Turkmen, K. Neutrophil-to-lymphocyte ratio as a possible indicator of epicardial adipose tissue in patients undergoing hemodialysis. Arch. Med. Sci. 2017, 1, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Cordova, R.; Viallon, V.; Fontvieille, E.; Peruchet-Noray, L.; Jansana, A.; Wagner, K.-H.; Kyrø, C.; Tjønneland, A.; Katzke, V.; Bajracharya, R.; et al. Consumption of ultra-processed foods and risk of multimorbidity of cancer and cardiometabolic diseases: A multinational cohort study. Lancet Reg. Health-Eur. 2023, 35, 100771. [Google Scholar] [CrossRef] [PubMed]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Saturated Fatty Acid and Trans-Fatty Acid Intake for Adults and Children: WHO Guideline; WHO Guidelines Approved by the Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2023; ISBN 978-92-4-007363-0.
- de Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schünemann, H.; Beyene, J.; et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015, 351, h3978. [Google Scholar] [CrossRef] [PubMed]
- Te Morenga, L.; Montez, J.M. Health effects of saturated and trans-fatty acid intake in children and adolescents: Systematic review and meta-analysis. PLoS ONE 2017, 12, e0186672. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.E.; Matthan, N.R.; Goldbaum, A.; Meng, H.; Lamon-Fava, S.; Lakshman, S.; Jang, S.; Molokin, A.; Solano-Aguilar, G.; Urban, J.F.; et al. Dietary patterns influence epicardial adipose tissue fatty acid composition and inflammatory gene expression in the Ossabaw pig. J. Nutr. Biochem. 2019, 70, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Jung, J.; Mintz, G.S.; Jin, U.; Park, J.-S.; Park, B.; Shin, H.-B.; Seo, K.-W.; Yang, H.-M.; Lim, H.-S.; et al. Epicardial Adipose Tissue Thickness Is Related to Plaque Composition in Coronary Artery Disease. Diagnostics 2022, 12, 2836. [Google Scholar] [CrossRef]
- Pacifico, L.; Bonci, E.; Di Martino, M.; Versacci, P.; Andreoli, G.; Silvestri, L.M.; Chiesa, C. A double-blind, placebo-controlled randomized trial to evaluate the efficacy of docosahexaenoic acid supplementation on hepatic fat and associated cardiovascular risk factors in overweight children with nonalcoholic fatty liver disease. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Rabkin, S.W.; Campbell, H. Comparison of reducing epicardial fat by exercise, diet or bariatric surgery weight loss strategies: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Feng, J.; Zhang, G.; Yuan, X.; Li, F.; Yang, T.; Hao, S.; Huang, D.; Hsue, C.; Lou, Q. Visceral adipose tissue is more strongly associated with insulin resistance than subcutaneous adipose tissue in Chinese subjects with pre-diabetes. Curr. Med. Res. Opin. 2018, 34, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Muniyappa, R.; Sowers, J.R. Role of insulin resistance in endothelial dysfunction. Rev. Endocr. Metab. Disord. 2013, 14, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Budoff, M. Triglycerides and Triglyceride-Rich Lipoproteins in the Causal Pathway of Cardiovascular Disease. Am. J. Cardiol. 2016, 118, 138–145. [Google Scholar] [CrossRef]
- Genovesi, S.; Giussani, M.; Orlando, A.; Orgiu, F.; Parati, G. Salt and Sugar: Two Enemies of Healthy Blood Pressure in Children. Nutrients 2021, 13, 697. [Google Scholar] [CrossRef]
- Cena, H.; Fonte, M.L.; Casali, P.M.; Maffoni, S.; Roggi, C.; Biino, G. Epicardial fat thickness: Threshold values and lifestyle association in male adolescents. Pediatr. Obes. 2015, 10, 105–111. [Google Scholar] [CrossRef]
- Velázquez-López, L.; Santiago-Díaz, G.; Nava-Hernández, J.; Muñoz-Torres, A.V.; Medina-Bravo, P.; Torres-Tamayo, M. Mediterranean-style diet reduces metabolic syndrome components in obese children and adolescents with obesity. BMC Pediatr. 2014, 14, 175. [Google Scholar] [CrossRef]
- Grosso, G.; Mistretta, A.; Frigiola, A.; Gruttadauria, S.; Biondi, A.; Basile, F.; Vitaglione, P.; D’Orazio, N.; Galvano, F. Mediterranean Diet and Cardiovascular Risk Factors: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 593–610. [Google Scholar] [CrossRef]
- Rosendo-Silva, D.; Viana, S.; Carvalho, E.; Reis, F.; Matafome, P. Are gut dysbiosis, barrier disruption, and endotoxemia related to adipose tissue dysfunction in metabolic disorders? Overview of the mechanisms involved. Intern. Emerg. Med. 2023, 18, 1287–1302. [Google Scholar] [CrossRef]
- Pisano, E.; Bugli, F.; Severino, A.; Pedicino, D.; Paroni Sterbini, F.; Martini, C.; De Maio, F.; Vinci, R.; Sacconi, A.; Canonico, F.; et al. Microbial signature of plaque and gut in acute coronary syndrome. Sci. Rep. 2023, 13, 14775. [Google Scholar] [CrossRef] [PubMed]
- Hamer, M.; Venuraju, S.M.; Urbanova, L.; Lahiri, A.; Steptoe, A. Physical Activity, Sedentary Time, and Pericardial Fat in Healthy Older Adults. Obesity 2012, 20, 2113–2117. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.; Di Renzi, P.; Pirro, M.R.; Borgognoni, F.; Vincentelli, G.M. New evidences about the strict relationship between the epicardial fat and the aerobic exercise. IJC Metab. Endocr. 2015, 6, 55–58. [Google Scholar] [CrossRef]
- Launbo, N.; Zobel, E.H.; Von Scholten, B.J.; Færch, K.; Jørgensen, P.G.; Christensen, R.H. Targeting epicardial adipose tissue with exercise, diet, bariatric surgery or pharmaceutical interventions: A systematic review and meta-analysis. Obes. Rev. 2021, 22, e13136. [Google Scholar] [CrossRef]
- Kim, M.-K.; Tomita, T.; Kim, M.-J.; Sasai, H.; Maeda, S.; Tanaka, K. Aerobic exercise training reduces epicardial fat in obese men. J. Appl. Physiol. 2009, 106, 5–11. [Google Scholar] [CrossRef]
- Chuensiri, N.; Suksom, D.; Tanaka, H. Effects of High-Intensity Intermittent Training on Vascular Function in Obese Preadolescent Boys. Child. Obes. 2018, 14, 41–49. [Google Scholar] [CrossRef]
- Seo, Y.-G.; Lim, H.; Kim, Y.; Ju, Y.-S.; Lee, H.-J.; Jang, H.; Park, S.; Park, K. The Effect of a Multidisciplinary Lifestyle Intervention on Obesity Status, Body Composition, Physical Fitness, and Cardiometabolic Risk Markers in Children and Adolescents with Obesity. Nutrients 2019, 11, 137. [Google Scholar] [CrossRef]
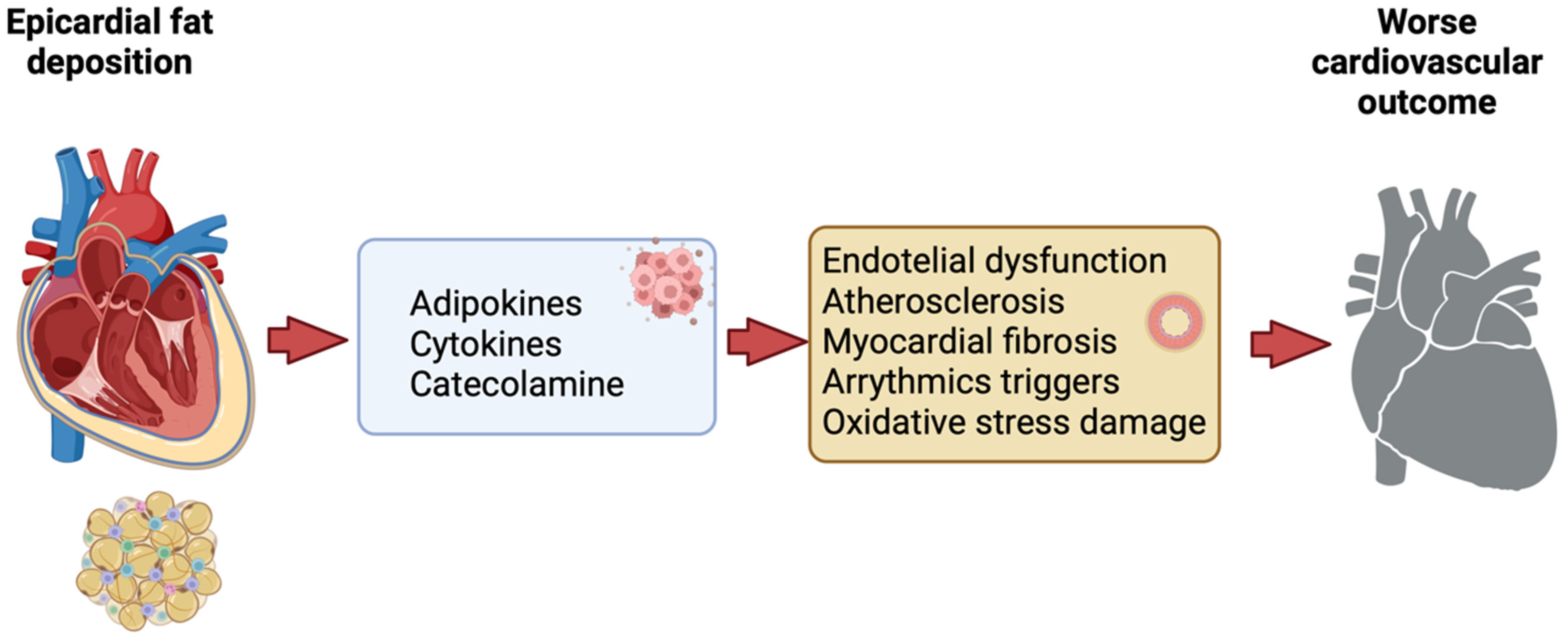
| First Author, Year of Publication | Study Type | Population | Main Result | References |
|---|---|---|---|---|
| Ozdemir et al. (2010) | Cross-sectional study | 106 obese and 62 lean children (aged 11–14 years) | Increased thickness of EAT in obese children (6.99 ± 1.45 mm vs. 3.93 ± 0.68 mm in lean subjects). Positive correlation between EAT thickness and BMI, left atrial diameter and left ventricular mass. | [9] |
| Schusterova et al. (2014) | Observational study | 25 overweight and obese subjects and 24 lean controls (aged 10–16 years) | In obese children, EAT thickness is correlated with an unfavorable cardiometabolic profile; it is positively with blood pressure, triglycerides, uric acid, apoprotein B, and hepatic enzymes and negatively correlated with HDL levels. EAT is a more accurate metabolic predictor than waist circumference but is not a stronger indicator than BMI. | [22] |
| Mazur et al. (2010) | Cross-sectional observational study | 52 obese children and 54 controls (aged 11–15 years) | No statistically significant correlation was found between EAT thickness and the HOMA index. Similarly, there was no significant difference in EATT between obese children with or without metabolic syndrome. | [27] |
| Barbaro et al. (2016) | Longitudinal pilot study | 73 prepuberal children (average age of 8.22 years) | For patients with a weight >90th percentile and a family history of metabolic syndrome risk factors, EAT values correlate positively with anthropometric parameters and metabolic markers (triglycerides, insulin, HOMA index) and negatively with HDL levels. | [28] |
| Abaci et al. (2009) | Cross-sectional study | 46 obese children and 30 lean controls (10.2 ± 2.5 years of age) | No significant correlation was observed between EAT and insulin resistance (r = 0.170, p = 0.253), but there was a significant correlation between EAT and BMI, age, and IMT. | [31] |
| Güneş et al. (2020) | Prospective and cross-sectional study | 94 obese patients (aged 8–18 year) | A significant association was established between EAT and insulin resistance (IR). The identified optimal cut-off value for EAT to predict IR was >3.85 mm (92.5% specificity and 68.5% sensitivity) | [30] |
| Schweighofer et al. (2023) | Prospective case–control study | 72 children and adolescents with normal BMI (aged 12–19 years) | Hypertensive patients have a higher volume (16.5 ± 1.9 cm3 and 10.9 ± 1.5 cm3) and thickness (0.8 ± 0.3 cm and 0.4 ± 0.1 cm) of EAT compared to their healthy peers. The volume of EAT might be a potential predictor of arterial hypertension in children. | [35] |
| Elshorbagy et al. (2016) | Prospective cohort study | 60 obese adolescents and 25 controls (aged 8–16 years) | EAT increased in MS patients compared to the control group. EATT emerged as a predictor for carotid IMT. | [33] |
| Bedir et al. (2013) | Cross-sectional study | 138 obese adolescents and 63 lean subjects (aged 9–18 years) | Close association between EAT and carotid IMT, along with early cardiac dysfunction, in obese adolescents with MS. Echocardiographic EAT appears to be a more effective indicator of premature atherosclerosis than waist circumference in MS patients. | [36] |
| Calcaterra et al. (2018) | Cross-sectional study | 32 disabled patients (12.4 ± 6.3 years) | EAT values in neurologically impaired children were higher than those in the control group (p = 0.02). EAT showed correlations with gender, age, pubertal stage, and WHtR. Significantcorrelations were found between EAT levels and abnormal triglycerides and HOMA-IR. | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calcaterra, V.; Cena, H.; Garella, V.; Loperfido, F.; Chillemi, C.; Manuelli, M.; Mannarino, S.; Zuccotti, G. Assessment of Epicardial Fat in Children: Its Role as a Cardiovascular Risk Factor and How It Is Influenced by Lifestyle Habits. Nutrients 2024, 16, 420. https://doi.org/10.3390/nu16030420
Calcaterra V, Cena H, Garella V, Loperfido F, Chillemi C, Manuelli M, Mannarino S, Zuccotti G. Assessment of Epicardial Fat in Children: Its Role as a Cardiovascular Risk Factor and How It Is Influenced by Lifestyle Habits. Nutrients. 2024; 16(3):420. https://doi.org/10.3390/nu16030420
Chicago/Turabian StyleCalcaterra, Valeria, Hellas Cena, Vittoria Garella, Federica Loperfido, Claudia Chillemi, Matteo Manuelli, Savina Mannarino, and Gianvincenzo Zuccotti. 2024. "Assessment of Epicardial Fat in Children: Its Role as a Cardiovascular Risk Factor and How It Is Influenced by Lifestyle Habits" Nutrients 16, no. 3: 420. https://doi.org/10.3390/nu16030420
APA StyleCalcaterra, V., Cena, H., Garella, V., Loperfido, F., Chillemi, C., Manuelli, M., Mannarino, S., & Zuccotti, G. (2024). Assessment of Epicardial Fat in Children: Its Role as a Cardiovascular Risk Factor and How It Is Influenced by Lifestyle Habits. Nutrients, 16(3), 420. https://doi.org/10.3390/nu16030420










